Highlight
ABA has a central role in repression of grape bud meristem activity, and both the natural dormancy cycle and artificial dormancy release stimuli act via regulation of ABA metabolism.
Key words: ABA 8′-hydroxylase, abscisic acid, bud, 9-cis-epoxycarotenoid dioxygenase, dormancy, grapevine.
Abstract
In warm-winter regions, induction of dormancy release by hydrogen cyanamide (HC) is mandatory for commercial table grape production. Induction of respiratory stress by HC leads to dormancy release via an uncharacterized biochemical cascade that could reveal the mechanism underlying this phenomenon. Previous studies proposed a central role for abscisic acid (ABA) in the repression of bud meristem activity, and suggested its removal as a critical step in the HC-induced cascade. In the current study, support for these assumptions was sought. The data show that ABA indeed inhibits dormancy release in grape (Vitis vinifera) buds and attenuates the advancing effect of HC. However, HC-dependent recovery was detected, and was affected by dormancy status. HC reduced VvXERICO and VvNCED transcript levels and induced levels of VvABA8’OH homologues. Regulation of these central players in ABA metabolism correlated with decreased ABA and increased ABA catabolite levels in HC-treated buds. Interestingly, an inhibitor of ethylene signalling attenuated these effects of HC on ABA metabolism. HC also modulated the expression of ABA signalling regulators, in a manner that supports a decreased ABA level and response. Taken together, the data support HC-induced removal of ABA-mediated repression via regulation of ABA metabolism and signalling. Expression profiling during the natural dormancy cycle revealed that at maximal dormancy, the HC-regulated VvNCED1 transcript level starts to drop. In parallel, levels of VvA8H-CYP707A4 transcript and ABA catabolites increase sharply. This may provide initial support for the involvement of ABA metabolism also in the execution of natural dormancy.
Introduction
In warm-winter regions, dormancy release poses a major obstacle to commercial viticulture. Artificial substitutes for chilling are thus mandatory in these regions to avoid prolonged dormancy, thereby allowing co-ordinated and early production of economically viable yields. The only practical means currently available for effective artificial dormancy release in vineyards involves treatment with hydrogen cyanamide (HC), used by the table grape industry worldwide (Lavee and May, 1997; Or, 2009). The ability of HC to induce respiratory stress, which initiates a biochemical cascade that leads to effective dormancy release, is also responsible for its toxicity, both to the vines and to the environment (Ophir et al., 2009; Or, 2009; Pérez et al., 2009; Vergara et al., 2012). Development of safe alternatives may rely on the manipulation of targets that are affected by the artificial stimuli downstream of the respiratory stress, which stands a much better chance of being plant specific and harmless. A detailed characterization of such targets is currently unavailable.
The results of a large-scale comparative analysis of grape (Vitis vinifera) bud responses to two artificial stimuli of bud dormancy release, HC and heat shock (HS), allowed a working model of the events occurring during artificially induced bud dormancy release to be proposed (Ophir et al., 2009) (Supplementary Fig. S1 available at JXB online). According to this model, perturbation of cytochrome pathway activity in the mitochondria leads to respiratory and oxidative stress, expressed as an increased level of reactive oxygen species, decreased activity of the tricarboxylic acid cycle, and decreased production of ATP. To address this energy crisis, the alternative oxidase pathway, glycolysis, pyruvate metabolism, and anaerobic respiration are induced, in an order that has yet to be defined. In parallel, the cellular antioxidant machinery and related pathways are up-regulated to cope with the oxidative burst. Changes resulting from the above reprogramming under conditions that mimic hypoxia may affect the interplay between ethylene and abscisic acid (ABA) in a way that allows removal of ABA repression of meristem activity and growth resumption. This hypothesis was inspired by similar scenarios played out in deepwater rice and Rumex palustris under low oxygen conditions (Benschop et al., 2006; Steffens et al., 2006; Lasanthi-Kudahettige et al., 2007; Hattori et al., 2009), and, as recently observed, during seed dormancy release as detailed below (Linkies et al., 2009; Arc et al., 2013).
The results of subsequent analyses supported the predictive power of the model: treatment with sodium azide (AZ), a well-known inhibitor of mitochondrial respiration, stimulated bud dormancy release in a manner similar to HC, and treatment with HC, a well-known dormancy release agent, inhibited O2 uptake by isolated grape bud mitochondria (Ophir et al., 2009; Pérez et al., 2009). Treatment with HC induced a temporary increase in hydrogen peroxide levels (Pérez et al., 2008) and alternative oxidase transcripts (Ophir et al., 2009). HC and HS transiently up-regulated various oxidative stress-related genes (Keilin et al., 2007; Halaly et al., 2008). HC and HS up-regulated expression of GDBRPK, a sucrose nonfermenting (SNF)-like protein kinase, which is a sensor of elevated AMP levels in stressful situations, as well as that of sucrose synthase (Halaly et al., 2008), pyruvate decarboxylase, and alcohol dehydrogenase (Or et al., 2000a ; Keilin et al., 2007; Halaly et al., 2008; Ophir et al., 2009). Production of both acetaldehyde and ethanol was detected following the application of dormancy release stimuli such as HC, HS, and AZ (Ophir et al., 2009), and hypoxic conditions induced dormancy release (Vergara et al., 2012). Enhancement of bud break by HC was shown to be dependent on calcium signalling, and HC induced changes in the transcription and phosphorylation of regulators of calcium signalling (Pang et al., 2007). Moreover, various dormancy release stimuli temporarily induced endogenous ethylene production, and exogenous ethylene stimulated dormancy release, whereas treatment with an inhibitor of ethylene signalling inhibited dormancy release (Ophir et al., 2009) and eliminated the enhancing effect of HC, AZ, and HS (E. Or et al., unpublished). In the current study, the model was further tested by investigating the hypothesis that ABA is involved in dormancy maintenance, and that HC stimulates the removal of this repression.
ABA and seed dormancy
ABA produced by zygotic tissues at late maturation stages appears to be a central regulator of seed dormancy and germination, and modifications in its metabolism or signalling lead to significant dormancy-related phenotypes (Karssen et al., 1983; Frey et al., 2004; Arc et al., 2013). In general, deficiency in ABA and its synthesis, as well as interference in ABA signalling, lead to dormancy loss, while suppression of ABA inactivation leads to increased depth of dormancy (Nambara and Marion-Poll, 2005; Nambara et al., 2010).
Carotenoid cleavage by 9-cis-epoxycarotenoid dioxygenase (NCED) has been proven to constitute a key regulatory step in the control of ABA synthesis, which affects seed dormancy and germination (Iuchi et al., 2001; Qin and Zeevaart, 2002; Cadman et al., 2006; Lefebvre et al., 2006). Accordingly, (i) an Arabidopsis nced6nced9 double mutant exhibited reduced ABA content and reduced seed dormancy (Lefebvre et al., 2006); (ii) overexpression of NCED increased the ABA level and dormancy in tomato seeds, and delayed germination in imbibed tobacco seeds (Qin and Zeevaart, 2002); and (iii) induction of NCED was sufficient to suppress germination of imbibed seeds despite their exposure to dormancy release treatment (Martínez-Andújar et al., 2011). Additional ABA-deficient mutants with impaired synthesis, such as aba1, aba2, and aao3, lacked the primary dormancy associated with mature Arabidopsis seeds (Leon-Kloosterziel et al., 1996; Finkelstein et al., 2002; Himmelbach et al., 2003), and overexpression of XERICO, another positive regulator of the ABA level, also resulted in repression of seed germination (Ko et al., 2006).
An additional key regulatory step for the control of ABA levels appears to be ABA inactivation by its hydroxylation at the 8′ position, catalysed by CYP707A ABA 8′-hydroxylase (ABA8’OH) (Cutler and Krochko, 1999; Nambara and Marion-Poll, 2005; Cutler, 2007; Nambara et al., 2010). The significant effect of this step on both the ABA level and seed dormancy is reflected by (i) increased ABA levels in dry and imbibed seeds of Arabidopsis cyp707a2 mutants and their reduced germination (Okamoto et al., 2006); and (ii) the higher ABA levels and increased dormancy of transgenic ABA8′OH1 RNAi (RNA interference) barley grains (Gubler et al., 2008).
ABA signalling is also important in the control of seed dormancy. The interaction between protein phosphatase 2C (PP2C) and SNF1-related protein kinase 2 (SnRK2), which negatively affect ABA signalling, is disrupted following binding of ABA to its pyr/pyl receptors and formation of ABA receptor–PP2C complexes. This allows activation of SnRK2, which then activates downstream transcription factors that induce ABA-responsive gene expression (Hubbard et al., 2010). In agreement with the role suggested for ABA in seed dormancy, germination of a pyr/pyl sextuple mutant was highly insensitive to ABA, and the triple mutant snrk2.2snrk2.6snrk2.3 also exhibited loss of dormancy (Fujii and Zhu, 2009; Nakashima et al., 2009; Gonzalez-Guzman et al., 2012). PP2C functions also regulate germination ability. Accordingly, germination of pp2c mutants was slower than in the wild type and was inhibited by very low ABA concentrations, in agreement with its negative role in ABA signalling (Kuhn et al., 2006; Rubio et al., 2009). Overexpression of AtPP2CA, however, resulted in significantly improved germination at ABA concentrations that completely inhibited wild-type seed germination (Kuhn et al., 2006).
