Summary
Anthocyanin biosynthesis is regulated by a transcription factor complex. Here, it is determined that the potential bHLH partners in this complex function in a hierarchy to control each other and the anthocyanin biosynthesis pathway.
Key words: bHLH, MYB, Solanaceae, tobacco, transcriptional control, transcription factors.
Abstract
The anthocyanin biosynthetic pathway is regulated by a transcription factor complex consisting of an R2R3 MYB, a bHLH, and a WD40. Although R2R3 MYBs belonging to the anthocyanin-activating class have been identified in many plants, and their role well elucidated, the subgroups of bHLH implicated in anthocyanin regulation seem to be more complex. It is not clear whether these potential bHLH partners are biologically interchangeable with redundant functions, or even if heterodimers are involved. In this study, AcMYB110, an R2R3 MYB isolated from kiwifruit (Actinidia sp.) showing a strong activation of the anthocyanin pathway in tobacco (Nicotiana tabacum) was used to examine the function of interacting endogenous bHLH partners. Constitutive expression of AcMYB110 in tobacco leaves revealed different roles for two bHLHs, NtAN1 and NtJAF13. A hierarchical mechanism is shown to control the regulation of transcription factors and consequently of the anthocyanin biosynthetic pathway. Here, a model is proposed for the regulation of the anthocyanin pathway in Solanaceous plants in which AN1 is directly involved in the activation of the biosynthetic genes, whereas JAF13 is involved in the regulation of AN1 transcription.
Introduction
Anthocyanins are the major pigmented group of flavonoid compounds responsible for a range of colours varying from pink to blue, through to red and violet in flowers, fruits, or leaves. The anthocyanin biosynthetic pathway is one branch of flavonoid metabolism (Winkel-Shirley, 2001) and its regulatory system has been extensively studied. The anthocyanin biosynthetic genes are transcriptionally regulated by a MYB–bHLH–WD40 (MBW) complex that contains R2R3 MYB and basic helix-loop-helix (bHLH) transcription factors and a WD40 protein (Feller et al., 2011). The expression patterns of the MBW components thus define the variety of pigmentation patterns of plant tissues (Davies et al., 2012),
The R2R3 MYBs are numerically the largest class of transcription factors in plants. The C1 MYB (Zea mays) was the first transcription factor identified to regulate anthocyanin biosynthesis (Paz-Ares et al., 1987); subsequently a large number of MYBs controlling anthocyanin colour in flowers (Quattrocchio et al., 1999; Schwinn et al., 2006; Albert et al., 2011) and fruit (Kobayashi et al., 2004; Takos et al., 2006; Espley et al., 2007; Lin-Wang et al., 2010) have been identified.
Basic helix-loop-helix proteins are the second largest class of transcription factor. Generally, the first 200 amino acids of the protein are involved in the interaction with the MYB partner, whereas the following 200 amino acids interact with the WD40 protein. The bHLH domains are involved in the formation of homo- or heterodimers with other bHLH proteins, which often is a prerequisite for DNA recognition and contributes to DNA-binding specificity (Feller et al., 2011; Hichri et al., 2011). The bHLH group of transcription factors have been divided into 26 subgroups (Pires and Dolan, 2010), but if ‘atypical’ bHLHs are included this is extended to 32 subgroups (Carretero-Paulet et al., 2010). Flavonoid related bHLHs have been grouped into subgroup IIIf.
The first bHLHs regulating the flavonoid pathway were identified in maize, Booster1 (B) and Red1 (R) (Chandler et al., 1989). Subsequently a number of additional bHLH transcription factors able to regulate the flavonoid pathway have been identified: these include, AN1 and JAF13 in Petunia (Petunia hybrida) (Quattrocchio et al., 1998; Spelt et al., 2000), Delila and Mutabilis in snapdragon (Antirrhinum majus) (Martin et al., 1991), TT8 and GLABRA3 in Arabidopsis (Nesi et al., 2000; Feyissa et al., 2009), VvMYC1 and VvMYCA1 in grape (Vitis vinifera) (Hichri et al., 2010) and A in pea (Pisum sativum) (Hellens et al., 2010). Many of these have been shown to regulate different physiological or morphological events including different branches of the flavonoid pathway, vacuole acidification, and epidermal cell fate (Hichri et al., 2011).
The interaction between the MYB and bHLH proteins has often been used to study combinatorial gene regulation in plants. In maize the presence of an amino acid motif in the R3 region of the MYB, identified as (DE)Lx2(RK)x3Lx6Lx3R, determines the binding specificity of the MYB to the bHLH partner and is responsible for the different ability of ZmC1 and a related R2R3 MYB, ZmP1, to interact with the bHLH R. The ability of the MYB to interact with the bHLH triggers the activation of the biosynthetic gene ZmBz1, with R playing a key role in the target specificity of the MBW complex (Grotewold et al., 2000; Zimmermann et al., 2004).
In Petunia, the MBW complex AN2–AN1–AN11 (MYB–bHLH–WD40, respectively) regulates anthocyanin biosynthesis in the flower petals (deVetten et al., 1997; Quattrocchio et al., 1999; Spelt et al., 2000). AN1 and AN11 are also involved in the control of other physiological events such as acidification of the vacuole when associated in a MBW complex that involves the MYB Ph4 (Quattrocchio et al., 2006). In Petunia, two bHLHs have been identified: JAF13 and AN1. Both bHLHs have been shown to regulate anthocyanin biosynthesis in Petunia flowers (Quattrocchio et al., 1998; Spelt et al., 2002) and they have often been used interchangeably in functional assays. However, their role in anthocyanin regulation has not been fully resolved, and they have been shown to have functional differences. In mutant lines, such as the an1 – mutant (Spelt et al., 2000) and in over-expression lines, JAF13 is not able to complement the lack of AN1.
