Abstract
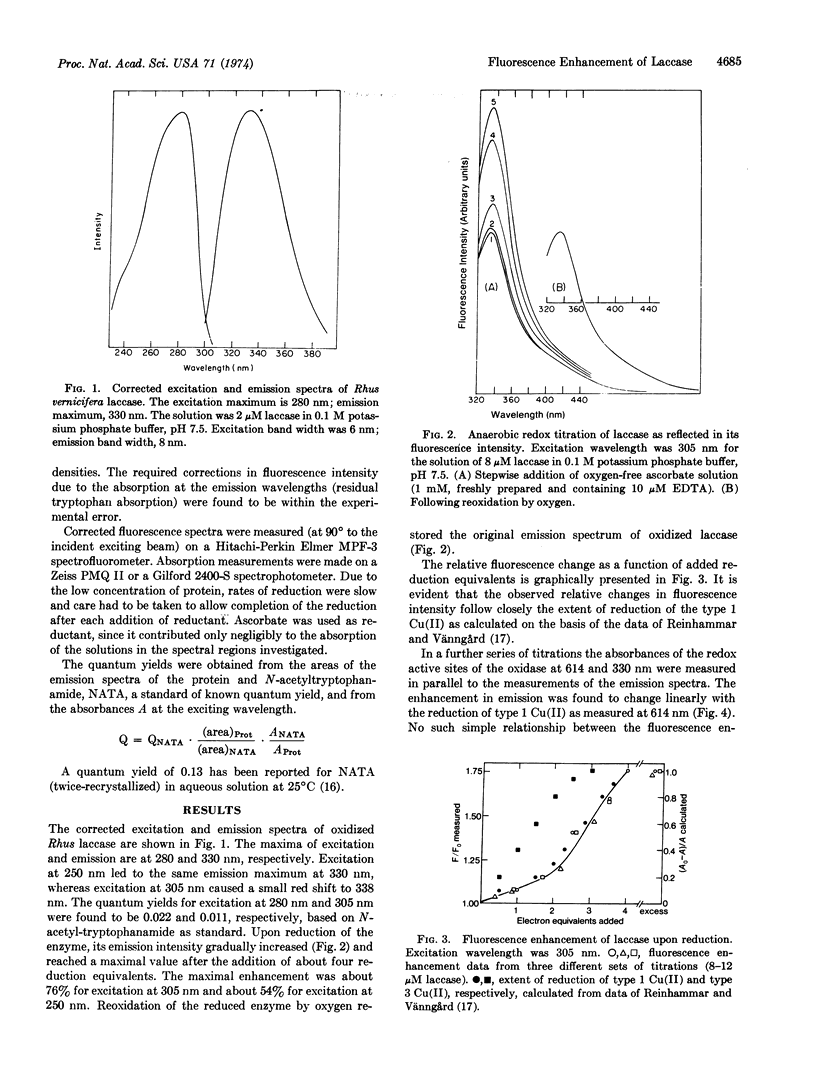
The intrinsic fluorescence of laccase (p-diphenol:O2 oxidoreductase, EC 1.10.3.2), emitted by its tyrosinyl and tryptophanyl residues, underwent significant enhancement upon reduction of the enzyme redox sites. The increase in quantum yield reached its maximum after the addition of approximately four reduction equivalents and depended on the wavelength of excitation:
54% increase for λex = 250 nm and 76% for λex = 305 nm.
A linear correlation between this enhancement and the reduction of the type 1 Cu(II) was observed both by direct measurement and by calculation on the basis of added reductant.
The implications of the fluorescence enhancement and its correlation with the reduction of the type 1 copper are discussed in terms of the possible quenching mechanisms. The possibility of a redox-induced structural transition in the protein is suggested.
Keywords: “blue” copper proteins, redox-related quenching
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE PURIFICATION AND AMINO ACID COMPOSITION OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:341–349. doi: 10.1042/bj0890341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson L. E., Malmström B. G., Strömberg C., Vänngård T. The kinetics of the anaerobic reduction of fungal laccase B. Eur J Biochem. 1973 May 2;34(3):434–439. doi: 10.1111/j.1432-1033.1973.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Finazzi-Agrò A., Rotilio G., Avigliano L., Guerrieri P., Boffi V., Mondovì B. Environment of copper in Pseudomonas fluorescens azurin: fluorometric approach. Biochemistry. 1970 Apr 28;9(9):2009–2014. doi: 10.1021/bi00811a023. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Rudikoff S., Potter M., Glaudemans C. P. Spectral changes on binding of oligosaccharides to murine immunoglobulin A myeloma proteins. Biochemistry. 1973 Jul 31;12(16):3039–3044. doi: 10.1021/bi00740a015. [DOI] [PubMed] [Google Scholar]
- Luk C. K. Quenching of the emission of tryptophan, tyrosine, and serum albumins by cupric ion. Biopolymers. 1971;10(7):1229–1242. doi: 10.1002/bip.360100712. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. The state of copper in stellacyanin and laccase from the lacquer tree Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):48–57. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- McMillin D. R., Holwerda R. A., Gray H. B. Preparation and spectroscopic studies of cobalt(II)-stellacyanin. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1339–1341. doi: 10.1073/pnas.71.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morpurgo L., Finazzi-Agrò A., Rotilio G., Mondovì B. Studies of the metal sites of copper proteins. IV. Stellacyanin: preparation of apoprotein and involvement of sulfhydryl and tryptophan in the copper chromophore. Biochim Biophys Acta. 1972 Jul 21;271(2):292–299. doi: 10.1016/0005-2795(72)90203-6. [DOI] [PubMed] [Google Scholar]
- Pollet R., Edelhoch H. The binding peoperties of anti-phosphorylcholine mouse myeloma proteins as measured by protein fluorescence. J Biol Chem. 1973 Aug 10;248(15):5443–5447. [PubMed] [Google Scholar]
- Reinhammar B. R., Vänngård T. I. The electron-accepting sites in Rhus vernicifera laccase as studied by anaerobic oxidation-reduction titrations. Eur J Biochem. 1971 Feb;18(4):463–468. doi: 10.1111/j.1432-1033.1971.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Rist G. H., Hyde J. S., Vänngård T. Electron-Nuclear Double Resonance of a Protein That Contains Copper: Evidence for Nitrogen Coordination to Cu(II) in Stellacyanin. Proc Natl Acad Sci U S A. 1970 Sep;67(1):79–86. doi: 10.1073/pnas.67.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklai N., Daniel E. Fluorescence properties of hemocyanin from Levantina hierosolima. Biochemistry. 1970 Feb 3;9(3):564–568. doi: 10.1021/bi00805a016. [DOI] [PubMed] [Google Scholar]
- Steinberg I. Z. Long-range nonradiative transfer of electronic excitation energy in proteins and polypeptides. Annu Rev Biochem. 1971;40:83–114. doi: 10.1146/annurev.bi.40.070171.000503. [DOI] [PubMed] [Google Scholar]