ABA and bud dormancy
A role for ABA in the regulation of bud endodormancy has been discussed in the literature, and it has been suggested that ABA levels increase in the autumn and act as a signal of shorter day-length. This, in turn, hypothetically results in inhibition of cell proliferation and shoot growth, promotion of terminal bud set, and induction of endodormancy. Accordingly, after 3–4 weeks of short days, regulators of ABA biosynthesis (NCED3, ABA1, and ABA2) and ABA signal transduction components (PP2C, ABI1, AREB3, among others) were induced in poplar buds, and ABA levels in the apex peaked (Arora et al., 2003; Rohde and Bhalerao, 2007; Ruttink et al., 2007). ABA application accelerated growth cessation in seedlings of two birch tree ecotypes (Li et al., 2003), ABA levels were highest in deeply dormant potato tubers (Korableva et al., 1980; Biemelt et al., 2000; Destefano-Beltrán et al., 2006a ), and endogenous ABA levels also increased during onset of grape bud dormancy (Düring and Bachmann, 1975; Koussa et al., 1994; Or et al., 2000b ). Decreased levels of ABA were recorded in leafy spurge during the transition from endodormancy to ecodormancy (Horvath et al., 2006).
Based on the above, a role for ABA in the regulation of dormancy maintenance and release was considered, and then questioned due to conflicting results in the limited number of reported studies. In support of ABA’s role, a decrease in endogenous ABA level preceded bud dormancy release in birch, grapevine, and potatoes (Koussa et al., 1994; Or et al., 2000b ; Li et al., 2004; Destefano-Beltrán et al., 2006a ), and delayed bud break was reported following ABA application in birch (Rinne et al., 1994a ), apple (Dutcher and Powell, 1972), kiwi fruit (Lionakis and Schwabe, 1984), and sour cherry (Mielke and Dennis, 1978). However, spring application of ABA on grapes had little effect on bud break (Hellman et al., 2006), and the effect of chilling on the endogenous ABA level is not clear. In agreement with the suggested role of ABA, chilling-induced dormancy release of birch was accompanied by alterations in endogenous ABA levels (Li et al., 2004). However, no clear effect of chilling on birch bud ABA content was detected in another study (Rinne et al., 1994a ). Among the findings that put ABA’s role in dormancy maintenance/release into question are the similar decline in ABA levels of chilled and non-chilled apple buds despite induction of dormancy release only in the chilled buds, and the higher ABA content in chilled cherry buds compared with non-chilled controls (Saure, 1985; Powell, 1987; Crabbe, 1994).
In potato, declining ABA content throughout the dormancy cycle was correlated with decreased expression of NCED1/2 (Destefano-Beltrán et al., 2006a ), and treatment of microtubers with an ABA biosynthesis inhibitor shortened the dormancy period (Suttle and Hultstrand, 1994). The level of ABA8′OH expression in tubers was inversely correlated to ABA levels and positively correlated to bud break (Debast et al., 2011). Nevertheless, application of ABA to dormant tubers had no marked effect, whereas treatment of non-dormant tubers only transiently inhibited sprout growth, suggesting that variations in ABA degradation ability may play a central role in bud behaviour (Suttle et al., 2012). An ~2-fold increase in the minituber ABA level following chemical inhibition of ABA8′OH activity only partially (but not significantly) delayed minituber dormancy release (Suttle et al., 2012).
In the current study, the hypothesis was tested that ABA is involved in the regulation of grape bud dormancy maintenance/release and that HC exerts its enhancing effect, at least in part, by affecting the bud ABA level.
Materials and methods
Plant material
The experiments were conducted with mature buds collected from cordon-trained grapevines (Vitis vinifera cv. Early sweet) in a commercial vineyard located in the Jordan Valley. All plants were subjected to the cultural practices commonly used in commercial vineyards.
The grape bud-break response in single-node cuttings appears to be well correlated with bud behaviour on the vine, and it is therefore used as a common and reliable indicator of the dormancy depth of grapevines under forcing conditions (Shulman et al., 1983; Koussa et al., 1994; Lavee and May, 1997; Or and Viloznyi, 1999; Or et al., 2000a ; Pérez and Lira, 2005; Pérez et al., 2008). Use of this system enables the study of issues related to true dormancy (endodormancy), without the interference of paradormant and ecodormant effects (Lang, 1987). Another advantage is the possibility of working with a large number of buds, providing a proper representation of the dormancy status of a given bud population at a specific point in time during the dormancy cycle. Hence, vines were pruned to three-node spurs, and the detached canes, each carrying nine buds (in positions 4–12), were transferred to the lab. Canes were cut into single-node cuttings, randomly mixed, and groups of 10 cuttings were prepared. Nine groups were used for each treatment.
Analysis of the effect of ABA on bud break
For ABA treatments, cuttings were both sprayed and immersed in vases with 10 μM or 100 μM ABA (Protone 20 SG™, Valent BioSciences, 20% active S-ABA, Libertyville, USA Israel) solution (150ml in each vase with three groups of cuttings), with addition of 0.02% (v/v) Triton X-100 (Sigma-Aldrich, St Louis, MO, USA). The vases were transferred to a growth chamber and forced at 22 °C under a 14h/10h light/dark regime. After incubation in ABA for 48h or 96h, cuttings were transferred to tap water. The control was treated similarly with 0.02% Triton X-100 solution.
Induction of dormancy release by chemical and physical stimuli
Following 48h pre-incubation as described above in 100 μM ABA or water, a second treatment was applied, considered as 0h for bud-break monitoring and bud sampling. The treated groups were then returned to water-containing vases and incubated under the above-described forcing conditions for an additional 28 d for bud-break monitoring. Cuttings that were pre-treated with Triton X-100 solution were used for control, HC, AZ, HC, and hypoxia treatments. For control treatments, the cuttings were sprayed again with tap water. For the HC treatment, cuttings were sprayed with 3% (v/v) ‘Dormex’ (SKW, Trostberg, Germany), a commercial formulation containing 49% (w/v) HC. For the AZ treatment, cuttings were sprayed with 2% (w/v) sodium azide (NaN3; Sigma-Aldrich). All solutions were formulated in water containing 0.02% Triton X-100 as the wetting agent. For the HS treatment, cuttings were immersed in 50 °C water for 1h. For the hypoxia treatment, cuttings were placed in glass jars containing 150ml of water and equipped with a rubber plug (80 cuttings per 2 litre jar). Jars were flushed with N2 to reduce the O2 level to 1%. Cuttings were removed from the sealed jars 48h later and transferred to vases as described above.
For the combined ABA–HC, ABA–AZ, ABA–HS, and ABA–hypoxia treatments, cuttings were initially treated with 100 μM ABA for 48h, and then treated with HC, AZ, HS, or hypoxia as described above.
The chemical 2,5-norbornadiene (NBD) binds specifically to ethylene receptors and competes with ethylene for the ethylene-binding sites (Sisler and Serek, 2003). NBD–HC and the relevant HC control were set up in sealed jars under the conditions described above. NBD (Sigma-Aldrich) was placed in a perforated container within each NBD treatment jar (5ml l–1) and jars were left sealed for 48h. Cuttings were then removed from the jars, treated with HC or water as described above, and transferred to vases in a growth chamber under the conditions described above.
Bud break was monitored 7, 11, 14, 18, 21, 25, and 28 d after treatment under the forcing conditions described above. Bud break was defined as the stage at which green tissue becomes visible underneath the bud scales. For gene expression and hormone analyses, identical treatments were carried out and buds were sampled at 12, 24, 48, and 96h, frozen in liquid nitrogen, and kept at –80 °C. Buds from jar-based treatments were only sampled at 48h from sealing time.
Quantitative real-time PCR analyses
Relative transcript levels were measured by quantitative real-time PCR (qRT-PCR) with ABsolute Blue QPCR SYBR Green Low ROX Mix (Thermo Fisher Scientific, Waltham, MA, USA) on a Corbett Rotor-Gene 6000 (Qiagen, Hilden, Germany). Total RNA was extracted from 2g sampled after grinding 20 buds as described previously (Or et al., 2000a ), and treated with RQ DNase (Promega, Madison, WI, USA) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 2.5 μg of total RNA using Moloney murine leukaemia virus reverse transcriptase (M-MLV RT; Promega) according to the manufacturer’s instructions. VvActin primers, characterized and optimized by Reid et al. (2006), were used for normalization.
The 10 μl reaction mixture consisted of 0.1 μM of forward and reverse primers, 5 μl of SYBR-Green (ABsolute Blue qPCR SYBR Green Mixes, Thermo Fisher Scientific), and 4 μl of cDNA diluted 1:32. PCRs were run under the following conditions: 15min at 95 °C and 40 cycles of 15 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. No-template controls consisted of all of the above components with the exception of cDNA. For each sample, six independent quantitation analyses comprising three biological repeats with two technical repeats were carried out. All of the primers (Supplementary Table S1 at JXB online) were designed by Primer3 software (http://frodo.wi.mit.edu/primer3/).
Quantitation of endogenous ABA and its catabolites
Triplicate samples of 10 frozen buds for each biological replicate were homogenized in liquid nitrogen, and 0.5g of the homogenized powder was sampled. The sample was extracted with 3ml of 80% methanol containing 1% acetic acid and deuterium-labelled ABA, neophaseic acid (neoPA), phaseic acid (PA), dihydrophaseic acid (DPA), and ABA glucosyl ester (ABA-GE) as internal standards, for 1h at 4 °C. Samples were centrifuged at 3000 g for 10min and filtered through an LRC-2 Frits Bond Elut Reservoir (Agilent Technologies, Santa Clara, CA, USA) to remove residual plant materials. The solvent (80% methanol, 1% acetic acid) extraction was repeated for 10min, and samples were centrifuged and filtered as before. The two extracts were combined and evaporated to dryness at 35 °C using a Savant SpeedVac Concentrator (Thermo Fisher Scientific). Dried samples were redissolved in 1ml of 80% acetonitrile, 1% acetic acid. The acetonitrile was removed by evaporation in vacuo. ABA and its catabolites were purified and measured as previously described with slight modification (Seo et al., 2011). After purification with a reverse phase column cartridge (Oasis HLB 30mg, 1ml, Waters, Milford, MA, USA), extracts were completely dried for sequential purification with a weak anion exchange column cartridge (BondElut DEA, 100mg, 1ml, Agilent Technologies). Dry residues were dissolved in 1ml of methanol and loaded onto BondElut DEA. Flow through which contains ABA-GE was corrected and then the eluent of methanol containing 1% acetic acid which contains ABA and other catabolites was corrected. The prominent ions for each compound were analysed by a liquid chromatography–tandem mass spectrometry system consisting of an ultra high performance liquid chromatograph (Agilent 1200 UHPLC; Agilent Technologies) and a triple quadrupole mass spectrometer (Agilent 6410; Agilent Technologies) equipped with an ODS column (ZORBAX XDB-C18, 2×50mm, 1.8 μm; Agilent Technologies). Analysis parameters are detailed in Supplementary Table S2 at JXB online. The endogenous ABA and catabolite contents were calculated from the peak area ratios of these endogenous compounds to internal standards.
