Abstract
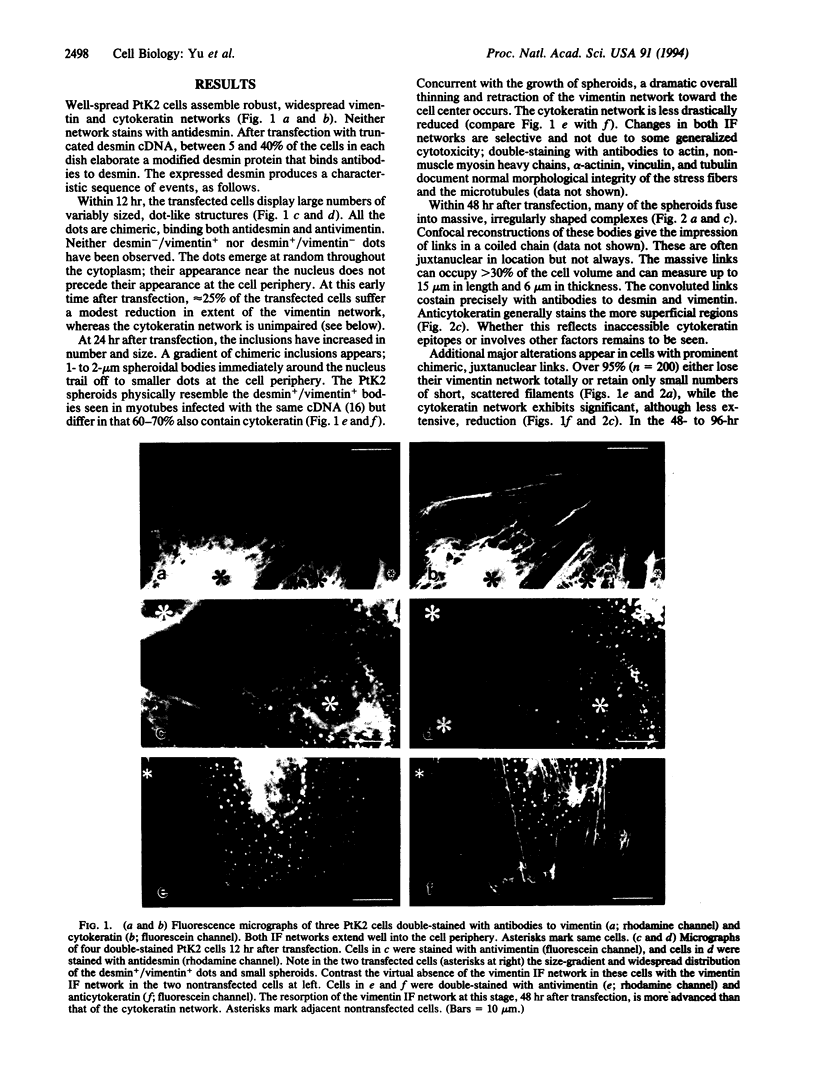
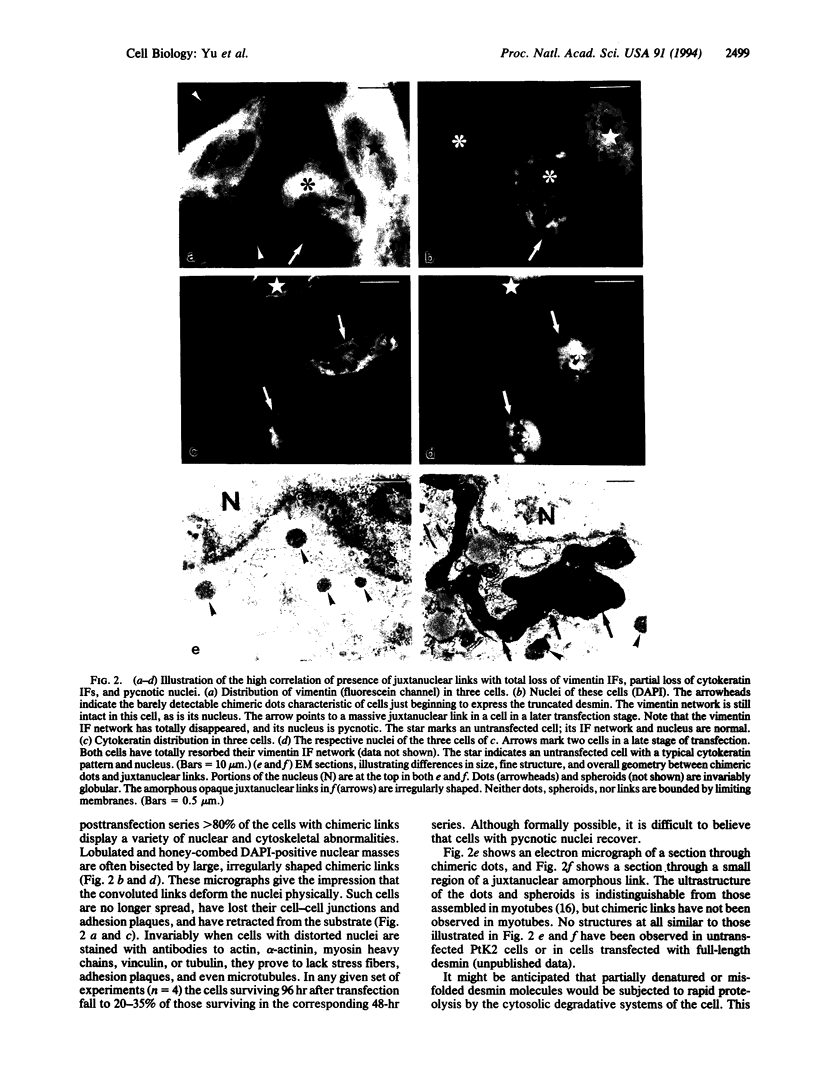
The earliest expression of truncated desmin in transfected PtK2 cells results in the formation of dispersed microprecipitates containing not only the truncated desmin, but also endogenous vimentin and cytokeratin proteins. Desmin microprecipitates without vimentin or vimentin microprecipitates without desmin are not observed. The microprecipitates involving cytokeratin invariably are also positive for desmin and vimentin. Over time, the precipitates enlarge into 1- to 2-microns spheroids and then fuse into amorphous chimeric juxtanuclear masses that can occupy > 30% of the cell volume. Concurrently, first the vimentin and then the cytokeratin networks are resorbed. The chimeric precipitates are not recognized or marked for degradation by the lysosomal system. Ultimately the cell nucleus fragments and the cell dies. Similar protein complexes appear in many human and animal pathologies, suggesting that a similar protein-precipitation sequence initiated by the introduction of a mutationally or environmentally altered protein molecule is at work.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K., Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987 Aug;105(2):791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K., Fuchs E. The molecular biology of intermediate filament proteins. Int Rev Cytol. 1992;134:243–279. doi: 10.1016/s0074-7696(08)62030-6. [DOI] [PubMed] [Google Scholar]
- Anton-Lamprecht I. Genetically induced abnormalities of epidermal differentiation and ultrastructure in ichthyoses and epidermolyses: pathogenesis, heterogeneity, fetal manifestation, and prenatal diagnosis. J Invest Dermatol. 1983 Jul;81(1 Suppl):149s–156s. doi: 10.1111/1523-1747.ep12540961. [DOI] [PubMed] [Google Scholar]
- Behe M. J., Englander S. W. Mixed gelation theory. Kinetics, equilibrium and gel incorporation in sickle hemoglobin mixtures. J Mol Biol. 1979 Sep 5;133(1):137–160. doi: 10.1016/0022-2836(79)90254-7. [DOI] [PubMed] [Google Scholar]
- Bennett G. S., Fellini S. A., Toyama Y., Holtzer H. Redistribution of intermediate filament subunits during skeletal myogenesis and maturation in vitro. J Cell Biol. 1979 Aug;82(2):577–584. doi: 10.1083/jcb.82.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blose S. H. Ten-nanometer filaments and mitosis: maintenance of structural continuity in dividing endothelial cells. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3372–3376. doi: 10.1073/pnas.76.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Carragher B., Bluemke D. A., Gabriel B., Potel M. J., Josephs R. Structural analysis of polymers of sickle cell hemoglobin. I. Sickle hemoglobin fibers. J Mol Biol. 1988 Jan 20;199(2):315–331. doi: 10.1016/0022-2836(88)90316-6. [DOI] [PubMed] [Google Scholar]