It seems likely that the MBW complex includes different classes of MYBs and bHLHs with specific functions in regulating the transcription of the flavonoid pathway. Alternatively, different bHLHs may be involved as heterodimers in the MBW complex to regulate the anthocyanin pathway. This specificity of functions amongst similar transcription factors, or involvement of multiple bHLH classes, would allow for more subtle regulation of the transcriptional mechanism through different combinations of transcription factors within the MBW complex. It is therefore necessary to understand how apparently similar MYB or bHLH transcription factors within the MBW complex can have differing regulatory activities.
Here it is shown that AcMYB110, an R2R3 MYB from kiwifruit (Fraser et al., 2013), elicits strong induction of anthocyanin when transiently expressed in the model plant tobacco (Nicotiana tabacum). This response enables us to further define the specific roles of the bHLH proteins NtAN1 and NtJAF13 and to present a model that explains the hierarchical interactions of the MYB and bHLH partners within the MBW complex.
Materials and methods
Phylogenetic analysis
Protein alignment used ClustalW within the software package Geneious 6.1.7 (Biomatters Ltd., Auckland, New Zealand), with manual refinement. A phylogenetic tree was formed from the alignment within the Geneious package using PhyML v2.2 and the maximum likelihood method (Guindon and Gascuel, 2003), with the default settings (NNIs tree topology search, BioNJ initial tree, LG model for amino acids substitutions, and 1000 bootstrap replicates).
Transient assay
Transient assays were performed as described previously (Hellens et al., 2005) using tobacco (Nicotiana tabacum) ‘Samsun’ and Nicotiana benthamiana plants grown in a glasshouse, using natural light with daylight extended to 16h. Transient dual-luciferase and colour assay were executed infiltrating approximately 300 µl of Agrobacterium tumefaciens GV3101 (MP90) mixture into young leaves. Agrobacterium were cultured on Lennox agar plates supplemented with selection antibiotics and incubated at 28 °C. The freshly grown bacteria were re-suspended in infiltration media and incubated at room temperature for at least 2h (Hellens et al., 2005) before infiltration.
To generate overexpression vectors the open reading frames of AcMYB110 and NtAN1 were amplified using specific primers (Supplementary Table S1) and cloned into pENTR-TOPO and subsequently a Gateway reaction was performed with destination expression vector pHEX2 (http://www.lifetechnologies.com). PhAN1 expression vector, as described in Albert et al. (2011), was provided by Nick Albert (Plant and Food Research, Palmerston North, NZ).
To generate the RNA interference constructs, small fragments were amplified of NtAN1 and NtJAF13 (respectively 383bp and 197bp long) with specific primers (Supplementary Table S1) and the cloned products were put into pENTR-TOPO, followed by a Gateway reaction with RNAi destination vector pTKO2 (http://www.lifetechnologies.com).
For the colour assay, a mixture of Agrobacterium transformed with constructs constitutively expressing transcription factors fused to the 35S promoter in pHEX2 and silencing the endogenous bHLHs NtAN1 and NtJAF13 by RNAi using a pTKO2 vector was used. Final colour development was assayed at four days and seven days after infiltration.
Tobacco AN1, CHS, and DFR promoter sequences were obtained by a genome walking strategy; approximately 1kb of target promoter was amplified using specific primers (Supplementary Table S1) and inserted into pGreen 0800-LUC. Promoter activation assays were performed in tobacco leaves. Agrobacterium containing the promoter–LUC fusions were mixed with Agrobacterium containing the transcription factor to be tested (ratio 1:3) and used to infiltrate tobacco leaves (Hellens et al., 2005). The resulting luminescence was measured four days after infiltration.
Pigment analysis
Five independent replicates were collected for each infiltration. Leaf samples were freeze-dried and ground. Anthocyanins were extracted in acidified MeOH (0.1% HCl) and centrifuged at 3000g. Aliquots were dried down and re-suspended in 20% v/v aqueous MeOH (0.2ml). The re-dissolved extracts were analysed by reversed phase HPLC using a method previously described (Andre et al., 2012). Anthocyanin peaks were identified by comparison of retention times with authentic standards and absorption at 520nm. Total anthocyanin content was calculated using standard solutions of cyanidin-3 galactoside (Extrasynthese, Genay, France) and expressed as cyanidin galactoside equivalents ng mg–1 of fresh weight.
Real time qPCR analysis
Gene expression analysis was performed on infiltrated leaves, 4 d after infiltration. RNA was isolated using TRIzol® (http://www.lifetechnologies.com) and DNAse-treated with DNA-free:Ambion (http://www.ambion.com/). Reverse transcription was performed using oligo(dT) according to the manufacturer’s protocol (Transcriptor: Roche Diagnostics, http://www.roche.com/). Quantitative real-time PCR was carried out using the LightCycler 480 System, using 480 SYBR® Green I Master (Roche Diagnostics). Reactions were performed in quadruplicate and a non-template control was included in each run. The data were analysed using the LightCycler® 480 software 1.5 normalised to tobacco actin (GQ281246) and calibrated to the control (empty vector) infiltration (primer sequences in Supplementary Table S1).
Yeast one-hybrid and yeast two-hybrid assays
Yeast one-hybrid experiments were conducted using the Matchmaker Gold Y1H library Screening System (Clontech). 490bp and 562bp of the NtAN1 and NtDFR promoters, respectively, were cloned in the pAbAi vector and transformed into Y1HGold strain to generate the bait reporter strains. NtAN1 and NtJAF13 full-length coding sequence were cloned into pDEST22 (Life Technologies) and transfected into yeast as a fusion to the GAL4 activating domain (AD). MYB110 was cloned using Gateway recombination technology (Life Technologies) in pGW6, a Gateway-adapted version of pTFT1 vector (Egea-Cortines et al., 1999) which allows expression of an untagged protein in yeast. The different pairwise combinations of MYB and bHLHs and corresponding empty vector controls were co-transformed into the Y1HGold strain containing the promoter construct. Growth on minimal synthetic defined media (SD) lacking adenine, uracil, and tryptophan and supplemented with 50ng ml–1 aureobasidin A was scored after 5 d at 30 °C.
