Abstract
Marchigian-Sardinian alcohol-preferring (msP) rats exhibit innate preference for alcohol along with anxious phenotype. In these animals, two single nucleotide polymorphisms in position −1836 and −2097 from the first start codon of the CRF1-R transcript have been found. Here we examined whether these point mutations account for the heightened anxiety-like behavior and stress responsiveness of msP rats. We re-derived the msP rats to obtain two distinct lines carrying the wild type (GG) and point mutations (AA), respectively. CRF1-R gene expression analysis revealed significant dysregulation of the system in the extended amygdala of AA rats. At the behavioral level, using the elevated plus maze, we found that both AA and GG lines had higher basal anxiety compared to Wistar rats. In the defensive burying test, AA rats showed decreased burying behavior compared to the GG and the unselected Wistar lines. Freezing/immobility did not differ among AA and GG but was higher than that of Wistars. The selective CRF1-R antagonist antalarmin (0, 10, 20 mg/kg) reduced burying behavior in Wistar animals. However, antalarmin (10 mg/kg) tended to increase rather than reducing this behavior when tested in the msP lines, an effect that appeared more marked in the GG as compared to the AA line. The present data suggest that rats with msP genetic background are more anxious and show different sensitivity to stress and CRF1-R blockade than Wistars. The point mutations occurring in the CRF1-R gene do not seem to influence basal anxiety while they appear to affect active responses to stress.
Keywords: CRF, SNP, anxiety, msP, defensive burying, restraint, alcoholism, abuse
INTRODUCTION
Dysregulation of the brain corticotropin releasing factor (CRF) system appears to be one of the major elements common to depression, anxiety and alcohol addiction (Markou et al. 1998; Muller and Wurst 2004; Nemeroff and Vale 2005). CRF is a 41 amino acid peptide that integrates many of the endocrine, behavioral, and autonomic responses to stress (Sarnyai et al. 2001; Vale et al. 1981). Its arousing and anxiogenic-like properties have been attributed to an enhanced CRF1 receptor (CRF1-R) activation at extrahypothalamic sites (Heinrichs et al. 1997; Muller and Wurst 2004; Skutella et al. 1998; Zorrilla and Koob 2004) whereas there is no consistency in reports on the role of CRF2-Rs in anxiety (Zhao et al. 2007). Preclinical studies using conventional and conditional knockout strategies showed decreased anxiety-like behavior in CRF1-R deficient mice, an effect which was independent of hypothalamic-pituitary-adrenocortical system function (Muller et al. 2003; Smith et al. 1998; Timpl et al. 1998). In agreement with this, pharmacological blockade at CRF1-R produced anxiolytic-like effects in animal models of anxiety including reduced acoustic startle responses (Schulz et al. 1996), conditioned fear (Hikichi et al. 2000), and defensive burying (DB) behavior (Richardson et al. 2008; Zhao et al. 2007).
Increased CRF-like immunoreactivity in the extended amygdala during alcohol withdrawal (Merlo Pich et al. 1995; Olive et al. 2002; Roberto et al. 2010; Zorrilla et al. 2001), and long-term upregulation of CRF1-Rs following alcohol dependence induction have also been documented (Sommer et al. 2008). In addition, hyperactivation of the CRF1-R system has been linked to excessive alcohol drinking and vulnerability to relapse. For instance, it has been documented that selective CRF1-R antagonists are highly efficacious in reducing alcohol self-administration and stress-induced relapse to alcohol seeking in post-dependent rats (Ciccocioppo et al. 2009; Funk et al. 2006; Gehlert et al. 2007; Hansson et al. 2006). These agents also attenuated alcohol “hangover”- and withdrawal-induced anxiety-like behavior in rats (Gehlert et al. 2007; Overstreet et al. 2004). Altogether, these findings corroborate the notion that a prolonged history of alcohol exposure leads to enhanced CRF/CRF1-R system activity that, in turn, sustains uncontrolled alcohol consumption motivated by the attempt to relief of negative emotional state such as anxiety and depression (Breese et al. 2011; Heilig and Koob 2007; Koob 2010).