Results
Effect of exogenous ABA on dormancy release of grapevine buds
To test the hypothesis that ABA regulates dormancy release of grapevine buds, the responses of dormant buds to application of ABA, a known inducer of dormancy release (HC), or water were compared. HC application led to the expected enhancement of bud dormancy release relative to the control. ABA, however, had a significant inhibitory effect on dormancy release of the tested bud population (Fig. 1A). Incubation of single-node cuttings with 10 μM ABA for 48h resulted in decreases of 18, 23, 25, and 16% in bud-break percentage relative to the control population at 11, 14, 18, and 21 d after treatment, respectively. Similar treatment with 100 μM ABA resulted in even stronger inhibition, with decreases of 25, 48, 46, and 26% in bud-break percentage relative to the control at the same time points. Incubation in 100 μM ABA for the longer period of 96h resulted in a higher degree of inhibition compared with incubation for 48h under identical conditions (Fig. 1B).
Fig. 1.
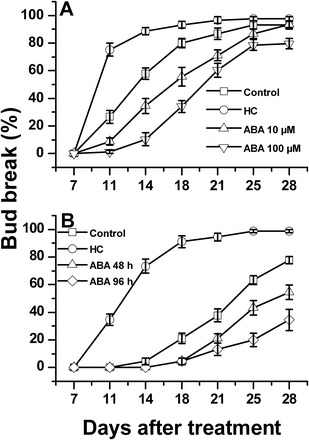
ABA delays bud break in a concentration- and duration-dependent manner. Vines of Vitis vinifera cv. Early Sweet from a vineyard at Gilgal, located in the Jordan Valley, were pruned to three-node spurs. The detached canes were cut into single-node cuttings, randomly mixed, and groups of 10 cuttings were prepared. (A) Four treatments were carried out, each with nine groups of 10 cuttings. The bases of the cuttings were immersed in vases containing 10 μM ABA, 100 μM ABA (with 0.02% Triton), or only 0.02% Triton (for control and HC treatments). The vases were placed in a growth chamber and forced at 22 °C under a 14h/10h light/dark regime. After 48h, the solutions were replaced with tap water, and sprayed with 0.02% Triton instead, apart from the HC-treated buds which were sprayed with 3% ‘Dormex’ as detailed in the Materials and methods. The treated groups were forced under the above conditions for another 28 d. Bud break was monitored at 7, 11, 14, 18, 21, 25, and 28 d after spraying. Values are averages of the nine groups in each treatment ±SE. (B) Both ABA treatments were carried out using 100 μM ABA. In the ABA 96h treatment, the cuttings were returned to ABA solution for an additional 48h after spraying. All other details are as in (A).
Effect of ABA on the enhancing effect of various dormancy release stimuli
Based on its inhibitory effect on bud dormancy release, it was suggested that exogenous ABA might also slow the advancing effect of HC on dormancy release of grapevine buds. Compared with the HC treatment, combined treatment with ABA and HC (ABA–HC) attenuated the bud-break rate, producing a ΔBud Break of 50% and 25% at 11 d and 14 d after HC treatment, respectively (Fig. 2A). Interestingly, recovery from such inhibition was evident 21 d after the ABA–HC treatment, compared with the HC-treated buds, whereas no recovery was evident for ABA-treated buds compared with the control. It should be noted that the ABA–HC treatment produced higher levels of bud break than the controls at all analysed time points.
Fig. 2.
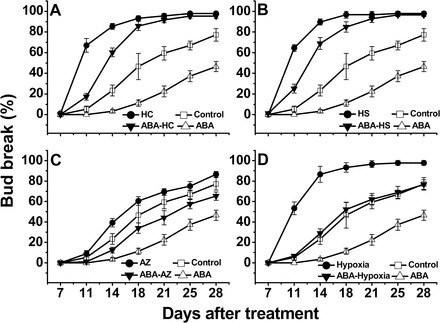
ABA attenuates the enhancing effect of various dormancy release stimuli. (A) In the combined ABA–HC treatment, buds were treated with ABA for 48h prior to HC treatment. All other details are as in Fig. 1. The experimental scheme used in (B), (C), and (D) is identical to that described in (A) apart from the indicated changes. (B) HC treatment was replaced with HS treatment where cuttings were immersed in 50 °C water for 1h as detailed in the Materials and methods. (C) HC treatment was replaced with AZ treatment where cuttings were sprayed with 2% sodium azide and 0.02% Triton. (D) HC treatment was replaced with hypoxia treatment where cuttings were incubated for 48h in sealed jars which were flushed with N2 to reduce O2 level to 1%.
Similar analyses were carried out to test the effect of ABA on the effect of other known stimuli of dormancy release. Incubation in 100 μM ABA for 48h prior to treatment with AZ, application of HS, or incubation under hypoxia for 48h attenuated bud break compared with the respective treatments with the relevant inducer of dormancy release without ABA (Fig. 2B–D). Similar to ABA–HC, recovery was evident for the ABA–HS-treated buds. However, the ABA–AZ and ABA–hypoxia buds failed to reach the bud-break percentage of the AZ or hypoxia treatments during the analysed period. Unlike the ABA–HC- and ABA–HS-treated bud populations, the ABA–AZ and ABA–hypoxia populations did not present higher levels of bud break than their respective controls at any analysed time point.
Effect of HC on expression of central components of ABA metabolism in grape buds
In light of the described findings, it was speculated that ABA might be involved in repression of primordial growth, and that stimuli of dormancy release, such as HC, may be involved in diminishing its repression potential via modification of ABA metabolism. To test this assumption, comparative transcript profiling of central regulators of ABA synthesis and degradation was carried out.
Previous bioinformatics analyses identified three putative grape homologues of NCED (VvNCED) and eight homologues of the Arabidopsi s ABA8′OH gene (VvA8H-CYP707A), encoding rate-limiting enzymes in ABA biosynthesis and catabolism, respectively (Young et al., 2012). In the current study, a single homologue of XERICO, termed VvXERICO, was identified (Supplementary Fig. S2 at JXB online). In mature grape buds, expression of all three homologues of NCED (hereafter referred to as VvNCED1, VvNCED2, and VvNCED3) was detected, but the levels of the latter two were very low compared with that of VvNCED1. Expression of VvXERICO, VvA8H-CYP707A1, and VvA8H -CYP707A4 was also recorded; expression of VvA8H-CYP707A2 was also detected, but only at very low levels.
Analyses of the effect of HC on the transcript levels of VvXERICO and the bud-expressed members of the VvNCED and VvA8H-CYP707A gene families were carried out using qRT-PCR. In agreement with a previous microarray analysis (Ophir et al., 2009), HC treatment seemed to down-regulate the expression of VvNCED1 and VvXERICO significantly, with a maximum difference at 48h (Fig. 3A, B). In contrast, HC led to a significant increase in the transcript level of VvA8H-CYP707A4, which peaked at 48h (Fig. 3C). A similar trend, but with less pronounced differences, was recorded for transcript levels of VvNCED2 (Supplementary Fig. S3A at JXB online), VvNCED3 (Supplementary Fig. S3B), and VvA8H-CYP707A1 (Supplementary Fig. S3C). Parallel profiling of VvNCED1 (Fig. 3D), VvXERICO (Fig. 3E), and VvA8H-CYP707A4 (Fig. 3F) in HS-treated buds presented very similar results relative to their controls. In AZ-treated buds, however, only the profiles of VvXERICO (Fig. 3H) and VvA8H-CYP707A4 (Fig. 3I) agreed with the patterns presented for HC and HS, whereas transcript levels of VvNCED1 were higher in the AZ-treated buds than in the control (Fig. 3G). At 12h, no difference was detected apart from down-regulation of VvNCED1 transcript levels by HC and up-regulation by AZ.
Fig. 3.
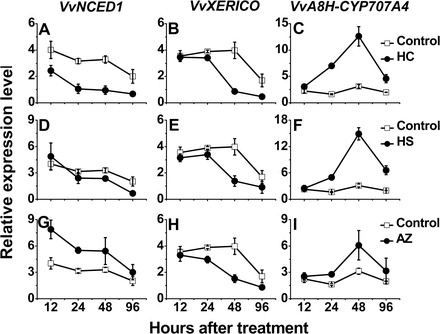
Dormancy release stimuli modulate transcription of regulators of ABA metabolism. Total RNA was extracted from control, HC-, HS-, and azide-treated buds sampled 12, 24, 48, and 96h after treatment. Relative expression levels of VvNCED1, VvXERICO, and VvA8H-CYP707A4 were determined by qRT-PCR as described in the Materials and methods and normalized against VvActin. The values represent the mean ±SE of three biological repeats, each with two technical repeats. Relative expression levels are presented for HC versus control buds (A–C), HS versus control buds (D–F), and AZ versus control buds (G–I) sampled 12, 24, 48, and 96h after treatment.