- Choi J., Costa M. L., Mermelstein C. S., Chagas C., Holtzer S., Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Vassar R., Fuchs E. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J Cell Biol. 1991 Dec;115(6):1661–1674. doi: 10.1083/jcb.115.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Vassar R., Fuchs E. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J Cell Biol. 1991 Dec;115(6):1661–1674. doi: 10.1083/jcb.115.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croop J., Dubyak G., Toyama Y., Dlugosz A., Scarpa A., Holtzer H. Effects of 12-O-tetradecanoyl-phorbol-13-acetate on Myofibril integrity and Ca2+ content in developing myotubes. Dev Biol. 1982 Feb;89(2):460–474. doi: 10.1016/0012-1606(82)90334-7. [DOI] [PubMed] [Google Scholar]
- Dlugosz A. A., Tapscott S. J., Holtzer H. Effects of phorbol 12-myristate 13-acetate on the differentiation program of embryonic chick skeletal myoblasts. Cancer Res. 1983 Jun;43(6):2780–2789. [PubMed] [Google Scholar]
- Epstein E. H., Jr Molecular genetics of epidermolysis bullosa. Science. 1992 May 8;256(5058):799–804. doi: 10.1126/science.1375393. [DOI] [PubMed] [Google Scholar]
- Forry-Schaudies S., Murray J. M., Toyama Y., Holtzer H. Effects of colcemid and taxol on microtubules and intermediate filaments in chick embryo fibroblasts. Cell Motil Cytoskeleton. 1986;6(3):324–338. doi: 10.1002/cm.970060309. [DOI] [PubMed] [Google Scholar]
- Forry-Schaudies S., Murray J. M., Toyama Y., Holtzer H. Effects of colcemid and taxol on microtubules and intermediate filaments in chick embryo fibroblasts. Cell Motil Cytoskeleton. 1986;6(3):324–338. doi: 10.1002/cm.970060309. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Grund C., Geiger B. Intermediate filament proteins in nonfilamentous structures: transient disintegration and inclusion of subunit proteins in granular aggregates. Cell. 1982 Aug;30(1):103–113. doi: 10.1016/0092-8674(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Lazarides E. The synthesis and distribution of desmin and vimentin during myogenesis in vitro. Cell. 1980 Jan;19(1):263–275. doi: 10.1016/0092-8674(80)90408-0. [DOI] [PubMed] [Google Scholar]