For the yeast two-hybrid assays, full length sequences of the candidate genes were inserted in pDEST22 and pDEST32 vectors from the ProQuest™ Two-Hybrid System (Life Technologies) and transfected in the PJ69-4A and PJ69-4alpha yeast strains (James et al., 1996). Pairwise combinations of MYBs and bHLHs, expressed as fusion proteins to the GAL4 activating (AD) and binding (BD) domains respectively, were obtained by mating and screened for growth after up to 3 d at 30 °C on SD media lacking tryptophan (T), leucine (L) and histidine (H) and supplemented by increasing amount of 3-amino-1,2,4-triazole (3AT) (10, 25, 50, 75, and 100mM) to increase the stringency of the histidine selection (Supplementary Fig. S1).
Statistical analysis
Analysis of variance (ANOVA) using the Genstat 14th edition, Tukey test (P<0.05) was used to separate the treatment means.
Results
Phylogenetic analysis of anthocyanin-related bHLHs
Phylogenetic analysis, performed on the deduced amino acid sequences of more than 30 flavonoid-related bHLHs belonging to the subgroup IIIf, clearly separates them in two clades (Fig. 1): clade A and clade B, as described in Davies et al. (2012). Clade A included JAF13 (Petunia), R (maize), Delila (snapdragon), and GL3/EGL3 (Arabidopsis), whereas clade B contained AN1 (Petunia), Intensifier (maize), Mutabilis (snapdragon), and TT8 (Arabidopsis). Members of the same subgroups are often involved in the same biological process, although it is not always clear if their functions overlap. For example; GL3, EGL3, and TT8 are partially redundant in the control of anthocyanin biosynthesis in Arabidopsis. However, each of these have also more specific functions such as PA biosynthesis (TT8), trichome formation (GL3/EGL3), and production of seed coat mucilage (TT8/EGL3) (Feller et al., 2011). Moreover, in Petunia the lack of AN1 (clade B) cannot be complemented by JAF13 (clade A), despite both being able to regulate anthocyanin biosynthesis (Spelt et al., 2000). On the other hand, in snapdragon Delila and Mutabilis, respectively from clade A and clade B, seem to be functionally redundant and able to complement for the loss of the other factor (Schwinn et al., 2006).
Fig. 1.
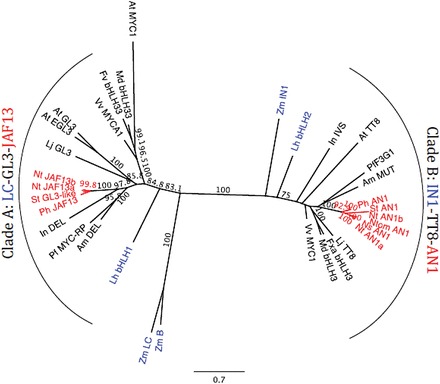
Phylogenetic tree of bHLH transcription factors involved in the regulation of the flavonoid pathway from a range of species. Amino acid sequences were aligned using ClustalW and a maximum likelihood tree formed from the alignment. Numbers indicate bootstrap values for 1000 replicates, with only values above 70 shown. Accession details are: Antirrhinum majus DELILA (AAA32663), MUTABILIS (Schwinn et al., 2006); Arabidopsis thaliana EGL3 (Q9CAD0), GL3 (NP_680372), MYC1 (Q8W2F1), TT8/bHLH42 (Q9FT81); Fragaria vesca bHLH33 (XP_004308377); Fragaria × ananassa bHLH3 (AFL02463); Ipomoea nil DEL (BAE94393), IVS (ivory seeds, BAE94394); Lilium (hybrid division I) bHLH1 (BAE20057), bHLH2 (BAE20058); Lotus japonicus GL3 (AB492284), TT8 (AB490778); Malus domestica bHLH3 (ADL36597), bHLH33 (ABB84474); Nicotiana sylvestris AN1 (HQ589210); Nicotiana tabacum AN1a (HQ589208), AN1b (HQ589209), JAF13a (KF305768), JAF13b (KF298397); Nicotiana tomentosiformis AN1 (HQ589211); Petunia x hybrida AN1 (AAG25928), JAF13 (AAC39455); Perilla frutescens F3G1 (AB103172), MYC-RP (AB024050); Solanum tuberosum AN1 (JX848660), GL3-like (NM_001288203); Vitis vinifera MYC1 (ACC68685), MYCA1 (ABM92332); Zea mays B-PERU (CAA40544), IN1 (AAB03841), LC (P13526). Sequences from Solanaceous species are shown in red and those from monocotyledonous species in blue.
Ectopic expression of AcMYB110 reveals different abilities to promote red pigmentation depending on the availability of endogenous bHLHs
When anthocyanin-related MYBs are ectopically expressed transiently in leaves of tobacco (Nicotiana tabacum) an anthocyanic patch can be generated (Espley et al., 2007). In the current study, kiwifruit AcMYB110 (Fraser et al., 2013) was used because of its strong ability to promote anthocyanin biosynthesis in tobacco leaves without the need to provide ectopic expression of a bHLH partner. This ability of AcMYB110 to activate the anthocyanin pathway of tobacco when transient expressed on its own is different from many other anthocyanin-related R2R3 MYBs (Lin-Wang et al., 2010). Four days after infiltration red pigmentation was evident, and this developed into a dark red patch after 7 days (Fig. 2).
Fig. 2.