Genetically selected Marchigian Sardinian alcohol preferring (msP) rats have been proposed as a phenocopy of animals in a post-dependent state (Ciccocioppo 2013; Ciccocioppo et al. 2006). They have an innate high preference for alcohol, show excessive alcohol drinking [6–8 g/kg body weight per day (Ciccocioppo et al. 2006)], are highly sensitive to stress and stress-induced alcohol seeking (Ciccocioppo 2013; Ciccocioppo et al. 2006), have depressive-like symptoms that recover following alcohol consumption (Ciccocioppo et al. 1999) and demonstrate an anxious-like phenotype (Hansson et al. 2006). In previous studies, we demonstrated that msP rats have an innate hyperfunction of the CRF system that is associated with the occurrence of two single nucleotide polymorphisms (SNPs) in the promoter region (position −1836 and −2097) of the gene encoding the CRF1-R (Gehlert et al. 2007; Hansson et al. 2007; Hansson et al. 2006). This genetic variation might have parallels in adolescent drinkers and adult alcohol-dependent subjects where similar mutations were found to be associated with patterns of excessive alcohol consumption (Schmid et al. 2010; Treutlein et al. 2006).
Starting from the original msP line we re-derived two distinct lines, one carrying the two point mutations (AA) and the other being the wild type line (GG). We have recently shown that the two observed mutations at the CRF1-R locus do not seem to play a major role in the expression of the msP excessive drinking phenotype, while they appear to be associated with decreased threshold for stress-induced reinstatement to alcohol seeking and an enhanced sensitivity to alcohol drinking inhibition by CRF1-R antagonism (Ayanwuyi et al. 2013). To follow up these initial observations, here we examined whether these mutations are responsible for the high sensitivity to stress and the heightened anxiety-like behavior of msP rats. Specifically, we first studied CRF1-R gene expression in various brain regions known to play a relevant role in stress, anxiety and alcohol abuse. Then, we investigated the phenotypic characteristics of the AA and the GG lines in preclinical models of human anxiety and fear comparing their behavior with that of unselected Wistar rats. Lastly, we evaluated their response to CRF1-R inhibition following administration of the selective antagonist, antalarmin.
MATERIAL AND METHODS
Animals
Subjects were adult males from two distinct sub-lines derived from the original msP line (65th generation). Animals were bred at the animal facility of the University of Camerino, Italy. Breeding started following genetic screening of the promoter region encoding for CRF1-Rs as previously described (Ayanwuyi et al. 2013). In brief, sequence variation AA versus GG in position −1836 and −2097, respectively, from the first start codon of the CRF1-R transcript, distinguished the two msP lines. Eighty msP rats were sequenced using Taqman-PCR analysis of tail DNA to identify animals carrying (AA) or not carrying (GG) both variants. The homozygous male and female AA and GG were then bred to obtain re-derived lines selectively carrying the AA and the GG types. They were bred for 2 more generations and then animals from the third and fourth generations were used for experiments. Male Wistar rats (Charles River, Calco, Italy) were employed for comparisons between msP and heterogeneous rats because msP rats were originally selected for their high alcohol preference starting from a stock of heterogeneous Wistar animals. All rats (bw, 300–400g at the time of the experiments) were housed in groups of four or five on a reverse 12-hour light-dark cycle (lights off at 08:30 AM) at a constant temperature of 20±2°C and relative humidity of 45–55%, with free access to tap water and food pellets (4RF18, Mucedola, Settimo Milanese, Italy). Animals were handled three times before each experiment and used only once. All procedures followed the EU Directive for Care and Use of Laboratory Animals and were approved by the Ethical Committee of the University of Camerino, Italy.
Drugs
The selective CRF1-R antagonist antalarmin (N-butyl-N-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin4-yl]-amine (Webster et al. 1996) was obtained from the National Institute on Alcohol Abuse and Alcoholism (NIAAA/NIH). Antalarmin was suspended in a vehicle composed of 10% Tween 80 and distilled water and was administered intraperitoneally (i.p.) in a 1 ml/kg volume injection. Doses and time of injection were as described elsewhere (Cippitelli et al. 2012). Physiological saline was injected three times prior to drug testing for habituation to the experimental procedures.