- Gill S. R., Wong P. C., Monteiro M. J., Cleveland D. W. Assembly properties of dominant and recessive mutations in the small mouse neurofilament (NF-L) subunit. J Cell Biol. 1990 Nov;111(5 Pt 1):2005–2019. doi: 10.1083/jcb.111.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. S., Duran S., Lin Z. X., Weber K., Holtzer H. Titin and myosin, but not desmin, are linked during myofibrillogenesis in postmitotic mononucleated myoblasts. J Cell Biol. 1986 Dec;103(6 Pt 1):2185–2196. doi: 10.1083/jcb.103.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. D., Lee V. M., Hurtig H. I., Murray J. M., Trojanowski J. Q. Epitopes located in spatially separate domains of each neurofilament subunit are present in Parkinson's disease Lewy bodies. J Comp Neurol. 1991 Jul 1;309(1):150–160. doi: 10.1002/cne.903090111. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Croop J., Dienstman S., Ishikawa H., Somlyo A. P. Effects of cytochaslasin B and colcemide on myogenic cultures. Proc Natl Acad Sci U S A. 1975 Feb;72(2):513–517. doi: 10.1073/pnas.72.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B., Kupfer H., Eshhar Z., Geiger B. Reorganization of arrays of prekeratin filaments during mitosis. Immunofluorescence microscopy with multiclonal and monoclonal prekeratin antibodies. Exp Cell Res. 1981 Aug;134(2):281–290. doi: 10.1016/0014-4827(81)90427-4. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A., McGrath J. A., Chapman S. J., Leigh I. M., Lane E. B., Eady R. A. Epidermolysis bullosa simplex (Dowling-Meara type) is a genetic disease characterized by an abnormal keratin-filament network involving keratins K5 and K14. J Invest Dermatol. 1991 Dec;97(6):959–968. doi: 10.1111/1523-1747.ep12491885. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima Y., Jokura Y., Yaoita H. Epidermolysis bullosa simplex, Dowling-Meara type. A report of two cases with different types of tonofilament clumping. Br J Dermatol. 1993 Jan;128(1):79–85. doi: 10.1111/j.1365-2133.1993.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Bachant J. B., Domingo A. Functions of intermediate filaments. Cell Motil Cytoskeleton. 1989;14(3):309–331. doi: 10.1002/cm.970140302. [DOI] [PubMed] [Google Scholar]
- Murayama S., Bouldin T. W., Suzuki K. Immunocytochemical and ultrastructural studies of upper motor neurons in amyotrophic lateral sclerosis. Acta Neuropathol. 1992;83(5):518–524. doi: 10.1007/BF00310029. [DOI] [PubMed] [Google Scholar]
- Ngai J., Coleman T. R., Lazarides E. Localization of newly synthesized vimentin subunits reveals a novel mechanism of intermediate filament assembly. Cell. 1990 Feb 9;60(3):415–427. doi: 10.1016/0092-8674(90)90593-4. [DOI] [PubMed] [Google Scholar]
- Niemi K. M., Kero M., Kanerva L., Mattila R. Epidermolysis bullosa simplex. A new histologic subgroup. Arch Dermatol. 1983 Feb;119(2):138–141. [PubMed] [Google Scholar]