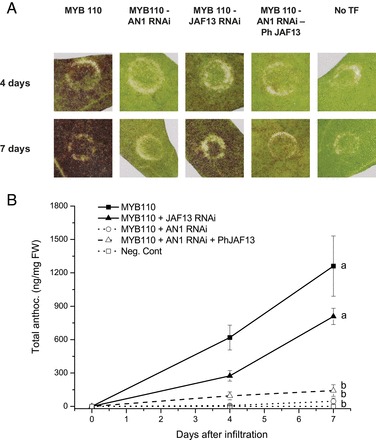
(A) Red colour development in Nicotiana tabacum leaves 7 days after ectopic expression of AcMYB110 in combination with bHLH RNAi constructs targeting tobacco NtAN1 and NtJAF13, and constructs constitutively expressing Petunia hybrida PhJAF13. (B) Anthocyanin content in infiltrated patches 4 and 7 days after infiltration. Anthocyanin content is expressed as cyanidin galactoside equivalents ng mg–1 of fresh weight. Error bars are SE for five replicates.
This ability of AcMYB110 to induce anthocyanin biosynthesis, without co-infiltration of a bHLH, could be due to an efficient interaction with the endogenous tobacco bHLH and WD40 proteins. To investigate these interactions, RNA interference (RNAi) was used to inhibit the activity of each of the endogenous tobacco bHLHs known to be involved in anthocyanin biosynthesis: NtAN1a (HQ589208), NtAN1b (HQ589209), and NtJAF13b (KF298397). When NtAN1 was silenced (the same RNAi construct was able to target both NtAN1a and NtAN1b) no red colour occurred, despite constitutive expression of AcMYB110 (Fig. 2A, B). In contrast constitutive expression of AcMYB110 and NtJAF13 RNAi still resulted in a red patch, although knock-down of NtJAF13b had the effect of delaying the development of the pigmentation (Fig. 2A, B). Four days after infiltration the anthocyanic patches where AcMYB110 and NtJAF13 RNAi were co-infiltrated were less intense compared with the darker pigmentation of the leaves infiltrated with AcMYB110 only, and even after 7 days they did not reach the same anthocyanin content of AcMYB110 only (Fig. 2A, B).
These results demonstrated that, in the tobacco system, AcMYB110 requires NtAN1 to activate anthocyanin biosynthesis, whereas the lack of NtJAF13b results in a slower development of pigmentation (Fig. 2A, B). A related phenotype is seen in colour development of flowers of stable RNAi tobacco lines targeting endogenous NtAN1(a and b) and NtJAF13b. NtAN1 RNAi lines developed white flowers, whereas the NtJAF13 RNAi lines developed less intense pink flowers compared with the wild type (Supplementary Fig. S2). To further characterize the functional interaction of the two bHLHs, the down-regulated NtAN1 (NtAN1 RNAi) was complemented by constitutively expressing the Petunia hybrida JAF13 (PhJAF13; AF020545). Using the Petunia PhJAF13 to complement the reduction in expression of the tobacco bHLH allows the monitoring by qPCR of expression of the endogenous NtbHLHs, as there is sufficient sequence difference between the genes from the two species. Constitutive expression of PhJAF13 was not able to complement the lack of tobacco NtAN1 (RNAi) and did not restore the ability of AcMYB110 to drive red pigmentation (Fig. 2). This demonstrates distinct functional properties for the two bHLHs and suggests differing roles in the anthocyanin regulatory system.
Ectopic expression of AcMYB110 up-regulates tobacco NtAN1 and the biosynthetic genes of the anthocyanin pathway in distinct ways
AcMYB110 is able to form a complex with either NtAN1 or NtJAF13, as confirmed by yeast two-hybrid assay (Fig. 3A). NtJAF13 and NtAN1 may target the MBW complex to different promoters within this pathway. The gene expression of chalcone synthase (NtCHS; AB213651) and dihydroflavonol 4-reductase (NtDFR; EF421429) were therefore examined, as representative of early and late stages of anthocyanin biosynthesis; as well as the expression of the endogenous NtJAF13b and NtAN1 genes. Ectopic expression of AcMYB110 in the tobacco leaf up-regulated the transcript level of the endogenous NtAN1 and the biosynthetic genes NtCHS and NtDFR (Fig. 3B). This is consistent with the development of a red pigmentation of the infiltrated leaf patches. In contrast, AcMYB110 did not have a significant effect on the expression of tobacco NtJAF13.
Fig. 3.

Interaction of MYB110 with different bHLH partners. (A) Analysis of MYB110, AN1, and JAF13 protein–protein interactions by yeast two-hybrid. Pairwise combinations (as indicated) were tested for their ability to activate the HIS3 reporter in the presence of 0 or 10mM 3AT. SD, minimal media; AD, activating domain only; BD, binding domain only; L, leucine; T, tryptophan; H, histidine. (B) Gene expression analysis of endogenous tobacco bHLHs: NtAN1, NtJAF13, and the anthocyanin biosynthetic genes chalcone synthase (CHS) and dihydroflavonol 4-reductase (DFR) 4 days after transient tobacco transformation constitutively expressing MYB110 with NtAN1 and NtJAF13 RNAi constructs. Error bars are SEM for four replicate reactions. Relative quantification is normalized to NtActin and calibrated to the ‘no MYB’ infiltration control
As expected, co-infiltration of the NtAN1 RNAi with AcMYB110 strongly reduced the transcript level of the endogenous NtAN1 and consequently the expression of the biosynthetic genes of the pathway and the colour of the leaf patch. On the contrary, NtJAF13 expression was slightly elevated when RNAi was directed at NtAN1. NtJAF13 RNAi did not completely silence the endogenous NtJAF13 transcript, but reduced its expression by approximately half. The reduced expression of NtJAF13 and ectopic expression of AcMYB110 resulted in a reduced up-regulation of NtAN1 and consequently of NtCHS and NtDFR (Fig. 3B).
