Highlight
AtDsPTP1 was found to regulate ABA accumulation and act as a negative regulator in osmotic stress signalling during Arabidospsis seed germination and seedling establishment.
Key words: Abscisic acid (ABA), Arabidopsis, dual-specificity protein phosphatase, osmotic stress, root elongation, seed germination.
Abstract
Dual-specificity protein phosphatases (DsPTPs) target both tyrosine and serine/threonine residues and play roles in plant growth and development. We have characterized an Arabidopsis mutant, dsptp1, which shows a higher seed germination rate and better root elongation under osmotic stress than the wild type. By contrast, its overexpression line, DsPTP1-OE, shows inhibited seed germination and root elongation; and its complemented line, DsPTP1-Com, resembles the wild type and rescues DsPTP1-OE under osmotic stress. Expression of AtDsPTP1 is enhanced by osmotic stress in seed coats, bases of rosette leaves, and roots. Compared with the wild type, the dsptp1 mutant shows increased proline accumulation, reduced malondialdehyde (MDA) content and ion leakage, and enhanced antioxidant enzyme activity in response to osmotic stress. AtDsPTP1 regulates the transcript levels of various dehydration-responsive genes under osmotic stress. Abscisic acid (ABA) accumulation in dsptp1 under osmotic stress is reduced with reduced expression of the ABA-biosynthesis gene NCED3 and increased expression of the ABA-catabolism gene CYP707A4. AtDsPTP1 also regulates the expression of key components in the ABA-signalling pathway. In conclusion, AtDsPTP1 regulates ABA accumulation, and acts as a negative regulator in osmotic stress signalling during Arabidospsis seed germination and seedling establishment.
Introduction
The signalling pathways of plants under osmotic stress have been intensively studied during the past two decades (Hasegawa et al., 2000; Xiong et al., 2002; Xiong and Zhu, 2002; Fujii and Zhu, 2012). Different protein kinases and transcription factors reportedly minimize damage caused by osmotic stress (Droillard et al., 2004; Gilmour et al., 2004). Abscisic acid (ABA) synthesis is significantly induced by the stress and its signalling has a crucial function in abiotic stresses responses (Agarwal and Jha, 2010; Fujita et al., 2011). The core module of the ABA-signalling pathway in response to osmotic stress, as well as other abiotic stresses, has been elucidated (Ye et al., 2012). However, many components in the signalling network of osmotic stress remain unknown (Cutler et al., 2010). Further analysis is needed to determine the exact functions and connections among the components involved in the regulation of plant responses to osmotic stress.
Similar to protein kinases, protein phosphatases (PPs) are also indispensable in stress signalling. According to their dephosphorylation targets, PPs can be divided into two groups, namely serine/threonine phosphatases and protein tyrosine phosphatases (PTPs), both of which have key functions in plant growth regulation and abiotic stresses responses (Schweighofer et al., 2004; Schweighofer and Meskiene, 2008; Bartels et al., 2010). Dual-specificity protein phosphatases (DsPTPs) can target both tyrosine and serine/threonine (Keyse, 1995). Except for the active site motif, DsPTPs do not share sequence similarity with PTPs. DsPTPs often have strict substrate specificity, and each member usually acts on only one or a limited number of target proteins (Keyse, 1995). In Arabidopsis, different DsPTPs and their associated protein kinases control plant growth and development under stress conditions. For example, mutation of the MKP1 gene results in plant hypersensitivity to stresses imposed by ultraviolet light and methyl methanesulfonate (Ulm et al., 2001). By contrast, mkp1 plants show hyposensitivity to salt stress (Ulm et al., 2002). PHS1, a DsPTP, controls microtubule organization and embryonic development (Naoi and Hashimoto, 2004). Furthermore, AtPTEN1, which encodes another DsPTP, is expressed exclusively in pollen and is essential for pollen development (Gupta et al., 2002). These studies suggest that DsPTPs have diverse functions in different biological processes.
Increasing evidence has shown that many protein kinases have positive functions in the osmotic stress-signalling pathway (Fujii and Zhu, 2012). However, PPs are also involved in the signalling process. In alfalfa, MP2C, a PP2C, suppresses the lethal phenotype induced by STE11, which is involved in the hyperosmotic HOG1 cascade (Meskiene et al., 1998). Furthermore, MP2C dephosphorylates and inactivates the stress-activated SIMK (Meskiene et al., 2003). In Arabidopsis, AtPTP1 dephosphorylates AtMAPK4 and AtMAPK6 in vitro, leading to the inactivation of these kinases that participate in osmotic stress signalling (Huang et al., 2000; Gupta and Luan, 2003). Two Arabidopsis PP2Cs, ABI1 and ABI2, negatively regulate ABA signalling in drought-induced stomatal closure (Schweighofer et al., 2004). However, the function of DsPTPs in osmoregulation is still unknown. One report on the identification of the mkp1 mutant, which exhibits enhanced salt stress tolerance, indicates that the DsPTPs have critical functions in osmoregulation (Droillard et al., 2004). Investigation of the functions of AtDsPTPs in response to osmotic stress is of great significance.
The AtDsPTP1 gene of Arabidopsis was identified more than a decade ago. The AtDsPTP1 protein is an active phosphatase which can inactivate AtMPK4 protein in vitro (Gupta et al., 1998). However, little is known about its biological functions under osmotic stress. In this study, the dsptp1 mutant shows enhanced tolerance to osmotic stress during seed germination and seedling establishment. This DsPTP positively regulates ABA accumulation and its signalling pathway in response to osmotic stress.
Materials and methods
Plant materials and growth conditions
The T-DNA insertion mutant dsptp1 (SALK_092811) was obtained from the Arabidopsis Biological Resource Centre (http://www.arabidopsis.org/). It was grown and screened for homozygosity. All seeds were of the Columbia ecotype: mutant, WT, overexpression (OE), and complemented (Com). Arabidopsis thaliana L. plants were kept in a growth chamber with a 16h photoperiod at a photon flux density of ~200 µmol m–2 s–1 at day/night temperature of 23/20°C, and a relative humidity of 90%.
Stress and ABA treatments
Two-week-old Arabidopsis seedlings on MS plates were transferred to filter papers saturated with different concentrations of mannitol or ABA solution and incubated for different times.
Seed germination assays
WT, dsptp1-mutant, OE, and Com line seeds (>100 seeds for each replicate) were surface sterilized. The seeds were sown on MS medium (Murashige and Skoog, 1962; 1 × MS salts, 10g l–1 sucrose, and 10g l–1 agar, pH 5.7) with or without either different osmotic stress-generator solutions or ABA solutions and then incubated at 23°C with a 16h light photoperiod. The number of planted and germinated seeds was recorded 7 d after being sown on the medium. Radicle emergence of >1mm indicated seed germination. Three replicate plates were used for each treatment.
Root elongation measurements
Arabidopsis seeds were sown on MS medium as above, stratified for 3d, and then incubated at 23°C for 7 d. For root elongation measurements, 15 seeds were used per replicate, and three replicates were made for each treatment. Seven-d-old seedlings with roots 1–1.5cm long were transferred from agar plates containing MS medium onto a second agar medium supplemented with different concentrations of osmotic stress-generator. Increases in root length were measured after 7 d of treatment (Rosado et al., 2006).