- Preisegger K. H., Zatloukal K., Spurej G., Denk H. Changes of cytokeratin filament organization in human and murine Mallory body-containing livers as revealed by a panel of monoclonal antibodies. Liver. 1991 Oct;11(5):300–309. doi: 10.1111/j.1600-0676.1991.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Sarria A. J., Nordeen S. K., Evans R. M. Regulated expression of vimentin cDNA in cells in the presence and absence of a preexisting vimentin filament network. J Cell Biol. 1990 Aug;111(2):553–565. doi: 10.1083/jcb.111.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. L., Lee V. M., Trojanowski J. Q. Analysis of epitopes shared by Hirano bodies and neurofilament proteins in normal and Alzheimer's disease hippocampus. Lab Invest. 1989 Apr;60(4):513–522. [PubMed] [Google Scholar]
- Schultheiss T., Choi J., Lin Z. X., DiLullo C., Cohen-Gould L., Fischman D., Holtzer H. A sarcomeric alpha-actinin truncated at the carboxyl end induces the breakdown of stress fibers in PtK2 cells and the formation of nemaline-like bodies and breakdown of myofibrils in myotubes. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9282–9286. doi: 10.1073/pnas.89.19.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss T., Lin Z. X., Ishikawa H., Zamir I., Stoeckert C. J., Holtzer H. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol. 1991 Sep;114(5):953–966. doi: 10.1083/jcb.114.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soellner P., Quinlan R. A., Franke W. W. Identification of a distinct soluble subunit of an intermediate filament protein: tetrameric vimentin from living cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7929–7933. doi: 10.1073/pnas.82.23.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stewart M. Intermediate filaments: structure, assembly and molecular interactions. Curr Opin Cell Biol. 1990 Feb;2(1):91–100. doi: 10.1016/s0955-0674(05)80037-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schmidt M. L., Shin R. W., Bramblett G. T., Rao D., Lee V. M. Altered tau and neurofilament proteins in neuro-degenerative diseases: diagnostic implications for Alzheimer's disease and Lewy body dementias. Brain Pathol. 1993 Jan;3(1):45–54. doi: 10.1111/j.1750-3639.1993.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Cleveland D. W. Characterization of dominant and recessive assembly-defective mutations in mouse neurofilament NF-M. J Cell Biol. 1990 Nov;111(5 Pt 1):1987–2003. doi: 10.1083/jcb.111.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal K., Denk H., Spurej G., Lackinger E., Preisegger K. H., Franke W. W. High molecular weight component of Mallory bodies detected by a monoclonal antibody. Lab Invest. 1990 Apr;62(4):427–434. [PubMed] [Google Scholar]