Elevated plus-maze test (EPM)
To measure anxiety-like responses, the EPM test was used as previously described (Cippitelli et al. 2011b). The apparatus consisted of two wooden open arms (50×10 cm) and two enclosed arms (50×10×40 cm) which were arranged so that the similar arms were opposite each other, and situated 60 cm above the floor. The 5-min procedure started by placing each animal in the center of the maze (10 × 10 cm) facing a closed arm. The test was conducted in a quiet room illuminated by dim red light. Six groups of animals were used. Three of them (N=24, 8 GG, 8 AA msPs and 8 Wistar rats) were subjected to the test procedure under basal conditions, whereas the other three groups (N=22, 6–8 per group) were previously exposed to 60-min restraint stress in cylindrical Plexiglass tubes right before rats were allowed to explore the maze. The percentage of time spent exploring the open arms and the percentage of open arm entries were used as measures of anxiety-like behavior, whereas the number of entries into the closed arms was used as an indicator of general motor activity (Pellow et al. 1985). An entry into an arm was defined as the animal placing all four paws over the line marking that area. The apparatus was cleaned with tap water between each rat performance.
Fear conditioning test
Fear conditioning was assessed as described (Bast et al. 2001; Hansson et al. 2006) by using operant conditioning chambers (Med Associates Inc., Georgia, VT). On the first day (conditioning phase), rats were placed individually in a chamber which was programmed to deliver five un-signaled 1-s foot-shocks (1.0 mA) through the grid floor at 5 minute intervals for a 30 minute period. The time of freezing was recorded at five minute intervals. On the following day (expression phase), rats were placed individually in the same experimental context with the exception that the foot-shock was no longer released. The time of freezing was recorded at 1-min intervals for an 8-min period. Data are reported as total time spent freezing during six consecutive 5-min blocks of the conditioning phase and 8-min re-exposure to the context previously associated with foot-shock (expression). N=26 rats (8 Wistar, 9 GG and 9 AA msP rats) were employed in this experiment.
Shock-probe defensive burying (DB) test
The shock-probe DB test (Pinel and Treit 1978) was carried out as previously described (Cippitelli et al. 2011a). The DB apparatus was a modified home cage with 5 cm high wood chip bedding material evenly distributed throughout the cage. One side of the cage contained a round hole of diameter 0.75 cm through which a probe delivering 1.5 mA electric shock was inserted. As for EPM, the DB test was conducted in a quiet room illuminated by dim red light. Rats were kept in the quiet test room for at least three hours on the day before the experiment for the purpose of acclimatization. On the test day, the shock probe was connected to a shocker instrument (Med associates, Inc.) and remained turned on throughout the test. Upon first contact with the shock-probe, the burying behavior of the rat was recorded for 15 minutes. Contacts with the probe resulted in the rat piling bedding material with treading-like movements of the forepaws and shoveling movements of the head, often directed toward the shock-probe. The latency to start burying and the duration of burying were dependent variables that served as measures of anxiety-/fear-like behaviors. The chip bedding material was changed before each rat performance. Three experiments were carried out by using this paradigm. DB behavior was first examined in the two msP rat lines (N=8 GG and N=8 AA) and compared to that of Wistar rats (N=8) and Wistar rats previously exposed to 60-min restraint stress (W/Restraint, N=8). In a second experiment, the effect of the selective CRF1-R antagonist antalarmin was tested. Antalarmin was administered to Wistar (N=23, 7–8 per treatment dose), GG msP (N=47, 14–17 per dose) and AA msP (N=40, 13–14 per dose) rats at doses of 0, 10 and 20 mg/kg, 30 min before the onset of the DB test. Finally, antalarmin was tested in additional Wistar rats (N=29) previously exposed to 60 min restraint stress. The CRF1-R antagonist (0, 10, 20 mg/kg) was administered 30 min prior to restraint and behavioral performance in the DB test was compared with that of Wistars receiving antalarmin 0 mg/kg not exposed to stress.