Histochemical analysis of GUS activity
A DNA fragment containing 1139bp of the upstream region of the ATDsPTP1 gene was amplified by PCR using oligonucleotides incorporating XbaI or BamHI sites at the 5’ ends [5’-TGTCTAGACCTTGGTTTCTGTTACTTGGCTC-3’
(XbaI site underlined) and 5′- AAGGATCCGAAGGA ATACGAATT CGCCAAAG-3′ (BamHI site underlined)]. The PCR fragments were digested with XbaI and BamHI and then cloned into the XbaI-BamHI site of a promoterless GUS expression vector, pBI101.3 (Clontech). The resulting construct, ATDsPTP1pro-GUS, was transferred from Escherichia coli DH5α into Agrobacterium tumefaciens GV3101. Arabidopsis was transformed by vacuum infiltration with Agrobacterium containing ATDsPTP1pro-GUS. Following the methods described by Bechtold et al. (1993), histochemical localization of independent transgenic lines was performed in 5-bromo-4-chloro-3-indolyl glucuronide (X-gluc) buffer (50mM sodium phosphate buffer, pH 7.0, 10mM EDTA, 0.1% Triton X-100, 2% DMSO, 0.5mM potassium ferrocyanide, 2mg ml–1 X-gluc) at 37°C for 6–12h. The stained plants were washed for 30min with 50–100% ethanol to remove the chlorophyll.
Isolation of total RNA and quantitative real-time RT-PCR
Two-week-old plants were transferred from MS agar plates to Petri dishes and placed on a filter paper soaked with 330mM mannitol solution osmotic treatment. Total RNA was isolated from Arabidopsis seeds or leaves using an RNeasy Plant mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. A 2 μg aliquot of RNA was reverse transcribed to cDNA with a SuperScript III RTS first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Transcript levels of each gene were measured by quantitative real-time RT-PCR (qRT-PCR) using an iCycler (Bio-Rad, Hercules, CA, USA) with iQ SYBR Green Supermix (Bio-Rad). To standardize the results, the amplification of AtACTIN was also determined and used as the internal standard. The data were normalized to the amplification of an Arabidopsis actin gene. For each sample, the mean value from three qRT-PCRs was used to calculate transcript abundance. The mean values were then plotted with their standard errors. The primers that were used in qRT-PCR are summarized in Supplementary Table S1.
Antioxidant enzyme assays
Frozen leaf segments (0.3g) were homogenized on ice with 1ml of 50mM potassium phosphate buffer (pH 7.0), containing 1mM EDTA and 1% polyvinylpyrrolidone (PVP). The buffer was mixed with 1mM ascorbate for the ascorbate peroxidase (APX) assay. The homogenate was centrifuged at 15 000g for 20min at 4°C and the supernatant was immediately used for the following antioxidant enzyme assays. Protein content was determined according to the method of Bradford (1976) with bovine serum albumin (BSA) as the standard.
The total activities of antioxidant enzymes were determined as described previously (Jiang and Zhang, 2001). Total superoxide dismutase (SOD) (EC 1.15.1.1) activity was assayed by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT). The 2.5ml reaction mixture contained 50mM potassium phosphate buffer (pH 7.8), 13mM methionine, 75mM NBT, 2mM riboflavin, 0.1mM EDTA, and 20 µl of enzyme extract. The reaction mixtures were illuminated for 15min at a light intensity of 5000 lux. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT as monitored at 560nm.
Total catalase (CAT) (EC 1.11.1.6) activity was assayed by measuring the decomposition rate of H2O2 (extinction coefficient 39.4mM–1 cm–1) at 240nm for 1min. The reaction mixture contained 50mM potassium phosphate buffer (pH 7.0), 10mM H2O2, and 20 µl of enzyme extract in a 3ml volume (Jiang and Zhang, 2001).
Total APX (EC 1.11.1.11) activity was measured by monitoring the decrease in absorbance at 290nm (extinction coefficient 2.8mM–1 cm–1) for 30 s in 1ml of a reaction mixture containing 50mM potassium phosphate buffer (pH 7.0), 0.5mM ascorbate, 0.1mM H2O2, and 20 µl of enzyme extract. The reaction was started with the enzyme extract. Correction was done for the low, non-enzymatic oxidation of ascorbate by H2O2 (Jiang and Zhang, 2001).
Total glutathione reductase (GR) (EC 1.6.4.2) activity was measured by following the oxidation of NADPH at 340nm (extinction coefficient 6.2mM–1 cm–1) for 1min in 1ml of an assay mixture containing 50mM potassium phosphate buffer (pH 7.8), 2mM Na2EDTA, 0.15mM NADPH, 0.5mM oxidized glutathione, and 100 µl of enzyme extract. The reaction was initiated by adding NADPH. Corrections were made for the background absorbance at 340nm without NADPH (Jiang and Zhang, 2001).
Proline assays
Four-week-old dsptp1 and WT (Col0) seedlings grown on soil were soaked with 330mM mannitol or 400mM sorbitol solution for different times. After treatment, the samples were frozen in liquid nitrogen and kept at –80°C for the proline assay. Proline concentration was determined following the methods described by Bates (1973).
Malondialdehyde (MDA) measurements
Lipid peroxidation was evaluated according to the method of Heath and Packer (1968) with slight modification; the reaction solution was heated in boiling water for 15min. The MDA content was measured from 0.5g of two-week-old Arabidopsis seedlings. The results are expressed as μmol g FW–1 of seedlings and correspond to means of measurements carried out with four extracts.
Ion leakage measurement
To measure ion leakage in mannitol-treated seedlings, two-week-old WT and dsptp1 seedlings grown on MS agar plates were carefully removed from the plate, rinsed briefly with distilled water, and placed in solutions containing 550mM mannitol for different times. After treatment, seedlings were rinsed briefly with distilled water and placed immediately in a tube with 5ml of water. The tube was agitated gently for 3h before the electrolyte content was measured. Three repetitions of each treatment were conducted.
Determination of endogenous ABA content
For the measurement of endogenous ABA, Arabidopsis leaves were ground in liquid nitrogen with the addition of 1ml of distilled water per 0.2g of frozen ground tissue, and then shaken at 4°C overnight. The homogenates were centrifuged at 12 000g for 20min at 4°C, and the supernatant was used directly for ABA assay. ABA analysis was carried out using RIA (radioimmune assay), as described by Quarrie et al. (1988). The reaction mixture (450 μl) contained 200 μl of phosphate buffer (pH 6.0), 100 μl of diluted antibody (Mac 252) (Abcam) solution (the antibody Mac252 was dissolved in PBS containing 5mg ml–1 BSA and 4mg ml–1 PVP), 100 μl of [3H]ABA (~8000 cpm) solution, and 50 μl of crude extract. The mixture was incubated at 4°C for 45min and bound radioactivity was measured in 50% saturated (NH4)2SO4-precipitated pellets with a liquid scintillation counter (Ye et al., 2011).