Effect of HC on expression of central components of ABA signalling in grape buds
The gene families of the central players in ABA signalling in V. vinifera have been recently identified and characterized (Boneh et al., 2012a, b ). In the current study, analyses are presented of the effect of HC on the transcript levels of members of the ABA receptors, PP2C and ABA-responsive element/ABA binding factor (AREB/ABF) gene families in the buds. Overall, the data suggest that HC triggered reprogramming of the expression of these ABA signalling components which are known to be regulated at the transcriptional level (Kuhn et al., 2006; Santiago et al., 2009; Yoshida et al., 2014). While levels of VvPP2C4 (Fig. 4B), VvPP2C9 (Fig. 4C), and VvRCAR1 (Fig. 4D) transcripts were significantly reduced in HC-treated buds, levels of VvPP2C2 (Fig. 4A), VvRCAR5 (Fig. 4E), and VvRCAR6 (Fig. 4F) transcripts were markedly induced.
Fig. 4.
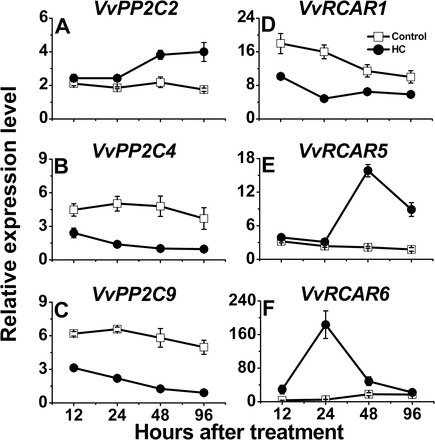
HC modulates transcription of central components of ABA signalling. Relative expression levels of VvPP2C2 (A), VvPP2C4 (B), VvPP2C9 (C), VvRCAR1 (D), VvRCAR5 (E), and VvRCAR6 (F) are presented in HC and control buds sampled at 12, 24, 48, and 96h after treatment. All other details are as described in Fig. 3.
Significant but smaller changes were recorded in the transcript levels of VvRCAR2 (Supplementary Fig. S4A at JXB online) and VvRCAR7 (Supplementary Fig. S4D), which were up-regulated in response to HC. No clear difference was observed in the level of VvRCAR3 (Supplementary Fig. S4B ) and VvRCAR4 (Supplementary Fig. S4C).
Analysis of the effect of HC on the transcript levels of AREB/ABF genes identified in grapevine (Boneh et al., 2012a ) indicated that both VvABF1 and VvABF2 are significantly down-regulated in response to HC (Fig. 5).
Fig. 5.
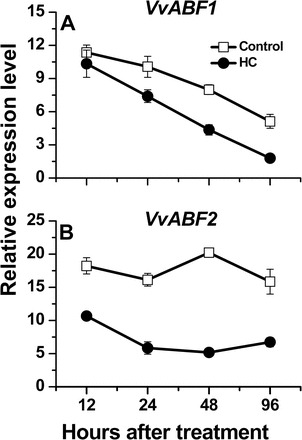
HC modulates expression of the central ABA response mediators VvABF genes. Relative expression levels of VvABF1 (A) and VvABF2 (B) are presented in HC and control buds sampled at 12, 24, 48, and 96h after treatment. All other details are as described in Fig. 3.
Effect of HC treatment on endogenous ABA and ABA catabolite content of grapevine buds
The levels of endogenous ABA and its catabolites neoPA, PA, and DPA were determined in HC-treated and control buds sampled at 48h and 96h after treatment (Fig. 6). Compared with controls, HC treatment resulted in a 35% decrease in endogenous ABA level (Fig. 6A). On the other hand, levels of neoPA were 1.8- and 1.4-fold higher in the HC-treated buds at 48h and 96h, respectively, compared with the control (Fig. 6D). PA and DPA levels were higher in HC-treated buds at 48h (1.72- and 1.2-fold, respectively), but at 96h their levels decreased and were similar to those of the control buds, which presented rather stable levels throughout the analysed period (Fig. 6B, C). It should be noted that levels of PA in the buds were 1000-fold lower than those of neoPA and DPA. Levels of ABA-GE were similar in HC-treated and control buds at the analysed time points (data not shown).
Fig. 6.
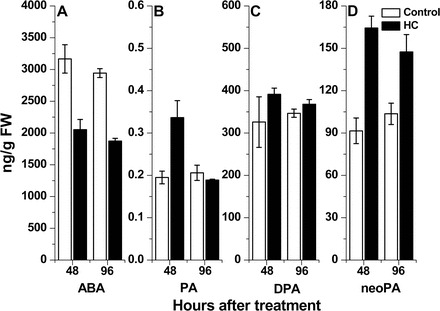
Effect of HC on ABA and ABA catabolite contents in grapevine buds. ABA (A), PA (B), DPA (C), and neoPA (D) levels were determined in HC-treated and control buds sampled 48h and 96h after treatment. The homogenized samples (0.5g) were used for hormone extraction as detailed in the Materials and methods, and 2H-labelled ABA, PA, DPA, and neoPA were spiked as internal standards. Levels of ABA and its catabolites were analysed by liquid chromatography–tandem mass spectrometry. The levels of the analysed molecules were calculated from the peak area ratios of the endogenous molecule to the relevant internal standard. Values represent means ±SE of three biological repeats (10–12 buds per repeat).
Interestingly, exposure of HC-treated buds to the ethylene signalling inhibitor NBD for 48h led to a 1.49-fold increase in ABA level (Fig. 7A), and a 1.1-fold decrease in ABA catabolites (Fig. 7B) compared with HC-treated buds. Transcription profiling revealed that, in accordance with the attenuation in ABA degradation exerted by NBD in HC-treated buds, the treatment also attenuated the HC-induced down-regulation of VvNCED1 (Fig. 7C) and up-regulation of VvA8H-CYP707A4 (Fig. 7D).
Fig. 7.
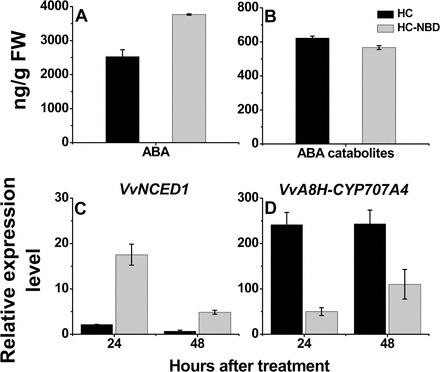
An inhibitor of ethylene signalling attenuates the enhancing effect of HC on ABA down-regulation. Levels of ABA (A) and ABA catabolites (B) were determined as described in Fig. 6 in HC- and NBD–HC-treated buds sampled 48h after treatment. Levels of VvNCED1 (C) and VvA8H-CYP707A4 (D) transcript were determined in the same bud samples as described in Fig. 3.
Profiling of VvNCED1 and VvA8H-CYP707A4 transcript levels during the dormancy cycle
To assess the potential involvement of ABA level and metabolism in the execution of natural dormancy, the dormancy status of buds was assessed from the beginning of November to the beginning of January (Fig. 8). An ~40% decrease in bud-break percentage from the beginning of November to 20 November might pinpoint this as the dormancy induction period. The period of dormancy maintenance, with bud-break percentages of 15–25%, lasts through the last third of November to 18 December, with maximal dormancy depth occurring in the middle of that period (4–11 December). During the last third of December, repression is alleviated, as reflected by the increasing percentage of bud break.
Fig. 8.
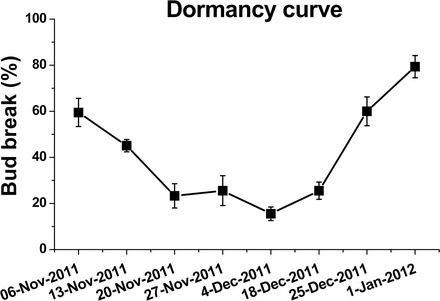
Changes in dormancy status of the bud population throughout the dormancy cycle. Canes from the vineyard under study were sampled weekly during the dormancy cycle. Single-node cuttings were prepared and bud break was monitored as described in Fig. 1. Bud-break percentages at 21 d are presented to describe the seasonal changes in dormancy status of the bud population in the vineyard. Values are averages of nine groups of replications, consisting of 10 buds each ±SE.
Levels of VvNCED1 and VvA8H-CYP707A4 transcripts were monitored in buds sampled throughout this natural dormancy cycle (Fig. 9). The level of VvNCED1 gradually increased and peaked in the last third of November (27 November), when bud-break percentage was ~25%. At the beginning of December (4 December), when the bud population reached its maximal dormancy (15% bud break for the analysed season), the level of VvNCED1 transcript started dropping, reaching its lowest level during maximum dormancy release in January. Concomitantly, the transcript level of VvA8H-CYP707A4, which was constantly low until the end of November, sharply increased and remained high during the period of dormancy release.
Fig. 9.
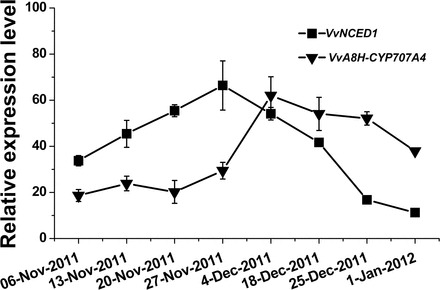
Expression profile of VvNCED1 and VvA8H-CYP707A4 throughout the dormancy cycle. Buds were sampled from canes that were harvested weekly during the dormancy cycle as described in Fig. 8. Levels of VvNCED1 and VvA8H-CYP707A4 transcripts were determined as described in Fig. 3.
Levels of ABA, neoPA, PA, and DPA in grape buds during the dormancy cycle
Levels of ABA and its catabolites were determined in grape buds sampled throughout the natural dormancy cycle, from mid-November to the beginning of January. ABA levels increased ~3-fold from 20 November to 18 December, and then decreased to 60% of maximum in the following 2 weeks (Fig. 10A) in parallel with an increase in bud-break percentage from 25% to 80% (Fig. 8).
Fig. 10.