AcMYB110 activates the promoters of NtAN1 and the biosynthetic genes NtCHS and NtDFR
Using a transient dual luciferase assay (Hellens et al., 2005), the ability of AcMYB110 to regulate the tobacco NtCHS, NtDFR, and NtAN1 promoters was tested. Different combinations of AcMYB110 and RNAi knock-down constructs directed at the endogenous bHLHs were co-infiltrated in tobacco leaves together with the pGREEN-LUC constructs, which contained the target promoters (tobacco CHS, DFR or AN1) driving the expression of the luciferase reporter gene (LUC). The results are presented relative to the level of the Renilla (REN) reporter gene, which is under control of a 35S promoter. As expected, AcMYB110 was able to activate the promoters of the biosynthetic genes, NtCHS and NtDFR (Fig. 4). However, when NtAN1 was down-regulated there was a significant drop in the activation of these promoters. Co-infiltration of NtJAF13 RNAi did not significantly affect AcMYB110 activation of the NtCHS and NtDFR promoters. Moreover, AcMYB110 was able to activate the NtAN1 promoter (Fig. 5A), and addition of the NtAN1 RNAi construct had no effect on this activation. In contrast, activation of the NtAN1 promoter was reduced in the presence of the NtJAF13 RNAi construct (Fig. 5A). Ectopic expression of PhJAF13 enhanced activation of the NtAN1 promoter, whereas over-expression of NtAN1 did not improve the activation of the NtAN1 promoter (Fig. 5B).
Fig. 4.
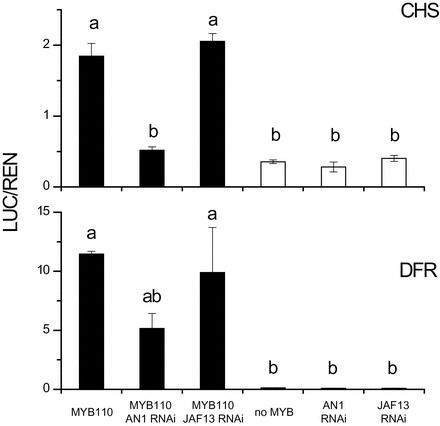
Promoter activation of the biosynthetic gene of the pathway NtCHS and NtDFR on a dual luciferase transient assay in Nicotiana tabacum. Tobacco leaves were infiltrated with 35S:MYB110 in different combination with RNAi constructs targeting the endogenous bHLHs AN1 and JAF13. The dual luciferase assay shows promoter activity expressed as a ratio of target promoter luciferase (LUC) to 35S Renilla (REN); a greater activity equates to an increase in LUC relative to REN. Error bars are the SEM for four replicate reactions.
Fig. 5.
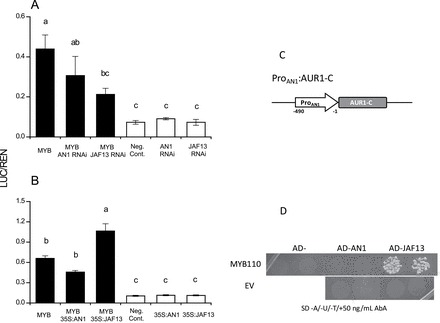
MYB110 interacts with the promoter of NtAN1. (A) NtAN1 promoter activation using a dual luciferase transient assay in tobacco leaves infiltrated with 35S:MYB110 in combination with RNAi constructs targeting the endogenous bHLHs AN1 and JAF13. Error bars are the SEM for four replicate reactions. (B) NtAN1 promoter activation using a dual luciferase transient assay in tobacco leaves infiltrated with 35S:MYB110 in combination with 35S:NtAN1 and 35S:JAF13. Error bars are the SEM for four replicate reactions. (C) Diagram of the yeast one-hybrid reporter construct containing 490bp of the NtAN1 promoter region. (D) Y1H analysis of MYB110, NtJAF13, and NtAN1 interactions with the AN1 promoter. Results obtained with two independent colonies for each combination are presented. SD, minimal media; A, adenine; U, uracil; T, tryptophan; AbA, Aureobasidin A; EV, empty vector.
Direct binding to the NtAN1 promoter by AcMYB110 was assayed by yeast one-hybrid (Fig. 5C). AcMYB110 was shown to bind the NtAN1 promoter only in presence of the NtJAF13, whereas no binding was demonstrated in the presence of NtAN1 (Fig. 5D). This result confirms the transient luciferase assay results where NtJAF13 seems to be involved in the regulation of NtAN1.
Comparative in silico analysis of the promoter regions of AN1, CHS, and DFR
The promoter sequences of N. tabacum NtAN1, NtCHS, and NtDFR were analysed using the PLACE database SignalScan program (Higo et al., 1999) and several potential MYB (Dare et al., 2008) and bHLH binding sites were identified (Fig. 6 and Supplementary Fig. S3). Nine different variants of the canonical CANNTG bHLH binding motif were identified. When focusing on the 650bp region upstream of the ATG, it was found that the CACGTG variant was only present in the promoters of the two biosynthetic genes whereas two other motifs, CATCTG and its reverse complement CAGATG, were only found in the NtAN1 promoter. The promoter analysis of AN1, CHS, and DFR was extended to three other species of the Solanaceae family (Petunia, potato, tomato) as well as to Arabidopsis. The results confirmed that the CACGTG motif was specific to the CHS and DFR biosynthetic genes and that either, or both, CATCTG and CAGATG motifs were present in the AN1-like promoters in the Solanaceae. However, this did not occur in the Arabidopsis promoters as the CATCTG motif was present in the AtCHS promoter and none of the two AN1-specific motifs were present in the TT8 promoter. Although the AN1-like promoters of the four Solanaceae share a low pairwise identity (48–55% with one another and 81% between tomato and potato AN1), the common CACGTG motif sits in a highly conserved stretch of 27bp which also contains conserved putative MYB binding sites. This suggests that this region is under high selective pressure and so may have an important regulatory function.