Generation of the DsPTP1-overexpressing (DsPTP1-OE) line and DsPTP1-complemented (DsPTP1-Com) line
Full-length Arabidopsis DsPTP1 cDNA was obtained by using reverse transcription-PCR, cloned into the pENTR-TOPO cloning vector (Invitrogen) and sequenced. After the LR reaction, DsPTP1 cDNA was inserted into the pGWB5 vector (a gift from Prof. Liang, YangZhou University) which had a 35S promoter; this vector was named pGWB5-DsPTP1. Transgenic Arabidopsis containing the cauliflower mosaic virus (CaMV) 35S promoter was generated using the floral-dipping method (Clough and Bent, 1998) and transferred into Col0 wild-type and dsptp1-mutant plants. Transformed plants were selected by growth on hygromycin-containing medium. Plants of the second generation after transformation were used for the experiments. The empty pGWB5 vector (the ccdb gene was substituted by a non-sense segment with a termination codon) which acted as control was also transferred into Col0 wild-type plants.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: DsPTP1, At3g23610; DREB2A, At5g05410; RD22, At5g25610; ICK1, At2g23430; ABF1, At1g49720; COR15A, At2g42540; ERD1, At5g51070; ZEP, At5G67030; NCED3, At3g14440; AAO3, At2G27150; CYP707A3, At5G45340; CYP707A4, At3g19270; ABI1, At4g26080; ABI3, At3g24650; ABI4, At2g40220; and ABI5, At2g36270.
Results
Phenotypic analyses of the dsptp1 mutant
To identify additional osmotic signalling components during seed germination, we obtained Arabidopsis T-DNA insertion mutants for the genes encoding various protein phosphatases. We also performed screening to identify any mutation that could lead to alteration in resistance to osmotic stress during seed germination. As shown in Fig. 1, dsptp1 shows enhanced tolerance to osmotic stress. The dsptp1 mutant carries a T-DNA insertion in the At3g23610 of the AtDsPTP1 gene. The results of real-time PCR and RT-PCR show that dsptp1 is a loss-of-function mutant because the AtDsPTP1 transcript level was hardly detected in the mutant, but it was obviously enhanced in the DsPTP1-OE line (OE-1, 2, 3, 4, 5, 6, 7) compared to the wild type, and it returned to a level similar to the wild type in the DsPTP1-Com line (Figs 1A, B; Fig. 2B). For this study we selected three independent transgenic lines (OE-5, 6, 7) that show high expression levels of DsPTP1 compared with the wild type. Under normal growth conditions in MS medium, no difference in germination percentage was observed among the WT, dsptp1 mutant, OE line (OE-5, 6, 7) and Com line seeds. However, the dsptp1-mutant seeds exhibited a significantly higher seed germination percentage than WT, while the OE line exhibited a lower seed germination percentage than WT (Figs. 1C, D; Supplementary Figure S1A, B). The Com line rescued the seed germination percentage to a level similar to that for WT seeds when compared with OE line seeds in MS medium supplemented with mannitol.
Fig. 1.
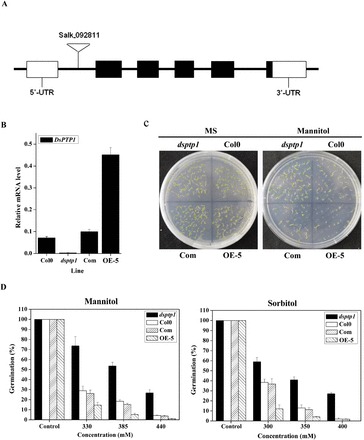
The dsptp1 mutant shows an increased germination percentage under osmotic stress. (A)
Structure of the AtDsPTP1 gene. The insertion of T-DNA in the dsptp1 mutant is shown as a triangle. Exons are shown as black boxes and introns as thin lines. (B) Real-time RT-PCR analysis for AtDsPTP1 transcript. The AtDsPTP1 transcript was present in total RNA from the WT, Com, and OE-5 lines but absent in the dsptp1-mutant plants. Relative expression levels of AtDsPTP1 are normalized to AtACTIN2 transcript levels. Values presented are mean ± SE of three different experiments. (C) Germination of WT (Col0), dsptp1-mutant, Com, and OE-5 line Arabidopsis seeds in normal and mannitol-containing media. (D) Percentage seed germination of dsptp1-mutant plants with and without different osmotic treatments. Arabidopsis seeds of WT (Col0) and DsPTP1 mutants were sown in MS medium with or without osmotic treatments including 330, 385, and 440mM mannitol (left) and 300, 350, and 400mM sorbitol. Values presented are mean ± SE of three different experiments.
To determine the sensitivity of the dsptp1 mutant to osmotic stress during seed germination, different concentrations of mannitol (330mM, 385mM, and 440mM) and sorbitol (300mM, 350mM, and 400mM) were supplied in the medium. The results show that the dsptp1 mutant exhibited an obviously higher seed germination percentage than WT, whereas the OE line exhibited the lowest seed germination percentage with the increasing mannitol and sorbitol concentrations (Fig. 1D; Supplementary Figures S1B, C).
Overexpression of DsPTP1 decreases osmotic tolerance in Arabidopsis
We generated the DsPTP1-OE lines and the DsPTP1-Com lines. The OE lines showed an increase in At3g23610 at the AtDsPTP1 transcript level relative to the WT plants, and the Com line showed a similar transcript level of AtDsPTP1 compared with the WT plants (Figs 1B, 2B). The seedlings of the OE lines, Com lines, dsptp1 mutant, and WT (Col0) were grown under osmotic stress. Their primary root growth was measured. Root growth of dsptp1-mutant plants was hyposensitive to mannitol (Fig. 2A, C; Supplementary Figure S1A, B) and sucrose (Fig. 2D; Supplementary Figure S1C) compared with that of WT plants (Col0). There was a similar result with a low concentration of sucrose in MS, which removes the sucrose (Supplementary Figure S4). By contrast, the OE lines were relatively more sensitive to osmotic agents than the WT (Fig. 2A, C, D; Supplementary Figure S1A, B, C), and the Com line rescued the root elongation of dsptp1 to a level similar to WT (Figs. 2A, C, D). These data indicate that AtDsPTP1 decreases osmotic stress tolerance in Arabidopsis.
Fig. 2.
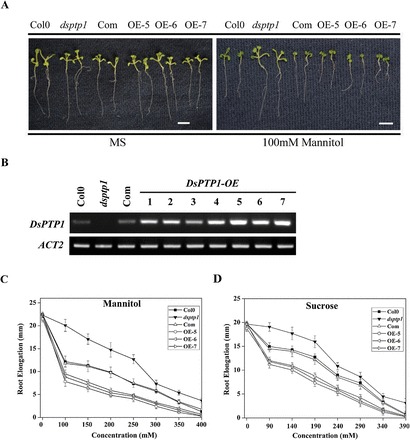
Root elongation in dsptp1 mutants is insensitive to osmotic stress. (A) Arabidopsis
root growth phenotypes of WT (Col0), dsptp1, and OE lines. Bar, 1cm. (B) The AtDsPTP1 transcript was present in total RNA from the WT, Com, and OE-1 to OE-7 plants, but absent in dsptp1-mutant plants. Relative expression levels of AtDsPTP1 were normalized to AtACT2 transcript levels. Values presented are mean ± SE of three different experiments. (C, D) dsptp1 seedlings show reduced root elongation inhibition under osmotic stress. Root lengths of Col0, dsptp1, and OE-5 line seedlings grown for 7 d on MS plates with mannitol (C) and sucrose (D) were measured to quantify their sensitivities to mannitol or sucrose. Seedlings were grown in MS medium for 1 week and then transferred to MS medium with or without various concentrations of mannitol and sucrose. Root elongation (i.e. increase in length after transfer) was determined after 7 d in Col0, dsptp1, and OE lines. Error bars indicate SE (n = 15). The experiments were repeated at least three times with similar results. Graphs correspond to one representative experiment.