Brain collection, reverse transcription and quantitative real time polymerase chain reaction (qPCR)
Brains (N=24) from Wistar (N=8), GG msP (N=8), AA msP (N=8) were collected and snap-frozen with isopentane for measurements of CRF1-R mRNA levels. The brains were sliced on a cryostat, and bilateral punches (300 µm thickness, 2 mm diameter) were collected from the medial region of the prefrontal cortex (mPFC), septum, nucleus accumbens (NAcc), bed nucleus of the stria terminalis (BNST), amygdala (Amy), paraventricular nucleus of the hypothalamus (PVN) and median raphe nucleus (MRN). Reverse transcription and qPCR were conducted as described elsewhere (Barbier et al. 2013). In brief, RNA was extracted and purified from brain tissue using the PureLink™ RNA Mini Kit (Ambion, Austin, TX) following the manufacturer’s instructions. cDNA was reverse transcribed from total RNA using the Superscript III First Strand Synthesis System (Invitrogen, Carlsbad, CA). Gene expression levels were determined by qPCR using a TaqMan Universal PCR Master Mix (Applied Biosystems Inc., Foster City, CA). cDNA concentrations of the CRF1-R transcript (CRF1-R) were calculated according to the relative quantification (ΔΔCt) method, corrected for differences in PCR efficiency, normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Primers used were as follows: TaqMan qPCR utilized commercially available CRF1-R (Rn00578611_m1) and Gapdh (Rn99999916_s1) primer/probe sets, (Applied Biosystems Inc., Foster City, CA), with PCR conditions according to the manufacturer protocol. Data as reported as percentage of control (Wistar group).
Statistical analysis
EPM data were analyzed by means of a two-way analysis of variance (ANOVA) where rat “line” and “restraint” were between-subject factors. Analysis of DB and fear conditioning data were carried out by means of one-way ANOVA with the rat “line” as between-subject factor. To evaluate CRF1-R mRNA expression levels, one-way ANOVA was also used to analyze the brain regions independently. The effect of antalarmin was analyzed by means of a two-way ANOVA with “rat line” and “drug treatment” as the between-subject factors. The effect of antalarmin on burying behavior of Wistar rats previously exposed to restraint was analyzed separately using one-way ANOVA with “group” as a between-subject factor. Accepted p-level was p<0.05 for behavioral data and p<0.01 for gene expression data. When appropriate, analyses of behavioral data were followed up by Fisher’s least significant difference (LSD) post hoc tests while qPCR data were followed by Tukey-Kramer post hoc tests.
RESULTS
CRF1-R gene expression levels in AA, GG and Wistar rats
Expression of the CRF1-R gene was evaluated on a network of structures densely populated in CRF1-Rs (Contarino and Gold 2002) or previously reported to be associated with anxiety- and fear-like behaviors as well as pathophysiological response to stress (De Boer and Koolhaas 2003). MsP lines showed different expression in CRF1-R mRNA levels in the Amy [F(2,20)=47.2, p<0.001] and the BNST [F(2,19)=7.8, p<0.01] compared to the unselected Wistar strain (Table 1). Post hoc testing revealed robust upregulation in the Amy of both GG and AA msP lines (p<0.001) and marked downregulation of CRF1-R in the BNST of the AA msP line compared to the Wistar line (p<0.01). A trend to an increased CRF1-R mRNA levels was seen in the mPFC [F(2,21)=3.6, p<0.05 ] while no significant alterations were observed in the Septum [F(2,19)=1.1, NS], the NAcc [F(2,19)=0.6, NS], the PVN [F(2,19)=2.0, NS] and the MRN [F(2,14)=1.8, NS].
Table 1.