Fig. 6.
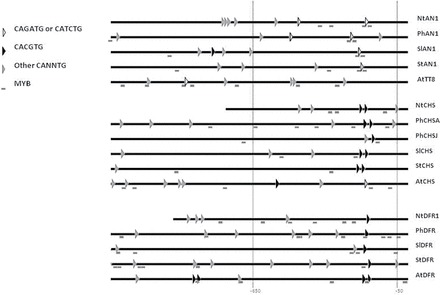
Schematic representation of bHLH and MYB motifs in the promoter regions of the anthocyanin biosynthetic genes (CHS and DFR) and AN1, or AN1-like, from Arabidopsis and four different Solanaceous species: tobacco, Petunia, tomato, and potato. Grey boxes indicate MYB binding sites corresponding to MYBPLANT (MACCWAMC), MYBPZM (CCWACC), MYBST1 (GGATA), and MYBCORE (CNGTTR) consensus motifs from PLACE database. bHLH binding sites corresponding to Ebox (CANNTG) motif are indicated by triangles. CAGATG or CACTG putative bHLH motifs (white triangles) were found specifically in the AN1 promoters of the four Solanaceae. Conversely, the CACGTG bHLH motifs (black triangles) were only found in the promoter region of the biosynthetic genes of the four Solanaceae and Arabidopsis.
Discussion
The transcriptional regulation of anthocyanin biosynthesis has been well described in plants and involves an MBW complex consisting of an R2R3 MYB, a bHLH, and a WD40 protein. MYBs and bHLHs that regulate the anthocyanin biosynthetic pathway have been extensively described in several plants of the Solanaceae family and are often found as small multi-gene families e.g. AN1, JAF13, AN2, DPL, and PHZ in Petunia (Quattrocchio et al., 1998; Quattrocchio et al., 1999; Spelt et al., 2000; Albert et al., 2011).
Although the different members of the bHLH and R2R3 MYB multi-gene families have often been thought of as having very similar roles, there is increasing evidence of specialization of function. Not only is there considerable amino acid sequence variability between the flavonoid-related MYB genes from the Poaceae (such as ZmC1) and those of dicots, but different gene family members within a species can vary in function, as seen from markedly different transgenic phenotypes resulting from over-expression of PhAN2, PhDPL, or PhPHZ in Petunia (Quattrocchio et al., 1999; Albert et al., 2011). Less information is available for the subfamily IIIf bHLHs belonging to the two different clades; one that includes sequences such as AN1 (Petunia), TT8 (Arabidopsis), and Mutabilis (snapdragon), and a second that contains sequences such as JAF13 (Petunia), Delila (snapdragon), and GL3 from Arabidopsis (Fig. 1).
Here, the roles of the two anthocyanin-related bHLH clades have been investigated using kiwifruit (Actinidia) AcMYB110, previously shown to activate anthocyanin biosynthesis (Fraser et al., 2013), and which is able to promote a red anthocyanic patch when expressed transiently without co-expression of an exogenous bHLH. Because of this strong activating ability, AcMYB110 proved to be a useful tool to study possible functional differences between the two flavonoid-related bHLH clades in tobacco.
The MYB–AN1 complex directly activates anthocyanin biosynthetic genes
Ectopic expression of the MYB NtAN2 in tobacco promotes anthocyanin biosynthesis and results in red pigmentation in both vegetative and floral tissues (Pattanaik et al., 2010). This is achieved by up-regulating the expression of the biosynthetic genes and the NtAN1 bHLH (Bai et al., 2011). Here, similar results were obtained when AcMYB110 was transiently expressed in tobacco leaves: NtAN1 was up-regulated, as were the biosynthetic genes of the pathway, resulting in dark red patches only a few days after infiltration (Fig. 2). However, constitutive expression of AcMYB110 did not affect the expression level of NtJAF13. Co-infiltration of AcMYB110 with the NtAN1 RNAi construct prevented activation of NtCHS and NtDFR and therefore red pigmentation (Figs 2, 3B, and 4), and the constitutive expression of PhJAF13 was not able to complement the lack of NtAN1 (Fig. 2). This is consistent with previous results where AN1 was shown to be necessary to activate the anthocyanin pathway: in Petunia, a white petal an1 mutant is due to the lack of PhAN1 and over-expressing PhJAF13 was not sufficient to restore anthocyanin biosynthesis (Spelt et al., 2000). A similar situation is found in pea, where loss of function of an AN1-like bHLH, corresponding to Mendel’s A gene, results in white flowers (Hellens et al., 2010). However, less specificity is seen in other species than that of Petunia or tobacco, where phenotypic redundancy between the bHLH family members is seen. For example, in snapdragon the bHLH Delila complements for the loss of another anthocyanin-related bHLH, Mutabilis (Schwinn et al., 2006). In contrast, co-infiltration of AcMYB110 with NtJAF13 RNAi resulted in a reduced level of NtJAF13 which did not seem to affect the ability of AcMYB110 to promote anthocyanin accumulation (Figs 2 and 3B).
The MYB–JAF13 complex has an indirect effect on anthocyanin biosynthesis through regulation of AN1 expression
Gene expression data (Fig. 3B) of the infiltrated leaves supported the role of NtJAF13 as an indirect regulator of the pathway by aiding the activation of NtAN1 expression by the MYB. Previous work had suggested that anthocyanin-related MYBs can regulate AN1 expression. In particular, MYB AN2 (or MYB AN4) and MYB75-PAP1 were shown to enhance the expression of PhAN1 and TT8 in Petunia and Arabidopsis, respectively (Spelt et al., 2000; Tohge et al., 2005). Moreover, there seems to be some level of functional redundancy amongst the flavonoid-related MYBs in regulating AN1 expression, given that PhAN1 is still expressed in Petunia an2 mutants (Spelt et al., 2000) and that in Arabidopsis TT2 also has been shown to promote TT8 expression (Baudry et al., 2006).