Expression patterns of AtDsPTP1
We constructed a chimeric gene consisting of the ATDsPTP1 promoter fused to a GUS reporter gene (ATDsPTP1pro-GUS) to analyse the expression patterns of GUS activity driven by the ATDsPTP1 promoter in transgenic Arabidopsis plants. Spatiotemporal expression analysis showed GUS expression in seed endosperm (Fig. 3B), pollen grains (Fig. 3C, G), and abscission zones of siliques (Fig. 3I), but not in mature embryo (Fig. 3A), rosette, and cauline leaf (Fig. 3E, F). Strong GUS activity was detected in leaf petioles and roots of rosette plants when the transgenic plants were exposed to high osmolarity solutions (Fig. 3I, J). Similarly, strong GUS staining was observed in the seed coat (Fig. 4E, G, H), but not in the embryo (Figs. 4A, C, D) of dry and 3-d-germinating seeds that were exposed to both hyperosmotic solutions. These results suggest that the expression of the AtDsPTP1 gene is upregulated in response to external osmotic changes.
Fig. 3.
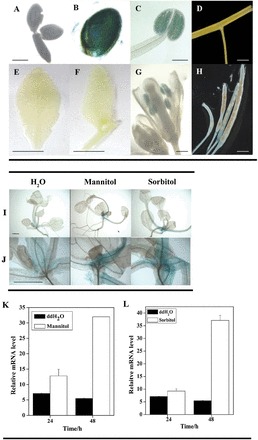
Expression patterns of the DsPTP1 gene in seedlings.
The AtDsPTP1 promoter-GUS activity was observed at various growth stages (A–J). (A) Embryo of dry seed (A). (B) Seed coat. (C) Pollen in anther. (D) Stems. (E) Mature rosette leaves. (F) Cauline leaves. (G) Flowers. (H) Siliques. Six independent lines were tested and observations repeated at least three times. (I) Twelve-day-old seedling. (J) Enlargement of (I); the right-hand photo in (J) is turned by 90° from the photo in (I). Six independent lines were tested and repeated at least three times. (A, B, C) Bar, 20 µm. (E, F) bar, 1cm; (D, G, H) bar, 1mm; (I, J); bar, 1mm. (K, L) Time course of mannitol-induced (K) and sorbitol-induced (L) gene expression of AtDsPTP1 in Arabidopsis seedlings. WT seedlings were treated with 440mM mannitol or 400mM sorbitol for various times. All seedlings treated with distilled water under the same conditions during the entire period served as controls. Relative expression levels of AtDsPTP1 were analysed by qRT-PCR and normalized to Atactin2 transcript levels. Values are presented are mean ± SE of three different experiments.
Fig. 4.
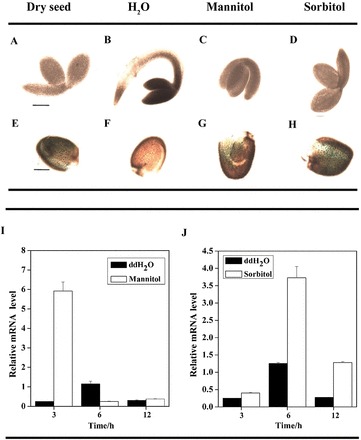
Induced expression patterns of the DsPTP1 gene in seeds under osmotic stress.
(A and E) Dry seed. (B–D). Three-day germinating seed. (F–H) Seed coat under different treatment conditions. Six independent lines were tested and repeated three times. Bar, 20 µm. (I, J) Time course of mannitol-induced (I) and sorbitol-induced (J) gene expression of AtDsPTP1 in Arabidopsis seeds. The WT Col0 seeds were treated with 440mM mannitol (I) and 400mM sorbitol (J) for various times. In all cases, seeds treated with distilled water under the same conditions during the entire period served as controls. Relative expression levels of AtDsPTP1 were analysed by qRT-PCR and normalized to Atactin2 transcript levels. Values presented are mean ± SE of three different experiments.
We also assayed the transcript level of DsPTP1 in germinating Arabidopsis seeds from 3 to 12h after imbibing and in 1 and 2 d seedlings under different treatments using qRT-PCR. In accordance with the results of GUS staining, the AtDsPTP1 expression in WT seeds was sharply induced by mannitol (Fig. 4I) and sorbitol (Fig. 4J) solutions. The transcript level of AtDsPTP1 in WT seedlings was also enhanced at 48h after mannitol (Fig. 3K) and sorbitol (Fig. 3L) treatments. These results indicate that AtDsPTP1 is induced by osmotic stress.
Increased proline content and resistance to oxidative damage in dsptp1 plants
Proline accumulation has adaptive functions in plant stress tolerance, acting as a compatible osmolyte that stores carbon and nitrogen (Hare et al., 1997). Previous studies in vitro showed that proline can be a reactive oxygen species (ROS) scavenger (Smirnoff and Cumbes, 1989), and a molecular chaperone that stabilizes protein structure. Proline accumulation can buffer cytosolic pH and balance cell redox status; it is also part of the stress signal that influences adaptive responses (Maggio et al., 2002). Proline accumulation in the dsptp1 mutant and WT plants treated with mannitol and sorbitol was measured. As shown in Fig. 5, the proline content in both dsptp1 mutant and WT plants increased in response to 330mM mannitol treatment. However, the increase in the dsptp1-mutant plants was more remarkable than that in the WT plants at 48h after mannitol treatment (Fig. 5A). A similar trend in proline content was observed between the dsptp1 mutant and WT plants treated with 400mM sorbitol for 36h (Fig. 5B).
Fig. 5.
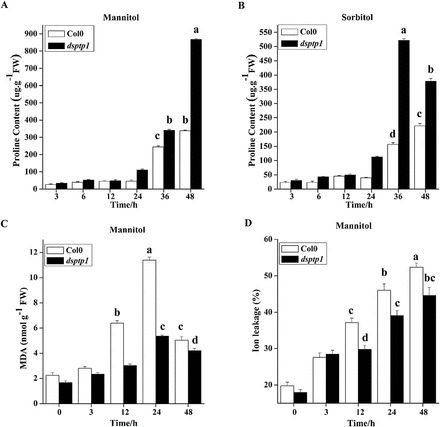
The dsptp1 mutant affects the physiological index under osmotic stress.
(A, B) Proline content determined in WT and dsptp1 mutants under mannitol treatment (A) and sorbitol treatment (B). (C, D) MDA content (C) and ion leakage (D) determined in WT and dsptp1 mutants under mannitol treatment. Values presented are mean ± SE of three different experiments. Means denoted by the same letter do not significantly differ at P < 0.05 according to Duncan’s multiple range test.