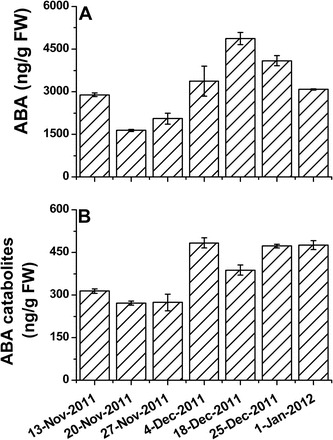
Changes in the contents of ABA and its catabolites throughout the dormancy cycle. The contents of ABA (A) and ABA catabolites (B) were determined as described in Fig. 6 in buds sampled at seven time points throughout the dormancy cycle. Sampling and dormancy status are described in Fig. 8.
The data presented in Fig. 10B suggest that the level of ABA catabolites was significantly increased between 27 November and 4 December, concomitant with the sharp increase in VvA8H-CYP707A4 transcript level (Fig. 9), and remained consistently high until the end of the analysed period.
Differential effect of ABA treatment on bud dormancy release during the natural dormancy cycle
To advance understanding of the potential role of ABA in regulating the dormancy cycle, the effect of exogenous ABA on natural and HC-stimulated dormancy release was analysed independently at several time points during the dormancy cycle, using the single-node cutting experimental system. In parallel with the actual bud-break data (Fig. 11A–G), the differences in bud-break percentage between pairs of relevant treatments are presented in Fig. 11H and I. As expected, the data indicated that compared with controls, HC enhancement of dormancy release increases as dormancy deepens, and its effect decreases toward natural dormancy release (see Fig. 8 for the seasonal dormancy curve, where deepest dormancy in the given season was represented by 15% bud break at 21 d). The data also indicated that compared with controls, the inhibitory effect of exogenous ABA decreases as dormancy deepens, and inhibition is not evident during natural dormancy release.
Fig. 11.
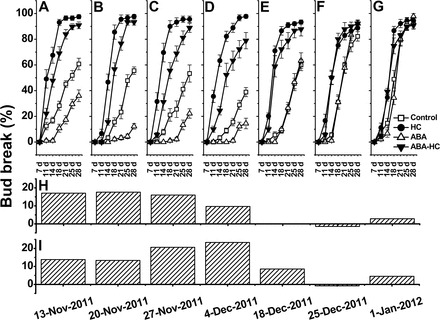
Differential effects of ABA treatment on bud dormancy release during the natural dormancy cycle. Single-node cuttings were prepared from canes that were harvested at seven sampling dates throughout the dormancy cycle as described in Fig. 8. Cuttings were grouped as described in Fig. 1 and used to analyse the effect of ABA on dormancy release of control and HC-treated buds as described in Fig. 2A. In parallel with the actual bud-break data (A–G), calculated values are presented as the difference in bud-break percentages between control and ABA-treated buds (H), and between HC- and ABA–HC-treated buds (I). These values represent the mean of differences for seven monitoring time points (7, 11, 14, 18, 21, 2, and 28 d) for each sampling date.
While exposure to exogenous ABA reduced the advancing effect of HC, the extent of this reduction increased until the onset of maximal dormancy, and then gradually decreased during the phase of dormancy maintenance and the initial stages of dormancy release. During the natural dormancy release phase, exogenous ABA lost its inhibitory effect on dormancy release of HC-treated buds. Earlier, it was seen that HC enables recovery from the inhibitory effect of ABA (Fig. 2A). The data presented in Fig. 11 suggest that the timing of the recovery from ABA repression is delayed as dormancy progresses.
Discussion
Exogenous ABA delays bud dormancy release
The hypothesis that ABA is involved in regulating the maintenance of grape bud dormancy and that HC exerts it enhancing effect, at least in part, by affecting bud ABA level was tested. In agreement with this hypothesis, the presented results suggest that exogenous ABA delays bud break of dormant buds (Fig. 1). The degree of inhibition seems to be dependent on ABA concentration, and on duration of incubation, supporting causal relationships between inhibition and ABA. These results are in agreement with the negative effects of exogenous ABA on seed germination (Rubio et al., 2009; Santiago et al., 2009; Ye et al., 2011), and on bud break in willow (Barros and Neill, 1989), apple (Dutcher and Powell, 1972), pear (Tamura et al., 2002), kiwi (Lionakis and Schwabe, 1984), and sour cherry (Mielke and Dennis, 1978).
Since ABA application to buds that are no longer dormant did not delay emergence of the primordial shoot (Fig. 11F, G), the reported inhibitory effect cannot be considered a non-specific and wide-ranging suppressive effect on bud primordial growth activity. Alternatively, such inhibition may be viewed as a component of a unique and complex mechanism that controls meristem activity during a specific developmental stage of the grapevine life cycle. In agreement with this, the degree of inhibition exerted by exogenous ABA seems to be affected by the dormancy status of the analysed bud population, as shown by ABA’s decreased ability to inhibit bud burst as buds reach deep dormancy, and then progress toward natural dormancy release. This further supports the assumption that the inhibitory effect of ABA is part of a wider regulatory network that operates during the dormancy cycle.
ABA limits the enhancing effect of HC and other dormancy release stimuli
The ability of HC and other artificial stimuli to enhance dormancy release of grape buds has been previously documented (Ophir et al., 2009 and references within) and was confirmed in the current study. The delay exerted by exogenous ABA on the advancing effect of HC, HS, AZ, and hypoxia (Fig. 2) supports the assumption that ABA has a critical role in maintaining grape bud dormancy, and suggests that it inhibits the cascade of biochemical changes activated by the artificial dormancy release stimuli that lead to dormancy release. In support of this, the stimulatory effect of H2O2 on seed dormancy release is negatively affected by exogenous ABA (Sarath et al., 2007). The recovery of ABA–HC- and ABA–HS-treated buds from this inhibitory effect 18 d or more post-treatment, in contrast to the behaviour of ABA-treated buds, suggests that HC- and HS-treated buds recruit the ability to deal with increased levels of ABA, possibly by affecting ABA metabolism and/or sensing.
HC affects ABA metabolism
HC induced a reduction in the transcript levels of VvXERICO and two VvNCED genes, as well as a parallel induction of two VvA8H-CYP707A homologues, suggesting that it exerts at least part of its enhancing effect through modification of ABA metabolism, resulting in a reduction in the total level of ABA. The mode of regulation suggested by the changes recorded in transcript levels is in agreement with the final outcome in metabolite level, as reflected by the decrease in bud endogenous ABA and parallel increase in ABA degradation products in HC-treated buds (Fig. 6). Taken together, these results support the hypothesis that HC treatment leads to a decrease in endogenous ABA level by promoting ABA degradation, inhibiting ABA synthesis, or both. This hypothesis is supported by the effects of dormancy release stimuli on the ABA degradation machinery in other systems. The stimulation of arabidopsis seed dormancy release by H2O2, nitrate, and nitric oxide (NO) is mediated largely by ABA8′OH, which catalyses the degradation of endogenous ABA (Liu and Zhang, 2009; Matakiadis et al., 2009; Liu et al., 2010). Bromoethane, which induces sprouting of dormant potato buds, led to a significant decrease in meristem ABA content and increase in ABA catabolism, which occurred predominantly via oxidation catalysed by ABA8′OH. The increased level of StCYP707A transcript in the meristem in response to bromoethane is consistent with the latter’s effect on ABA level (Destefano-Beltrán et al., 2006a ). Changes in ABA content in whole potato tubers were also recorded following enhancement of dormancy release by synthetic cytokinin or heat stress (Ji and Wang, 1988; van Den Berd et al., 1991). In agreement with this, increased exposure to controlled chilling, a natural stimulus of dormancy release, led to a decrease in ABA levels in pear vegetative buds (Tamura et al., 2002).
The inhibitory effect of ABA is sensitive to seasonal changes
As the season progressed, a decrease was recorded in the degree of inhibition exerted by exogenous ABA on dormancy release of both control and HC-treated buds (Fig. 11). The results are supported by the periodicity of the response to ABA in lateral buds of willow (Barros and Neill, 1989). Additional support stems from the inhibitory effect of ABA on bud break of dormant pear buds exposed to 200–500 chilling hours, and its inability to affect bud burst of similar buds that present shallow dormancy after exposure to 800–1000 chilling hours (Tamura et al., 2002). This decreased response, which supports the assumption that ABA’s effect is dependent on developmental stage, may be explained by each of the following scenarios, or some combination of them: (i) an increase in total ABA level beyond that required for maximal repression, as a result of an increased endogenous ABA level; (ii) an increase in ABA degradation capacity which facilitates more efficient removal of the added exogenous ABA; and (iii) developmental phase transition, which leads to the establishment of a new regulatory network, where ABA is no longer a regulator of primordial growth activity. Transition from one scenario to another is expected and assumed to be possible during the dormancy cycle. A gradual increase in VvNCED1 transcript and endogenous ABA levels up to a maximum at the stage of dormancy maintenance (Figs 8, 9) support the first scenario. The correlation between the degree of inhibition by exogenous ABA (reflected by ΔCon–ABA, presented in Fig. 11H) and the endogenous ABA levels also suggests that the effect of exogenous ABA decreases with a rise in endogenous ABA. A sharp increase in the levels of VvA8H-CYP707A4 transcript and ABA degradation products in the heart of the dormancy maintenance period supports the second scenario. The complete inability of ABA, as well as HC, to affect bud burst toward the phase of natural dormancy release (Fig. 11G) may favour the third scenario. The parallel decrease in the level of VvNCED1 transcript and increase in the levels of both VvA8H-CYP707A4 transcript and ABA degradation products from 27 November to 4 December may serve as an initial indication of the existence of a defined developmental window in the bud dormancy cycle when ABA can play a regulatory role in dormancy maintenance. In line with this, it is suggested that the maximal difference between HC and ABA–HC treatments (ΔHC–ABAHC, Fig. 11I) reflects both the deepest natural dormancy and maximal enhancing effect of HC, before the buds become sensitive and HC damage masks potential bud-break ability.