Our promoter activation analysis supports a hierarchical model where NtJAF13 is involved in the transcriptional activation of NtAN1, which in turn regulates the anthocyanin biosynthetic genes (Fig. 7). In tobacco, silencing of NtAN1 by RNAi, or over-expressing NtAN1 did not affect NtAN1 promoter activation, suggesting that NtAN1 is not involved in activating its own promoter, unlike in Arabidopsis, where TT8 in combination with PAP1 or TT2 is able to regulate its own expression (Baudry et al., 2006; Xu et al., 2013). In contrast, the NtJAF13 RNAi had a negative effect on NtAN1 promoter activation (Fig. 5A) and over-expression of NtJAF13 improved NtAN1 promoter activation (Fig. 5B). The role of NtJAF13 as a regulator of NtAN1 expression (in combination with AcMYB110) was further confirmed by yeast one-hybrid assay, in which AcMYB110 was able to bind the NtAN1 promoter only in the presence of the NtJAF13 (Fig. 5D). These results, in yeast and in planta, establish that NtJAF13 is required by the MYB to activate NtAN1 expression, whereas NtAN1 is not involved in its own regulation.
Fig. 7.
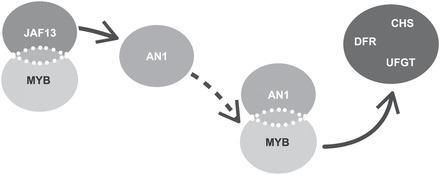
Schematic diagram representing the roles of AN1 and JAF13 in regulating anthocyanin biosynthesis and their relationships with MYB activators. Solid arrows represent direct transcriptional activation, whereas the dotted arrow shows an increased concentration of AN1 leading to the next step of transcriptional regulation. UFGT, UDP-flavonoid glycosyltransferase.
Given that AcMYB110 is able to activate all the promoters tested, our results suggest that the specificity of promoter recognition by the MBW complex is determined by the bHLH partner. The exact role of the bHLH in the MBW complex in different plant species is not yet clear. Studies in maize have suggested roles in reducing the inhibitory effect of single-repeat MYBs and/or in assisting transcription through aiding in chromatin accessibility (Feller et al., 2011; Kong et al., 2012). However, there is less known on the involvement of the bHLH in promoter cis-element recognition, some of which may be explained by the recent identification of further interacting proteins that alter DNA recognition by the bHLH (Kong et al., 2012). Our analysis of the promoter sequences from Arabidopsis and four different Solanaceous species found specific target bHLH cis-elements: a CACGTG bHLH-binding motif was present in the promoters of the two biosynthetic genes and surrounded by several potential MYB binding sites, whereas the CAGATG and/or CATCTG motifs were specific to the AN1 promoter (Fig. 6), supporting a model where the AN1–MYB and JAF13–MYB complexes would specifically recognise these motifs in the biosynthetic gene and the AN1 promoters, respectively. In Arabidopsis the CACGTG motifs was also found in the biosynthetic gene promoters; consistent with the fact that TT8 is able to regulate its own promoter (Baudry et al., 2006), neither of the two ‘AN1-like’-specific bHLH motifs were present in the TT8 promoter, suggesting that the model of hierarchical regulation is not well conserved outside the Solanaceae family. Future work to confirm this observation is required.
AN1 and JAF13 regulate anthocyanin biosynthesis through a hierarchical mechanism
In the Solanaceae, specificity of the anthocyanin-activating MYBs is defined by the interaction with the bHLH partners JAF13 and AN1. The MYB, to activate anthocyanin biosynthesis, needs to associate specifically to AN1 to induce transcription of the biosynthetic genes, whereas the MYB–JAF13 complex is not able to directly activate the genes of the pathway. The MYBs therefore rely on elevating AN1 expression so that the MYB–AN1–WD40 complex can then activate the anthocyanin response. The role of JAF13 is to enhance AN1 expression, boosting anthocyanin biosynthesis; this is achieved by forming a complex with the MYB and activating the AN1 promoter. JAF13 is suggested to act further upstream in the regulatory cascade in the control of anthocyanin biosynthesis.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Analysis of MYB110, AN1 and JAF13 protein–protein interactions by yeast two-hybrid.
Figure S2. Colour development in flowers of stable RNAi lines targeting endogenous NtAN1 and NtJAF13.
Figure S3. (A) Promoter sequence alignment of AN1 (A), CHS (B) and DFR (C) genes from tobacco, Petunia, tomato and potato.
Table S1. Oligonucleotide primer sequences.
Acknowledgements
We thank Nick Albert and Richard Espley for valuable discussion and advice. Nick Albert for providing the Petunia AN1 and JAF13 constructs; Hans Sommer for vector pTFT1. Tim Holmes and Darren Snaith for help with the pictures and the diagrams. This work was funded by the New Zealand Foundation for Research Science and Technology C06X0812 ‘Exploiting Opportunities from Horticultural Genomics’, and the Ministry of Science and Innovation.