MDA is produced by lipid peroxidation because of the effect of ROS (Halliwell and Chirico, 1993). As the best-characterized product of lipid peroxidation, MDA is often used as a biomarker to measure cell membrane injury and cell and tissue oxidative damage (Esterbauer et al., 1991). To determine the degree of cellular injury, we determined the MDA content in the dsptp1 mutant and WT plants with 550mM mannitol treatment. The MDA content increased in both dsptp1-mutant and WT plants after the treatment. However, the increase in the dsptp1-mutant seedlings was less than half of that in the WT seedlings after the treatment. Although the dsptp1-mutant seedlings had slightly lower MDA content than the WT seedlings even without stress treatment, the increase in the MDA level was significantly lower in the mutant than in the WT plants after 12 and 24h of mannitol treatment (Fig. 5C). Similar results were obtained with the 330mM mannitol treatment (Supplementary Figure S5).
After the dsptp1-mutant and WT seedlings were treated with 550mM mannitol, ion leakage was measured as an indicator of osmotic stress-induced cellular damage. Although the dsptp1-mutant seedlings had no obvious differences in ion leakage compared with the WT plants without stress treatment, mannitol treatment resulted in a sharper increase in electrolyte leakage in the WT plants than in the dsptp1-mutant plants (Fig. 5D). These data indicate that the dsptp1-mutant plants are less sensitive to osmotic stress.
To investigate the effects of AtDsPTP1 on the activities of antioxidant enzymes, we determined the total activities of antioxidant enzymes, including APX, SOD, GR, and CAT. Fig. 6 shows that the osmotic stress-induced APX (Fig. 6A), SOD (Fig. 6B), GR (Fig. 6C), and CAT (Fig. 6D) activities of the dsptp1-mutant plants are significantly higher than those of the WT plants. These findings suggest that dsptp1 has an enhanced ability to scavenge the extra ROS under osmotic stress to maintain the equilibrium between ROS production and scavenging. These data indicate that the dsptp1-mutant plants can attenuate cell damage caused by osmotic stress.
Fig. 6.
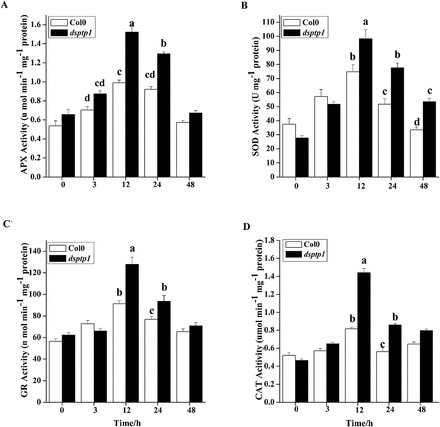
Effects of DsPTP1 on the activities of antioxidant enzymes in Arabidopsis seedlings exposed to mannitol treatment.
(A–D) Total activities of antioxidant enzymes APX (A), SOD (B), GR (C), and CAT (D). Seedlings were treated with mannitol for the times indicated. Values are mean ± SE of three different experiments. Means denoted by the same letter do not significantly differ at P < 0.05 according to Duncan’s multiple range test.
The dsptp1 mutant enhances expression of osmotic responsive genes
To determine the function of AtDsPTP1 in signalling pathways in response to osmotic stress, we analysed the expression of genes regulated by abiotic stress in WT and dsptp1-mutant plants with or without mannitol treatment. As shown in the real-time PCR results in Fig. 7, significant differences in the expression of general stress-responsive genes (e.g. RD22 and ICK1) and ABA-response genes (e.g. ABF1) were found between the WT and the dsptp1-mutant plants. In addition, osmotic stress-induced expression was different in the dsptp1-mutant and WT seedlings for ERD1 (early response to dehydration 1) (Fig. 7F), COR15A (Fig. 7E), and DREB2A (Fig. 7A). ERD1 encodes a chloroplast-targeted chaperonin possibly involved in proteolysis (Weaver et al., 1998), COR15A encodes a chloroplast-targeted LEA protein (Artus et al., 1996), and DREB2A encodes a transcription factor activated in the early stages of the ERD1 osmotic stress response (Kiyosue et al., 1994).
Fig. 7.
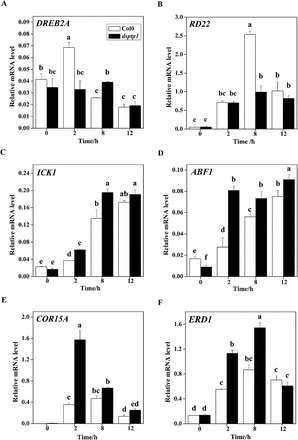
The dsptp1 mutant shows altered expression of several dehydration-responsive genes after osmotic stress: DREB2A (A), RD22 (B), ICK (C), ABF (D), COR15A (E), and ERD1 (F). The expression level of these genes in response to stress was analysed by qRT-PCR. Total RNA was isolated from 15-d-old WT, Col0, and dsptp1 seedlings treated with 385mM mannitol for 0h, 2h, 8h, and 12h. In all experiments, the expression of the constitutive Actin2 gene was used as the control. Three replicates were made for each treatment with similar results. Values are mean ± SE of three different experiments. Means denoted by the same letter do not significantly differ at P < 0.05 according to Duncan’s multiple range test.
Shortly after osmotic treatment, DREB2A and RD22 transcripts accumulated to significantly higher levels in the WT than in the dsptp1-mutant plants (Figs. 7A, B). By contrast, the induction of ICK1, ABF1, COR15A, and ERD1 expression was higher in the dsptp1-mutant plants than that in the WT plants after osmotic treatment (Figs 7C–F). The compromised induction of different dehydration-responsive genes together with reduced sensitivity to osmotic stress in dsptp1 indicated that DsPTP1 is possibly important in the dehydration-signalling pathway.
The dsptp1 mutant reduces ABA content and suppresses the ABA-signalling pathway under osmotic stress
To test the sensitivity of the dsptp1 mutant to ABA, we determined the effect of ABA on the germination, cotyledon expansion, and root elongation of dsptp1. The radicle emergence of seeds was observed after 7 d of sowing on MS medium supplemented with different ABA concentrations (Fig. 8A; Supplementary Figure S2A). The inhibition of seed germination by ABA in the dsptp1-mutant plants was less obvious than that in the WT plants, whereas the OE-line seeds were more sensitive to ABA than were the WT seeds. Seed germination percentage in the Com lines was similar to that of WT seeds. The effects of ABA on root elongation were also observed among dsptp1, WT, OE lines and Com lines. Root elongation was measured 7 d after 7-d-old seedlings were transferred to MS medium supplemented with different ABA concentration (Fig. 8B; Supplementary Figure S2B). The inhibition of root elongation by ABA was reduced in the dsptp1-mutant plants and enhanced by overexpression of DsPTP1 compared with the WT plants. Root elongation in the Com lines was similar to that of WT seeds (Fig. 8B).
Fig. 8.