Unfavourable light or temperature conditions have been shown to prevent germination by co-ordinated regulation of NCED and CYP707A gene expression in several species (Seo et al., 2006; Gubler et al., 2008; Toh et al., 2008; Leymarie et al., 2009; Argyris et al., 2011). Interestingly, a wider group of key Arabidopsis genes, which are involved in the regulation of ABA metabolism and signalling, has recently been shown to be highly sensitive to slow seasonal changes, and regulation of their expression in seeds in response to soil temperature results in continual and dramatic adjustments to the dormancy depth within the soil seed bank. Among these are NCED6, SnrK2.1, SnrK2.4, and ABI3, which are up-regulated when temperatures are low and lead to deep dormancy. The transition to shallow dormancy is linked to ABA catabolism and repression of ABA signalling, as evidenced by the increased expression of CYP707A2 and ABI2 in response to high soil temperature and dormancy release (Footitt et al., 2011). Co-ordinated regulation of the levels of both ABA and ABA metabolism regulators during the dormancy cycle has also been shown in a few bud studies. ABA levels in apical poplar buds increased significantly after 3–4 weeks of short days, which induce dormancy (Rohde et al., 2002), in parallel with significant up-regulation of genes encoding NCED and other enzymes catalysing ABA biosynthesis (Ruttink et al., 2007). Significant seasonal changes in ABA content, which negatively correlated with bud-burst ability, were also recorded in the apical buds of silver birch (Rinne et al., 1994b ). In potato tuber meristems, ABA content rose significantly as the natural dormancy cycle progressed, and then decreased steadily. These changes were positively correlated with changes in the expression of StNCED2, whereas expression of StCYP707A1 was up-regulated when the ABA level started to decrease (Destefano-Beltrán et al., 2006b ).
It should be noted that the changes in ABA level lagged somewhat behind the changes in VvNCED1 and VvA8H-CYP707A4 transcript levels, as well as the levels of ABA catabolites. The technical failure to determine the levels of ABA and its degradation products on 11 December may have prevented the detection of a potentially higher and earlier ABA peak. Another option is that VvNCED1 protein level or activity is not completely mirrored by the level of its transcript, allowing an extended period of ABA synthesis, and temporarily masking the effect of increased degradation ability. Along the same lines, changes in ABA levels in potato tuber meristems were reported to lag behind increased expression of StCYP707A, and it was speculated that ABA8′OH activity might also be regulated post-transcriptionally (Destefano-Beltrán et al., 2006a ). It is clear, however, that ABA quantity rises to a maximal level at the stage of dormancy maintenance (from 20 November to 18 December) and gradually decreases in parallel with increasing natural bud-break ability, in agreement with the initial hypothesis. Based on the data, it can be speculated that upon induction of dormancy by as yet unidentified environmentally regulated factors, preparation for ABA production starts at the level of transcription and actual accumulation of ABA starts later, serving as a master regulator of dormancy maintenance. Future production of NCED antibodies and/or analysis of NCED activity will enable this assumption to be tested.
Despite the delayed bud break with the combined treatments of ABA–HC and ABA–HS compared with HC, bud break occurred at higher levels compared with controls at all time points. This behaviour is in agreement with the suggested amplification of ABA degradation ability by HC and HS treatments, which may allow the buds to process higher levels of ABA (both endogenous and exogenous) with better efficiency than control buds. It is speculated that by increasing the level of exogenous ABA beyond the processing ability of the HC-treated buds, its inhibitory effect will be intensified. Experiments with ABA concentrations >100 μM were not conducted, but delayed recovery following ABA–HC treatment with extended incubation in ABA (6 d) supports this assumption (data not shown).
It is interesting to note that the inhibitory effect of ABA on enhancement of dormancy release by AZ and hypoxia was greater than that recorded for HC or HS, as reflected by (i) the inability of ABA–AZ- and ABA–hypoxia-treated buds to present bud-break levels that are higher than that of the control; and (ii) the absence of recovery of the AZ- and hypoxia-treated buds to comparable levels, which was evident in the ABA–HC and ABA–HS treatments. In the case of AZ, this may stem from a degree of phytotoxicity resulting from vigorous stress. This scenario agrees with the limited enhancement of dormancy release by 2% AZ (Fig. 2C), versus the better enhancement recorded at lower concentrations (E. Or et al., unpublished results). It is speculated that such harsh stress may increase ABA levels (as reflected by an increased VvNCED1 transcript level instead of the expected decrease), and thus delay removal of inhibition. Currently, there are no data that coincide with the potentially lower ability of the hypoxia treatment to enhance ABA degradation relative to HC and HS.
Potential involvement of ABA signalling components in the regulation of dormancy release
Although the reported results strongly support regulation at the level of ABA metabolism, potential changes in ABA signalling are also possible. Thus, several candidates from the gene families of central players in the ABA signalling machinery were selected for transcript profiling. The selection was based on (i) previous studies suggesting that ABA receptor, PP2C, and ABRE genes are regulated at the transcriptional level (Kuhn et al., 2006; Santiago et al., 2009; Raghavendra et al., 2010; Szostkiewicz et al., 2010; Footitt et al., 2011; Yoshida et al., 2014); (ii) previous identification of family members that are regulated by ABA and present potential protein–protein interactions with their targets in the ABA signalling cascade in grapevine (Boneh et al., 2012a, b ); and (iii) validation of the expression of the selected candidates in grapevine buds. It has been shown that under conditions that increase ABA levels, the transcript levels of the ABA receptors are down-regulated and the level of PP2C transcript is increased due to feedback regulation (Raghavendra et al., 2010; Szostkiewicz et al., 2010). The HC-induced reduction of VvNCED1 transcript on the one hand, and increase in level of VvA8H-CYP707A4 transcript, DPA, and neoPA on the other, were linked to the decrease in ABA level. In light of this, induction of all of the receptors except VvRCAR1, and down-regulation of VvPP2C4 and VvPP2C9 by HC was expected. These changes might reflect a feedback response to a decreased ABA level, and serve as additional validation for ABA-related changes in response to HC. Alternatively, they may be regulated by an as yet unknown master regulator of dormancy status and play a primary role in modifying the cell’s sensitivity to ABA. Notably, while changes in ABA levels were in line with dormancy depth, these changes could not fully explain the seasonal dormancy cycling in Arabidopsis seeds, and it was suggested that factors that regulate ABA signalling and sensitivity, such as DOG1 and MFT, must play important roles in seasonal cycling (Footitt et al., 2011). In terminal buds of poplar, ABA biosynthesis and part of the signal transduction pathway are activated concomitantly with the transition of the apex to a closed bud structure, before termination of meristematic activity (Ruttink et al., 2007). Interestingly, the suggestion was raised that ABA signalling might be involved in the regulation of poplar bud sensitivity to the sugar signals that regulate the dormancy cycle (Rohde et al., 2002). The experimental data presented in the current work may serve as an initial indication for possible involvement of ABA signalling in the regulation of grape bud dormancy. However, it should be clearly stated that further research is required to support this assumption fully.
Removal of ABA occurs downstream of the development of respiratory stress and ethylene signals
The fact that ABA inhibited dormancy release of buds subjected to anaerobic conditions is in agreement with the working model, which suggests that removal of ABA is downstream of the development of respiratory stress. Levels of ABA were not measured in buds subjected to anaerobic conditions, but the increased level of A8H-CYP707A4 transcript in these buds compared with controls (data not shown) further supports the model. Based on the present model, it is also assumed that ethylene signalling is required to induce ABA degradation. The higher level of ABA and lower levels of ABA catabolites in HC–NBD-treated buds compared with HC-treated buds, coupled with the fact that dormancy release is inhibited by NBD (Ophir et al., 2009), support this assumption. An antagonistic interaction between ethylene and ABA during seed germination has been shown in various species and was recently reviewed by Arc et al. (2013). In agreement with the present findings, seeds of ethylene-insensitive Arabidopsis mutants etr1 and ein2 exhibit a higher ABA content than the wild type and slower germination (Beaudoin et al., 2000; Ghassemian et al., 2000; Chiwocha et al., 2005; Wang et al., 2007). Moreover, NCED3 up-regulation and CYP707A2 down-regulation were recorded in ein2 and etr1-1 mutants (Cheng et al., 2009). Unlike the suggested antagonistic effect during dormancy release, a synergistic effect was suggested during preparation for bud dormancy, based on data reported for birch. Transgenic ethylene-insensitive birch trees exposed to short days did not accumulate ABA in apical buds, and formation of terminal buds was abolished as well, in contrast to the typical behaviour of birch exposed to such conditions (Ruonala et al., 2006).
Variability in the response of gene family members to dormancy release stimuli
The present results suggested that only some of the genes in the gene families under study are regulated in the buds during the dormancy cycle, and in response to dormancy release stimuli. Similar scenarios have been described previously in seeds and buds. In barley, transcript levels of HvNCED1, but not HvNCED2, vary during grain development, and modulate ABA accumulation at late maturation stages and in response to changes in environmental conditions (Chono et al., 2006). In Arabidopsis, NCED6, NCED9, and CYP707A2 seem to be major players in the regulation of seed dormancy, and opposite profiles were recorded for the receptors PYR1 and PYL7 (Lefebvre et al., 2006; Footitt et al., 2011; Frey et al., 2012). In potato tuber meristems, changes in ABA content during progression of the natural dormancy cycle and in response to bromoethane closely mirrored the expression of StNCED2, but not that of StNCED1. Similarly, decreases in ABA content correlated mainly with StCYP707A2, one of three members of the ABA 8′-hydroxylase gene family (Destefano-Beltrán et al., 2006a , b).