References
- Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM. 2011. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. Plant Journal 65, 771–784. [DOI] [PubMed] [Google Scholar]
- Andre CM, Greenwood JM, Walker EG, Rassam M, Sullivan M, Evers D, Perry NB, Laing WA. 2012. Anti-inflammatory procyanidins and triterpenes in 109 apple varieties. Journal of Agricultural and Food Chemistry 60, 10546–10554. [DOI] [PubMed] [Google Scholar]
- Bai Y, Pattanaik S, Patra B, Werkman J, Xie C, Yuan L. 2011. Flavonoid-related basic helix-loop-helix regulators, NtAn1a and NtAn1b, of tobacco have originated from two ancestors and are functionally active. Planta 234, 363–375. [DOI] [PubMed] [Google Scholar]
- Baudry A, Caboche M, Lepiniec L. 2006. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana . Plant Journal 46, 768–779. [DOI] [PubMed] [Google Scholar]
- Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martinez-Garcia JF, Bilbao-Castro JR, Robertson DL. 2010. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiology 153, 1398–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler VL, Radicella JP, Robbins TP, Chen JC, Turks D. 1989. Two regulatory genes of the maize anthocyanin pathway are homologous—isolation of B utilizing R genomic sequences. Plant Cell 1, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dare AP, Schaffer RJ, Lin-Wang K, Allan AC, Hellens RP. 2008. Identification of a cis-regulatory element by transient analysis of co-ordinately regulated genes. Plant Methods 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KM, Albert NW, Schwinn KE. 2012. From landing lights to mimicry: the molecular regulation of flower colouration and mechanisms for pigmentation patterning. Functional Plant Biology 39, 619–638. [DOI] [PubMed] [Google Scholar]
- deVetten N, Quattrocchio F, Mol J, Koes R. 1997. The an11 locus controlling flower pigmentation in Petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Genes & Development 11, 1422–1434. [DOI] [PubMed] [Google Scholar]
- Egea-Cortines M, Saedler H, Sommer H. 1999. Ternary complex formation between the MADS-box proteins SQUAMOSA, DEFICIENS, and GLOBOSA is involved in the control of floral architecture in Antirrhinum majus . Embo Journal 18, 5370–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. 2007. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant Journal 49, 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller A, Machemer K, Braun EL, Grotewold E. 2011. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. The Plant Journal 66, 94–116. [DOI] [PubMed] [Google Scholar]
- Feyissa DN, Lovdal T, Olsen KM, Slimestad R, Lillo C. 2009. The endogenous GL3, but not EGL3, gene is necessary for anthocyanin accumulation as induced by nitrogen depletion in Arabidopsis rosette stage leaves. Planta 230, 747–754. [DOI] [PubMed] [Google Scholar]
- Fraser L, Seal A, Montefiori M, et al. 2013. An R2R3 MYB transcription factor determines red petal colour in an Actinidia (kiwifruit) hybrid population. BMC Genomics 14, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. 2000. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proceedings of the National Academy of Sciences, USA 97, 13579–13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52, 696–704. [DOI] [PubMed] [Google Scholar]
- Hellens R, Allan A, Friel E, Bolitho K, Grafton K, Templeton M, Karunairetnam S, Gleave A, Laing W. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Moreau C, Lin-Wang K, et al. 2010. Identification of Mendel’s white flower character. Plos One 5, e13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V. 2011. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. Journal of Experimental Botany 62, 2465–2483. [DOI] [PubMed] [Google Scholar]
- Hichri I, Heppel SC, Pillet J, Leon C, Czemmel S, Delrot S, Lauvergeat V, Bogs J. 2010. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Molecular Plant 3, 509–523. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research 27, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Halladay J, Craig EA. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H. 2004. Retrotransposon-induced mutations in grape skin color. Science 304, 982–982. [DOI] [PubMed] [Google Scholar]
- Kong Q, Pattanaik S, Feller A, Werkman JR, Chai CL, Wang YQ, Grotewold E, Yuan L. 2012. Regulatory switch enforced by basic helix-loop-helix and ACT-domain mediated dimerizations of the maize transcription factor R. Proceedings of the National Academy of Sciences, USA 109, 2091–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC. 2010. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biology 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Prescott A, Mackay S, Bartlett J, Vrijlandt E. 1991. Control of anthocyanin biosynthesis in flowers of Antirrhinum majus . Plant Journal 1, 37–49. [DOI] [PubMed] [Google Scholar]
- Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L. 2000. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12, 1863–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanaik S, Kong Q, Zaitlin D, Werkman J, Xie C, Patra B, Yuan L. 2010. Isolation and functional characterization of a floral tissue-specific R2R3 MYB regulator from tobacco. Planta 231, 1061–1076. [DOI] [PubMed] [Google Scholar]
- Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H. 1987. The regulatory c1 locus of Zea mays encodes a protein with homology to myb protooncogene products and with structural similarities to transcriptional activators. The EMBO Journal 6, 3553–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires N, Dolan L. 2010. Origin and diversification of basic-helix-loop-helix proteins in plants. Molecular Biology and Evolution 27, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F, Verweij W, Kroon A, Spelt C, Mol J, Koes R. 2006. PH4 of Petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell 18, 1274–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F, Wing J, van der Woude K, Souer E, de Vetten N, Mol J, Koes R. 1999. Molecular analysis of the anthocyanin2 gene of Petunia and its role in the evolution of flower color. Plant Cell 11, 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F, Wing JF, van der Woude K, Mol JNM, Koes R. 1998. Analysis of bHLH and MYB domain proteins: species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant Journal 13, 475–488. [DOI] [PubMed] [Google Scholar]
- Schwinn KE, Venail J, Shang Y, et al. 2006. A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum . Plant Cell 18, 831–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelt C, Quattrocchio F, Mol J, Koes R. 2002. ANTHOCYANIN1 of Petunia controls pigment synthesis, vacuolar pH, and seed coat development by genetically distinct mechanisms. Plant Cell 14, 2121–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelt C, Quattrocchio F, Mol JNM, Koes R. 2000. anthocyanin1 of Petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. The Plant Cell Online 12, 1619–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR. 2006. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiology 142, 1216–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Nishiyama Y, Hirai MY, et al. 2005. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. The Plant Journal 42, 218–235. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. 2001. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology 126, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WJ, Grain D, Le Gourrierec J, et al. 2013. Regulation of flavonoid biosynthesis involves an unexpected complex transcriptional regulation of TT8 expression, in Arabidopsis . New Phytologist 198, 59–70. [DOI] [PubMed] [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF. 2004. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant Journal 40, 22–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