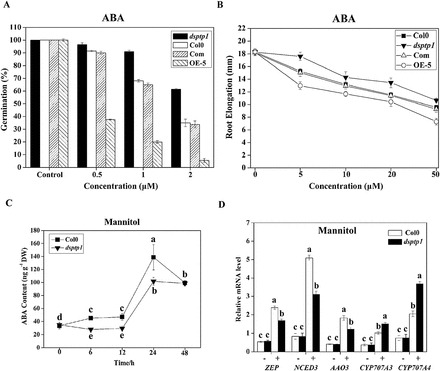
DsPTP1 is involved in osmotic stress- and ABA- signalling. (A) Percentage Arabidopsis seed germination with WT, dsptp1, OE, and Com lines in MS medium supplemented with or without 0.5, 1, and 2 μM ABA. (B) Seedlings were grown in MS medium for 1 week and then transferred to MS medium with or without 5, 10, 20, and 50 μM ABA. Root elongation (increase in length after transfer) was determined after 7 d. Error bars indicate SE (n = 15 for root elongation; n = 3 for germination; each experiment contained 100 seeds). Three replicates were made for each treatment with similar results, and graphs correspond to one representative experiment.
(C) Determination of endogenous ABA level in WT and dsptp1 seedlings after mannitol treatments. Expression differences of the ABA-biosynthesis key genes ZEP, NCED, AAO3, and catabolic gene CYP707A3 and CYP707A4, in WT and dsptp1 were analysed before or after osmotic stress. Values are mean ± SE of three different experiments. Means denoted by the same letter do not significantly differ at P < 0.05 according to Duncan’s multiple range test.
The ABA contents in the dsptp1-mutant and WT plants were also measured using a radioimmunoassay (RIA) method. In the absence of stress treatment, WT and dsptp1 leaves had similar ABA contents (Fig. 8C). When the plants were treated with 440mM mannitol for the indicated time, the endogenous ABA level in the WT plants was higher than that in the dsptp1-mutant plants.
To investigate the function of DsPTP1 in ABA accumulation in response to osmotic stress, we analysed the expression of genes encoding enzymes for ABA biosynthesis and catabolism in the WT and dsptp1-mutant plants (Nambara and Marion-Poll, 2005). The qRT-PCR results showed that the expressions of all the ABA-biosynthesis genes and the catabolic genes are similar without osmotic stress. The expression level of all the genes assayed for ABA biosynthesis (ZEP, NCED3, and AAO3) and catabolism (CYP707A3 and CYP707A4) increased after osmotic stress treatment. However, the magnitudes of the increases varied. The expression level of the biosynthesis gene NCED3 markedly increased to a higher level in the WT plants compared with the dsptp1-mutant plants (Fig. 7D). By contrast, the catabolic gene CYP707A4 was expressed at an obviously lower level in the WT plantscompared with the dsptp1 mutant (Fig. 7D). This result indicates that DsPTP1 affects the endogenous ABA content by regulating the genes for ABA biosynthesis and catabolism after osmotic stress treatment.
To explore the function of AtDsPTP1 in ABA-signalling pathways in response to osmotic stress, we analysed the expression of genes that regulate ABA responses in WT and dsptp1-mutant plants both before and after mannitol treatment. The results of real-time PCR showed significant differences between the WT and the dsptp1-mutant plants in terms of the expression of ABA-regulated genes, such as ABI1, ABI3, and ABI5 (Fig. 9). This indicates that AtDsPTP1 probably acts upstream of these genes in the ABA pathways. The induction of ABI3 (Fig. 9B) and ABI5 (Fig. 9D) expression was significantly lower in dsptp1-mutant plants than in the WT plants with mannitol treatment. By contrast, the induction of ABI1 increased in the dsptp1-mutant plants compared with the WT plants after osmotic stress (Fig. 9A). We also measured the expression of ABA-response genes (ABI1, 3, 4, 5) in the WT and dsptp1 mutant under 20 µM ABA treatment; the dsptp1 mutant showed less sensitivity to ABA compared with the WT (Supplementary Figure S3).
Fig. 9.
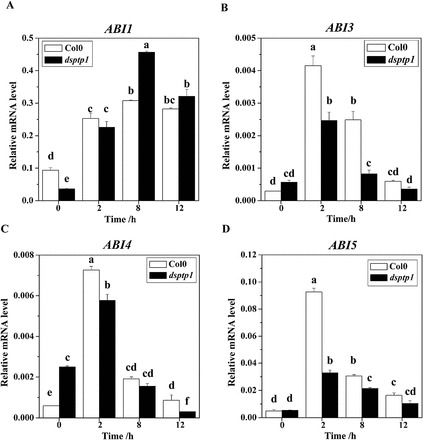
The dsptp1 mutant shows altered expression of ABA-regulated genes under osmotic stress. Expression levels of several genes in response to ABA signalling were analysed by qRT-PCR (A–D): ABI1 (A), ABI3 (B), ABI4 (C), and ABI5 (D). Total RNA was isolated from 15-d-old WT and dsptp1 seedlings treated with 385mM mannitol for the times indicated. In all experiments, the expression of the constitutive Actin2 gene was used as the control. Three replicates were made for each treatment with similar results. Values are mean ± SE of three different experiments. Means denoted by the same letter do not significantly differ at P < 0.05 according to Duncan’s multiple range test.
Discussion
Protein kinases and PPs have critical functions in plant development and stress responses (Fujii and Zhu, 2012). Members from different sub-families of PPs were studied for their biological functions (Schweighofer et al., 2004; Schweighofer and Meskiene, 2008; Bartels et al., 2010). As a member of the unique group that is capable of dephosphorylating both serine/threonine and tyrosine, DsPTP1 is little known, especially in plant stress responses. Thus, we characterized a dsptp1 mutant that is hyposensitive to osmotic stress during seed germination and root elongation of seedlings (Figs 1, 2; Supplementary Figure S1). With the genetic evidence, we studied the biological function of the DsPTP1 gene in response to stress conditions. The expression of the DsPTP1 gene is induced by osmotic stresses both in seedlings (Fig. 3K, L) and seeds (Fig. 4I, J). Knockout of the AtDsPTP1 gene results in enhanced tolerance, whereas overexpression of the AtDsPTP1 gene results in an attenuated tolerance compared with the wild type, and the AtDsPTP1-complemented lines rescued the tolerance of the dsptp1 mutant to the wild-type level under these stress conditions (Figs 1, 2; Supplementary Figure S1). Our results indicate that the AtDsPTP1 gene is involved in osmotic stress responses and possibly has a negative regulatory function.
A promoter-reported fusion analysis indicated that DsPTP1 is strongly expressed in the endosperm layer in the seed coat of dry seeds (Figs 3B, 4E), but that this disappears after imbibition (Fig. 4F). Seed coats have a key function in maintaining seed dormancy (Holdsworth et al., 2008), suggesting that DsPTP1 is involved in regulating seed germination; the induced expression of DsPTP1 occurs at the base and roots of rosettes, both of which are important for the maintenance of root elongation under stress conditions (Beemster and Baskin, 1998). These results further prove that AtDsPTP1 is a negative regulator in plant growth and development under stress conditions, such as seed germination and root elongation.
Although most protein phosphatases have many functions in controlling plant growth and development, the pathways they are involved in can vary. We studied the physiological and biochemical responses of the dsptp1 mutant to osmotic stress. As shown in Fig. 5, all physiological indexes, such as ion leakage, suggest a better performance of the dsptp1 mutant under osmotic stress than WT. Proline accumulates largely in cells as an osmolyte to help in osmotic adjustment under stress. The dsptp1-mutant plants had higher proline accumulation than the WT plants under osmotic stress. Proline accumulation modulates pH changes and reduces cell damage by osmotic stress (Zhu, 2002). The levels of MDA, a biomarker of cell membrane injury, and ion leakage, an indicator of osmotic-induced cellular damage, increase less under osmotic stress in the dsptp1 mutant than in WT.