Final remarks
To summarize, the following scenario is suggested: at early stages of the dormancy cycle, endogenous ABA levels are below the threshold needed to inhibit bud break, and thus a supply of exogenous ABA may have a significant additive effect on the dormancy level. Later, the level of endogenous ABA rises above that threshold, and therefore addition of exogenous ABA gradually loses it additive inhibitory effect. Once ABA degradation abilities are acquired (and levels of synthesis decrease), both endogenous and exogenous ABA are efficiently metabolized, promoting similar dormancy release in both ABA-treated and control buds. In the presence of HC, the degree of recovery from exogenous ABA inhibition, which is facilitated by HC-induced ABA degradation, depends on the endogenous ABA metabolism. Recovery slows down when endogenous ABA levels rise, due to the need for the limited ABA degradation capacity, induced by HC, to handle higher levels of ABA (from combined endogenous and exogenous sources). Later, when endogenous ABA synthesis decreases and ABA degradation naturally increases, the ability to recover is improved, until it becomes irrelevant due to regeneration of full bud-break capacity.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Reprograming during artificially induced dormancy release: model of current working hypothesis.
Figure S2. Identification of VvXERICO.
Figure S3. Transcription modulation of additional bud-expressed VvNCED and VvA8H-CYP707A genes by hydrogen cyanamide (HC).
Figure S4. Transcription modulation of additional bud-expressed VvRCAR genes by hydrogen cyanamide (HC).
Table S1. Primers used for gene expression analyses by qRT-PCR.
Table S2. Parameters for LC-ESI-MS/MS analysis.
References
- Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A. 2013. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Frontiers in Plant Science 4, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyris J, Truco MJ, Ochoa O, McHale L, Dahal P, Van Deynze A, Michelmore RW, Bradford KJ. 2011. A gene encoding an abscisic acid biosynthetic enzyme (LsNCED4) collocates with the high temperature germination locus Htg6. 1 in lettuce (Lactuca sp.). Theoretical and Applied Genetics 122, 95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Rowland LJ, Tanino K. 2003. Induction and release of bud dormancy in woody perennials: a science comes of age. HortScience 38, 911–921. [Google Scholar]
- Barros RS, Neill SJ. 1989. Status of abscisic acid in willow as related to the induction of bud dormancy. Acta Physiologiae Plantarum 11, 117–123. [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. 2000. Interactions between abscisic acid and ethylene signaling cascades. The Plant Cell 12, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Bou J, Peeters AJM, Wagemaker N, Guhl K, Ward D, Hedden P, Moritz T, Voesenek LACJ. 2006. Long-term submergence-induced elongation in Rumex palustris requires abscisic acid-dependent biosynthesis of gibberellin1. Plant Physiology 141, 1644–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemelt S, Hajirezaei M, Hentschel E, Sonnewald U. 2000. Comparative analysis of abscisic acid content and starch degradation during storage of tubers harvested from different potato varieties. Potato Research 43, 371–382. [Google Scholar]
- Boneh U, Biton I, Schwartz A, Ben-Ari G. 2012a. Characterization of the ABA signal transduction pathway in Vitis vinifera . Plant Science 187, 89–96. [DOI] [PubMed] [Google Scholar]
- Boneh U, Biton I, Zheng C, Schwartz A, Ben-Ari G. 2012b. Characterization of potential ABA receptors in Vitis vinifera . Plant Cell Reports 31, 311–321. [DOI] [PubMed] [Google Scholar]
- Cadman CSC, Toorop PE, Hilhorst HWM, Finch-Savage WE. 2006. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. The Plant Journal 46, 805–822. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Chiang MH, Hwang SG, Lin PC. 2009. Antagonism between abscisic acid and ethylene in Arabidopsis acts in parallel with the reciprocal regulation of their metabolism and signaling pathways. Plant Molecular Biology 71, 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, Kermode AR. 2005. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. The Plant Journal 42, 35–48. [DOI] [PubMed] [Google Scholar]
- Chono M, Honda I, Shinoda S, Kushiro T, Kamiya Y, Nambara E, Kawakami N, Kaneko S, Watanabe Y. 2006. Field studies on the regulation of abscisic acid content and germinability during grain development of barley: molecular and chemical analysis of pre-harvest sprouting. Journal of Experimental Botany 57, 2421–2434. [DOI] [PubMed] [Google Scholar]
- Crabbe JJ. 1994. Dormancy. In: Arntzen CJ, Ritter EM, eds. Encyclopedia of agricultural science , Vol. 1. New York: Academic Press, 597–611. [Google Scholar]
- Cutler AJ. 2007. Abscisic acid (ABA). Encyclopedia of life sciences 10.1002/9780470015902.a9780470020088. [Google Scholar]
- Cutler AJ, Krochko JE. 1999. Formation and breakdown of ABA. Trends in Plant Science 4, 472–478. [DOI] [PubMed] [Google Scholar]
- Debast S, Nunes-Nesi A, Hajirezaei MR, Hofmann J, Sonnewald U, Fernie AR, Börnke F. 2011. Altering trehalose-6-phosphate content in transgenic potato tubers affects tuber growth and alters responsiveness to hormones during sprouting. Plant Physiology 156, 1754–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destefano-Beltrán L, Knauber D, Huckle L, Suttle J. 2006a. Chemically forced dormancy termination mimics natural dormancy progression in potato tuber meristems by reducing ABA content and modifying expression of genes involved in regulating ABA synthesis and metabolism. Journal of Experimental Botany 57, 2879–2886. [DOI] [PubMed] [Google Scholar]
- Destefano-Beltrán L, Knauber D, Huckle L, Suttle J. 2006b. Effects of postharvest storage and dormancy status on ABA content, metabolism, and expression of genes involved in ABA biosynthesis and metabolism in potato tuber tissues. Plant Molecular Biology 61, 687–697. [DOI] [PubMed] [Google Scholar]
- Düring H, Bachmann O. 1975. Abscisic acid analysis in Vitis vinifera in the period of endogenous bud dormancy by high pressure liquid chromatography. Physiologia Plantarum 34, 201–203. [Google Scholar]
- Dutcher RD, Powell LE. 1972. Culture of apple shoots from buds in vitro . Journal of the American Society for Horticultural Science 97, 511–514. [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. The Plant Cell 14, S15–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE. 2011. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proceedings of the National Academy of Sciences, USA 108, 20236–20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A. 2012. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. The Plant Journal 70, 501–512. [DOI] [PubMed] [Google Scholar]
- Frey A, Godin B, Bonnet M, Sotta B, Marion-Poll A. 2004. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia . Planta 218, 958–964. [DOI] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. 2009. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proceedings of the National Academy of Sciences, USA 106, 8380–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. 2000. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. The Plant Cell 12, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernandez MA, Holdsworth MJ, Perez-Amador MA, Kollist H. 2012. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. The Plant Cell 24, 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Hughes T, Waterhouse P, Jacobsen J. 2008. Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiology 147, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaly T, Pang X, Batikoff T, Crane O, Keren A, Venkateswari J, Ogrodovitch A, Sadka A, Lavee S, Or E. 2008. Similar mechanisms might be triggered by alternative external stimuli that induce dormancy release in grape buds. Planta 228, 79–88. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H. 2009. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460, 1026–1030. [DOI] [PubMed] [Google Scholar]
- Hellman E, Shelby S, Lowery C. 2006. Exogenously applied abscisic acid did not consistently delay budburst of deacclimating grapevines. Journal of the American Pomological Society 60, 178–186. [Google Scholar]
- Himmelbach A, Yang Y, Grill E. 2003. Relay and control of abscisic acid signaling. Current Opinion in Plant Biology 6, 470–479. [DOI] [PubMed] [Google Scholar]
- Horvath DP, Anderson JV, Soto-Suárez M, Chao WS. 2006. Transcriptome analysis of leafy spurge (Euphorbia esula) crown buds during shifts in well-defined phases of dormancy. Weed Science 54, 821–827. [Google Scholar]
- Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. 2010. Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes and Development 24, 1695–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. 2001. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis . The Plant Journal 27, 325–333. [DOI] [PubMed] [Google Scholar]
- Ji ZL, Wang SY. 1988. Reduction of abscisic acid content and induction of sprouting in potato, Solanum tuberosum L., by thidiazuron. Journal of Plant Growth Regulation 7, 37–44. [Google Scholar]
- Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M. 1983. Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157, 158–165. [DOI] [PubMed] [Google Scholar]
- Keilin T, Pang X, Venkateswari J, et al. 2007. Digital expression profiling of a grape-bud EST collection leads to new insight into molecular events during grape-bud dormancy release. Plant Science 173, 446–457. [Google Scholar]
- Ko JH, Yang SH, Han KH. 2006. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. The Plant Journal 47, 343–355. [DOI] [PubMed] [Google Scholar]
- Korableva NP, Karavaeva KA, Metlitskii LV. 1980. Changes of abscisic acid content in potato tuber tissues in the period of deep dormancy and during germination. Fiziologiya Rastenii 27, 441–446. [Google Scholar]
- Koussa T, Broquedis M, Bouard J. 1994. Changes of abscisic acid level during the development of grapevine latent buds, particularly in the phase of dormancy break. Vitis 33, 63–67. [Google Scholar]
- Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI. 2006. The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiology 140, 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA. 1987. Dormancy: a new universal terminology. HortScience 22, 817–820. [Google Scholar]
- Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P. 2007. Transcript profiling of the anoxic rice coleoptile. Plant Physiology 144, 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavee S, May P. 1997. Dormancy of grapevine buds—facts and speculation. Australian Journal of Grape and Wine Research 3, 31–46. [Google Scholar]
- Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion Poll A. 2006. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. The Plant Journal 45, 309–319. [DOI] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JAD, Koornneef M. 1996. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. The Plant Journal 10, 655–661. [DOI] [PubMed] [Google Scholar]
- Leymarie J, Benech-Arnold RL, Farrant JM, Corbineau F. 2009. Thermodormancy and ABA metabolism in barley grains. Plant Signaling and Behavior 4, 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Junttila O, Heino P, Palva ET. 2003. Different responses of northern and southern ecotypes of Betula pendula to exogenous ABA application. Tree Physiology 23, 481–487. [DOI] [PubMed] [Google Scholar]
- Li C, Junttila O, Heino P, Palva ET. 2004. Low temperature sensing in silver birch (Betula pendula Roth) ecotypes. Plant Science 167, 165–171. [Google Scholar]
- Linkies A, Müller K, Morris K, Turečková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE. 2009. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana . The Plant Cell 21, 3803–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionakis SM, Schwabe WW. 1984. Bud dormancy in the kiwi fruit, Actinidia chinensis Planch. Annals of Botany 54, 467–484. [Google Scholar]
- Liu Y, Ye N, Liu R, Chen M, Zhang J. 2010. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. Journal of Experimental Botany 61, 2979–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang J. 2009. Rapid accumulation of NO regulates ABA catabolism and seed dormancy during imbibition in Arabidopsis. Plant Signaling and Behavior 4, 905–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Andújar C, Ordiz MI, Huang Z, Nonogaki M, Beachy RN, Nonogaki H. 2011. Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proceedings of the National Academy of Sciences, USA 108, 17225–17229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakiadis T, Alboresi A, Jikumaru Y, Tatematsu K, Pichon O, Renou JP, Kamiya Y, Nambara E, Truong HN. 2009. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiology 149, 949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke EA, Dennis FG. 1978. Hormonal control of flower bud dormancy in sour cherry (Prunus cerasus L.). III. Effects of leaves, defoliation and temperature on levels of abscisic acid in flower primordia. Journal of the American Society for Horticultural Science 103, 446–449. [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, Katagiri T, Umezawa T, Kidokoro S, Maruyama K, Yoshida T, Ishiyama K, Kobayashi M. 2009. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2. 2, SRK2E/SnRK2. 6/OST1 and SRK2I/SnRK2. 3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant and Cell Physiology 50, 1345–1363. [DOI] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. 2005. Abscisic acid biosynthesis and catabolism. Annual Review of Plant Biology 56, 165–185. [DOI] [PubMed] [Google Scholar]
- Nambara E, Okamoto M, Tatematsu K, Yano R, Seo M, Kamiya Y. 2010. Abscisic acid and the control of seed dormancy and germination. Seed Science Research 20, 55–67. [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. 2006. CYP707A1 and CYP707A2, which encode abscisic acid 8’-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiology 141, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir R, Pang X, Halaly T, Venkateswari J, Lavee S, Galbraith D, Or E. 2009. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene–ABA interplay and cell enlargement. Plant Molecular Biology 71, 403–423. [DOI] [PubMed] [Google Scholar]
- Or E. 2009. Grape bud dormancy release—the molecular aspect. In: Roubelakis-Angelakis KA, ed. Grapevine molecular physiology & biotechnology. Berlin: Springer, 1–29. [Google Scholar]
- Or E, Belausov E, Popilevsky I, Tal YB. 2000b. Changes in endogenous ABA level in relation to the dormancy cycle in grapevines grown in a hot climate. Journal of Horticultural Science and Biotechnology 75, 190–194. [Google Scholar]
- Or E, Viloznyi I. 1999. Timing of hydrogen cyanamide application to grapevine buds. Vitis 38, 1–6. [Google Scholar]
- Or E, Vilozny I, Eyal Y, Ogrodovitch A. 2000a. The transduction of the signal for grape bud dormancy breaking induced by hydrogen cyanamide may involve the SNF-like protein kinase GDBRPK. Plant Molecular Biology 43, 483–494. [DOI] [PubMed] [Google Scholar]
- Pang X, Halaly T, Crane O, Keilin T, Keren-Keiserman A, Ogrodovitch A, Galbraith D, Or E. 2007. Involvement of calcium signalling in dormancy release of grape buds. Journal of Experimental Botany 58, 3249–3262. [DOI] [PubMed] [Google Scholar]
- Pérez FJ, Lira W. 2005. Possible role of catalase in post-dormancy bud break in grapevines. Journal of Plant Physiology 162, 301–308. [DOI] [PubMed] [Google Scholar]
- Pérez FJ, Vergara R, Or E. 2009. On the mechanism of dormancy release in grapevine buds: a comparative study between hydrogen cyanamide and sodium azide. Plant Growth Regulation 59, 145–152. [Google Scholar]
- Pérez FJ, Vergara R, Rubio S. 2008. H2O2 is involved in the dormancy-breaking effect of hydrogen cyanamide in grapevine buds. Plant Growth Regulation 55, 149–155. [Google Scholar]
- Powell LE. 1987. The hormonal control of bud and seed dormancy in woody plants. In: Davies PJ, ed. Plant hormones and their role in plant growth and development. Berlin: Springer, 539–552. [Google Scholar]
- Qin X, Zeevaart JAD. 2002. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiology 128, 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. 2010. ABA perception and signalling. Trends in Plant Science 15, 395–401. [DOI] [PubMed] [Google Scholar]
- Reid KE, Olsson N, Schlosser J, Peng F, Lund ST. 2006. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biology 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne P, Saarelainen A, Junttila O. 1994a. Seasonal changes in bud dormancy in relation to ABA level in seedling and coppice shoots of Betula pubescens as affected by short photoperiod, water stress and chilling. Physiologia Plantarum 90, 451–458. [Google Scholar]
- Rinne P, Tuominen H, Junttila O. 1994b. Seasonal changes in bud dormancy in relation to bud morphology, water and starch content, and abscisic acid concentration in adult trees of Betula pubescens . Tree Physiology 14, 549–561. [DOI] [PubMed] [Google Scholar]
- Rohde A, Bhalerao RP. 2007. Plant dormancy in the perennial context. Trends in Plant Science 12, 217–223. [DOI] [PubMed] [Google Scholar]
- Rohde A, Prinsen E, De Rycke R, Engler G, Van Montagu M, Boerjan W. 2002. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. The Plant Cell 14, 1885–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim TH, Santiago J, Flexas J, Schroeder JI, Rodriguez PL. 2009. Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiology 150, 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruonala R, Rinne PLH, Baghour M, Moritz T, Tuominen H, Kangasjärvi J. 2006. Transitions in the functioning of the shoot apical meristem in birch (Betula pendula) involve ethylene. The Plant Journal 46, 628–640. [DOI] [PubMed] [Google Scholar]
- Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A. 2007. A molecular timetable for apical bud formation and dormancy induction in poplar. The Plant Cell 19, 2370–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park SY, Marquez JA, Cutler SR, Rodriguez PL. 2009. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. The Plant Journal 60, 575–588. [DOI] [PubMed] [Google Scholar]
- Sarath G, Hou G, Baird LM, Mitchell RB. 2007. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta 226, 697–708. [DOI] [PubMed] [Google Scholar]
- Saure MC. 1985. Dormancy release in deciduous fruit trees. Horticultural Reviews 7, 239–300. [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion Poll A, Sun T, Koshiba T. 2006. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. The Plant Journal 48, 354–366. [DOI] [PubMed] [Google Scholar]
- Seo M, Jikumaru Y, Kamiya Y. 2011. Profiling of hormones and related metabolites in seed dormancy and germination studies. Methods in Molecular Biology 773, 99–111. [DOI] [PubMed] [Google Scholar]
- Shulman Y, Nir G, Fanberstein L, Lavee S. 1983. The effect of cyanamide on the release from dormancy of grapevine buds. Scientia Horticulturae 19, 97–104. [Google Scholar]
- Sisler EC, Serek M. 2003. Compounds interacting with the ethylene receptor in plants. Plant Biology 5, 473–480. [Google Scholar]
- Steffens B, Wang J, Sauter M. 2006. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223, 604–612. [DOI] [PubMed] [Google Scholar]
- Suttle JC, Abrams SR, De Stefano-Beltrán L, Huckle LL. 2012. Chemical inhibition of potato ABA-8’-hydroxylase activity alters in vitro and in vivo ABA metabolism and endogenous ABA levels but does not affect potato microtuber dormancy duration. Journal of Experimental Botany 63, 5717–5725. [DOI] [PubMed] [Google Scholar]
- Suttle JC, Hultstrand JF. 1994. Role of endogenous abscisic acid in potato microtuber dormancy. Plant Physiology 105, 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostkiewicz I, Richter K, Kepka M, Demmel S, Ma Y, Korte A, Assaad FF, Christmann A, Grill E. 2010. Closely related receptor complexes differ in their ABA selectivity and sensitivity. The Plant Journal 61, 25–35. [DOI] [PubMed] [Google Scholar]
- Tamura F, Tanabe K, Itai A. 2002. Regulation of endodormancy in Japanese pear. Acta Horticulturae 2, 325–336. [Google Scholar]
- Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N. 2008. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology 146, 1368–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Den Berd JH, Vreugdenhil D, Ludford PM, Hillman LL, Ewing EE. 1991. Changes in starch, sugar, and abscisic acid contents associated with second growth in tubers of potato (Solanum tuberosum L.) one-leaf cuttings. Journal of Plant Physiology 139, 86–89. [Google Scholar]
- Vergara R, Parada F, Rubio S, Pérez FJ. 2012. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. Journal of Experimental Botany 63, 4123–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu C, Li K, Sun F, Hu H, Li X, Zhao Y, Han C, Zhang W, Duan Y. 2007. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Molecular Biology 64, 633–644. [DOI] [PubMed] [Google Scholar]
- Ye N, Zhu G, Liu Y, Zhang A, Li Y, Liu R, Shi L, Jia L, Zhang J. 2011. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. Journal of Experimental Botany 63, 1809–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Maruyama K, Mogami J, Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. 2015. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant, Cell and Environment, 38, 35–49 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PR, Lashbrooke JG, Alexandersson E, Jacobson D, Moser C, Velasco R, Vivier MA. 2012. The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genomics 13, 243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