ROS are important signalling molecules that accumulate under many stress conditions. However, high ROS concentrations can be highly toxic to plants (Apel and Hirt, 2004; Ye et al., 2011). Thus, we measured the antioxidant enzyme activities in the dsptp1-mutant and WT plants (Fig. 6). Increased proline content reduces the inhibitory effects of osmotic stress on enzyme activity, increases the thermal stability of enzymes, and prevents the dissociation of enzyme complexes (Hayashi et al., 1997). Therefore, we suspect that proline accumulation causes the enhanced activity of all four antioxidant enzymes in the dsptp1 mutant relative to the WT plants, which could explain why the MDA content in the mutant plant is lower than that in the WT plants.
The results demonstrate that DsPTP1 is induced by osmotic stress in the WT plants, thereby suppressing the accumulation of many metabolites that ameliorate the effects of osmotic stress and changing the expression pattern of a subset of dehydration-responsive genes (Fig. 7). Knockout of the AtDsPTP1 gene in the dsptp1-mutant plants results in better adaption to osmotic stress than in the WT plants. The compromised induction of different dehydration-responsive genes together with reduced sensitivity to osmotic stress in dsptp1 indicates that DsPTP1 is possibly important in the dehydration signalling pathway (Fig. 7).
As a stress phytohormone, ABA has a pivotal regulatory function in numerous stress responses, including osmotic stress (Boudsocq and Lauriere, 2005; Rosado et al., 2006; Cutler et al., 2010). To study how the DsPTP1 gene controls the responses of plant to osmotic stress, we examined the relationship between the DsPTP1 gene and ABA using the dsptp1-mutant plants. The inhibitory effects of ABA on seed germination and root elongation were suppressed by the mutation of the AtDsPTP1 gene but enhanced by the overexpression of the AtDsPTP1 gene (Fig. 8A, B; Supplementary Figs S2A, B). Meanwhile, the seed germination percentage (Fig. 8A) and root elongation (Fig. 8B) were rescued to a level similar to the wild type by complementing the corresponding gene in the dsptp1 mutant. Similar effects were noted under osmotic stress treatment (Figs 1, 2). These results strongly suggest that the AtDsPTP1 gene is involved in the Arabidopsis ABA-signalling pathway, mediating the inhibitory effect of ABA in plants under osmotic stress.
To study the relationship between AtDsPTP1 and the ABA pathway, we determined the ABA contents in the dsptp1 mutant and WT plants. Knockout of the DsPTP1 gene decreased the ABA content in the dsptp1-mutant plants compared with the WT plants. This finding may be attributed to the downregulated expression of the rate-limiting, ABA-biosynthesis enzyme gene NCED3, and the significantly upregulated expression of the ABA-catabolic enzyme gene CYP707A4 (Fig. 8).
Thus, downregulation of AtDsPTP1 can decrease ABA content by suppressing ABA biosynthesis and increasing ABA catabolism. The expression of genes in the ABA-response pathway show similar results (Fig. 9); downregulation of DsPTP1 decreases the gene expression of the positive regulators in ABA signalling, such as ABI3 and ABI5, and increases the expression of the negative regulator, ABI1, under osmotic stress conditions. This phenomenon can also explain why the mutant plant is less sensitive to osmotic stress as well as the ABA treatment. These findings suggest that the dsptp1-mutant plants are more tolerant to osmotic stress than the WT plants.
All of these results show that tolerance of dsptp1 to osmotic stress is mainly connected to proline accumulation. With a higher concentration of proline accumulation in dsptp1, there is less injury under osmotic stress. Thus, the dsptp1 mutant accumulated less ABA, which led to a decreased ABA response compared with the WT under osmotic treatment (Fig. 8C, D, Fig. 9; Supplementary Figure S5), just as previously reported (Lv et al., 2011). The dsptp1 mutant also showed less sensitivity to exogenous ABA, and this is also connected with the lower accumulation of, and decreased the sensitivity to, ABA (Fig. 8A, B; Supplementary Figure S2). These results show that DsPTP1 acts as a negative regulator in osmotic stress signalling during Arabidopsis seed germination and seedling establishment.
We characterized a dsptp1 mutant of Arabidopsis. The DsPTP1 gene is induced significantly by osmotic stress and has a negative regulatory function during seed germination and seedling establishment. Its mutation results in better performance in the dsptp1-mutant than WT plants under osmotic stress. The dsptp1-mutant plants accumulated more proline than WT plants under osmotic stress; proline functions in osmotic adjustment in cells. The dsptp1-mutant plants exhibit attenuated sensitivity to osmotic stress damage than the WT plants, resulting in reduced MDA content, reduced ion leakage, and enhanced antioxidant enzyme activities (APX, SOD, GR, and CAT). The accumulation of ABA in response to osmotic stress is also controlled by downregulation of DsPTP1 gene expression, which reduces expression of ABA-biosynthesis genes and increases expression of ABA-catabolism genes. The DsPTP1 gene is also involved in the ABA-signalling pathway, regulating the expression of ABI genes such as ABI1, ABI3, and ABI5. In addition, DsPTP1 regulates the expression of a series of dehydration-responsive genes under osmotic stress. The aforementioned results show that DsPTP1 acts as a negative regulator in osmotic stress signalling during Arabidopsis seed germination and seedling establishment.
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Table S1. Primers used for real- time RT-PCR assays.
Supplementary Figure S1. Comparison of WT, dsptp1-mutant, OE-6, and OE-7 lines in seed germination under osmotic stresses.
Supplementary Figure S2. Comparison of WT, dsptp1-mutant, OE-6, and OE-7 lines in seed germination and root elongation under ABA treatment.
Supplementary Figure S3. The dsptp1 mutant shows altered expressions of ABA-regulated genes under ABA treatment.
Supplementary Figure S4. Comparison of root elongation in the OE, WT, and dsptp1-mutant lines on medium with or without sucrose.
Supplementary Figure S5. Comparison of MDA content between the WT and dsptp1 mutant under treatment with 330mM mannitol.
Acknowledgements
We are grateful to Prof. Mee-len Chye of the University of Hong Kong for providing us with the pBI101.3-GUS vector. We also thank the National Basic Research Programme of China (2012CB114300), the National Natural Science Foundation of China (NSFC31101099 and 91317307), the Shenzhen Overseas Talents Innovation and Entrepreneurship Funding Scheme (The Peacock Scheme), Shandong Province agricultural breeding project, research subject of innovative use of agricultural biological resources, and Hong Kong Research Grant Council (CUHK2/CRF11G, CUHK3/CRF11G and AoE/M-05/12) for financial support.
References
- Agarwal PK, Jha B. 2010. Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Plant Biology 54, 201–212. [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Artus NN, Uemura M, Steponkus PL, Gilmour SJ, Lin C, Thomashow MF. 1996. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proceedings of the National Academic of Sciences, USA 93, 13404–13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels S, Besteiro MA, Lang D, Ulm R. 2010. Emerging functions for plant MAP kinase phosphatases. Trends in Plant Science 15, 322–329. [DOI] [PubMed] [Google Scholar]
- Bates LS. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39, 205–207. [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. 1993. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. Comptes Rendus de l Academie des Sciences Series III 316, 1194–1199. [Google Scholar]
- Beemster GTS, Baskin TI. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana . Plant Physiology 116, 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Lauriere C. 2005. Osmotic signaling in plants: multiple pathways mediated by emerging kinase families. Plant Physiology 138, 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annals of Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: Emergence of a core signaling network. Annual Review of Plant Biology 61, 651–679. [DOI] [PubMed] [Google Scholar]
- Droillard MJ, Boudsocq M, Barbier-Brygoo H, Lauriere C. 2004. Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Letters 574, 42–48. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine 11(1 ), 81–128. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. 2011. ABA-mediated transcriptional regulation in response to osmotic stress in plants. Journal of Plant Research 124, 509–525. [DOI] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. 2012. Osmotic stress signaling via protein kinases. Cellular and Molecular Life Sciences 69, 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Fowler SG, Thomashow MF. 2004. Arabidopsis transcriptional activators CBF1, CBF2, and CBF3 have matching functional activities. Plant Molecular Biology 54, 767–781. [DOI] [PubMed] [Google Scholar]
- Gupta R, Huang Y, Kieber J, Luan S. 1998. Identification of a dual-specificity protein phosphatase that inactivates a MAP kinase from Arabidopsis. The Plant Journal 16, 581–589. [DOI] [PubMed] [Google Scholar]
- Gupta R, Luan S. 2003. Redox regulation of protein tyrosine phosphatases and MAP kinases in higher plants. Plant Physiology 132, 1149–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Ting JT, Sokolov LN, Johnson SA, Luan S. 2002. A tumor suppressor homolog, AtPTEN1, is essential for pollen development in Arabidopsis. The Plant Cell 14, 2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Chirico S. 1993. Lipid peroxidation: its mechanism, measurement, and significance. The American Journal of Clinical Nutrition 57, 715S–725S. [DOI] [PubMed] [Google Scholar]
- Hare PD, Cress WA, Van Staden J. 1997. The involvement of cytokinins in plant responses to environmental stress. Plant Growth Regulation 23, 79–103. [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. 2000. Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51, 463–499. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Mustardy L, Deshnium P, Ida M, Murata N. 1997. Transformation of Arabidopsis thaliana with the coda gene for choline oxidase; accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. The Plant Journal 12, 133–142. [DOI] [PubMed] [Google Scholar]
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125, 189–198. [DOI] [PubMed] [Google Scholar]
- Holdsworth MJ, Finch-Savage WE, Grappin P, Job D. 2008. Postgenomics dissection of seed dormancy and germination. Trends in Plant Science 13, 7–13. [DOI] [PubMed] [Google Scholar]
- Huang YF, Li H, Gupta R, Morris PC, Luan S, Kieber JJ. 2000. ATMPK4, an Arabidopsis homolog of mitogen-activated protein kinase, is activated in vitro by AtMEK1 through threonine phosphorylation. Plant Physiology 122, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J. 2001. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant and Cell Physiology 42, 1265–1273. [DOI] [PubMed] [Google Scholar]
- Keyse SM. 1995. An emerging family of dual specificity MAP kinase phosphatases. Biochemica et Biophysica Acta 1265, 152–160. [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Yamaguchi-Shinozaki K, Shinozaki K. 1994. Cloning of cDNAs for genes that are early-responsive to dehydration stress (ERDs) in Arabidopsis thaliana: identification of three ERDs as HSP cognate genes. Plant Molecular Biology 25, 791–798. [DOI] [PubMed] [Google Scholar]
- Lv WT, Lin B, Zhang M, Hua XJ. 2011. Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiology 156, 1921–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio A, Matsumoto T, Hasegawa PM, Pardo JM, Bressan RA. 2002. The long and winding road to halotolerance genes. In: Lauchli A, Luttge U, eds. Salinity: environment–plants–molecules. The Netherlands: Kluwer Academic Publishers, 505–533. [Google Scholar]
- Meskiene I, Baudouin E, Schweighofer A, Liwosz A, Jonak C, Rodriguez PL, Jelinek H, Hirt H. 2003. Stress-induced protein phosphatase 2C is a negative regulator of a mitogen-activated protein kinase. The Biochemical Journal 278, 18945–18952. [DOI] [PubMed] [Google Scholar]
- Meskiene I, Bogre L, Glaser W, Balog J, Brandstotter M, Zwerger K, Ammerer G, Hirt H. 1998. MP2C, a plant protein phosphatase2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proceedlings of the National Academy of Sciences, USA 95, 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15, 473–497. [Google Scholar]
- Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism. Annual Review of Plant Biology 56, 165–185. [DOI] [PubMed] [Google Scholar]
- Naoi K, Hashimoto T. 2004. A semidominant mutation in an Arabidopsis mitogen-activated protein kinase phosphatase-like gene compromises cortical microtubule organization. The Plant Cell 16, 1841–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, et al. 1988. A monoclonal antibody to (S)-abscisic acid: its characterization and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta 173, 330–339. [DOI] [PubMed] [Google Scholar]
- Rosado A, Amaya I, Valpuesta V, Cuartero J, Botella MA, Borsani O. 2006. ABA and ethylene-mediated responses in osmotically stressed tomato are regulated by the TSS2 and TOS1 loci. Journal of Expermental Botany 57, 3327–3335. [DOI] [PubMed] [Google Scholar]
- Schweighofer A, Hirt H, Meskiene I. 2004. Plant PP2C phosphatases: emerging functions in stress signaling. Trends in Plant Science 9, 236–243. [DOI] [PubMed] [Google Scholar]
- Schweighofer A, Meskiene I. 2008. Protein phosphatases in plant growth signalling pathways. In: Boegre L, ed. Plant growth signalling. Heidelberg: Springer-Verlag, 277–297. [Google Scholar]
- Smirnoff N, Cumbes QJ. 1989. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28, 1057–1060. [Google Scholar]
- Ulm R, Ichimura K, Mizoguchi T, Peck SC, Zhu T, Wang X, Shinozaki K, Paszkowski J. 2002. Distinct regulation of salinity and genotoxic stress responses by Arabidopsis MAP kinase phosphatase 1. The EMBO Journal 21, 6483–6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R, Revenkova E, di Sansebastiano GP, Bechtold N, Paszkowski J. 2001. Mitogenactivated protein kinase phosphatase is required for genotoxic stress relief in Arabidopsis. Genes and Development 15, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM. 1998. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatments. Plant Molecular Biology 37, 455–469. [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker K, Zhu JK. 2002. Cell signaling during cold, drought and salt stress. The Plant Cell 14, S165–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Zhu JK. 2002. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell and Environment 25, 131–139. [DOI] [PubMed] [Google Scholar]
- Ye N, Jia L, Zhang J. 2012. ABA signal in rice under stress conditions. Rice 10, 1186/1939-8433-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye N, Zhu G, Liu Y, Li Y, Zhang J. 2011. ABA control H2O2 accumulation through the induction of OsCATB in Rice leaves under water stress. Plant and Cell Physiology 52, 689–698. [DOI] [PubMed] [Google Scholar]
- Zhu JK. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