Expression of the CRF1-R gene within a network of brain structures including the medial region of the prefrontal cortex (mPFC), septum, nucleus accumbens (NAcc), bed nucleus of the stria terminalis (BNST), amygdala (Amy), paraventricular nucleus of the hypothalamus (PVN) and median raphe nucleus (MRN) in Wistar (N=8), GG msP (N=8), AA msP (N=8) lines.
| Rat line |
|||
|---|---|---|---|
| Brain Structure | Wistar | GG msP | AA msP |
| mPFC | 100.9±5.3 | 136.8±9.2 | 125.0±12.8 |
| Septum | 104.7±12.9 | 109.3±8.2 | 90.8±8.6 |
| NAcc | 100.3±3.3 | 123.2±12.8 | 106.1±21.1 |
| BNST | 102.3±8.7 | 80.8±5.6 | 62.8±2.7** |
| Amy | 102.0±7.7 | 187.0±4.7*** | 188.8±8.7*** |
| PVN | 100.4±4.0 | 106.8±5.5 | 116.3±6.2 |
| MRN | 115.6±34.9 | 119.9±18.2 | 112.1±8.0 |
Data are reported as mean (±S.E.M) percent (%) of control (Wistar group).
p<0.01,
p<0.001 difference from the Wistar group. For detailed statistics, see Results.
Basal anxiety levels and stress-induced anxiety in the EPM test
EPM results indicated that under basal conditions both GG and AA msP lines showed increased anxiety-like behavior compared to unselected Wistar rats. However, no significant differences between the two alcohol preferring lines were observed. Further, EPM data showed that exposure to restraint stress produced heightened anxiety levels in all three rat lines examined. Overall ANOVA for the percentage of time spent exploring the open arms revealed a robust main effect of “line” [F(2,40)=14.1, p<0.001] accompanied by main effect of “restraint” [F(1,40)=16.4, p<0.001] but not by a significant interaction of “line x restraint” [F(2,40)=1.9, NS]. Post hoc analysis indicated that the collapsed variable of line was decreased in both GG and AA msP vs. Wistar rats (p<0.001). Similarly, restrained Wistar animals showed anxiogenic-like behavior compared with non-restrained (p<0.001, Figure 1A). Results of ANOVA with regard to the percentage of open arm entries paralleled to a lower extent those of time spent exploring the open arms. Thus, a main effect of “line” [F(2,40)=5.8, p<0.01] was accompanied by main effect of “restraint” [F(1,40)=6.3, p<0.05] with no significant interaction “line x restraint” [F(2,40)=0.4, NS]. Post hoc comparisons of the collapsed variable of “line” showed increased percentage of entries onto the open arms for Wistar vs. both the AA (p<0.01) and the GG (p<0.05) msP lines with restraint stress significantly altering this measure (p<0.05, Figure 1B). However, the observed difference in these anxiety-related variables was associated to a different number of entries into the closed arms since ANOVA showed overall significant difference for the main effect of “line” [F(2,40)=17.8, p<0.001] in absence of significant “restraint” effect [F(1,40)=3.0, NS] or interaction “line x restraint” [F(2,40)=2.0, NS]. Specifically, crossings onto closed arms were decreased in the GG and the AA lines (p<0.001 for both) vs. Wistars (Figure 1C).
Figure 1.
Elevated anxiety-like behavior of both GG and AA lines derived from the original msP line as assessed in the elevated-plus maze (EPM) test. GG (N=8) and AA (N=8) msP rats show lower (A) time spent exploring open arms and (B) open arm entries as compared with unselected Wistar (N=8) rats in basal (no-restraint) conditions. 1-hour restraint stress produces anxiogenic effects in all lines examined (Wistar N=6, GG msP N=8, AA msP N=8). The elevated anxiety of the msP lines is accompanied by (C) reduced exploration of the maze. Values are presented as mean percent (%, ±S.E.M.) of open arm time and entries, and mean (±S.E.M.) number of closed arm entries. *p<0.05, **p<0.01 and ***p<0.001, significant difference between both msP lines and the Wistar rat strain. **(AA) p<0.01,*(GG) p<0.05, significant difference between the AA and the GG line vs. the Wistar line, respectively. #p<0.05, ###p<0.001, difference from non-restrained groups. For detailed statistics, see Results.
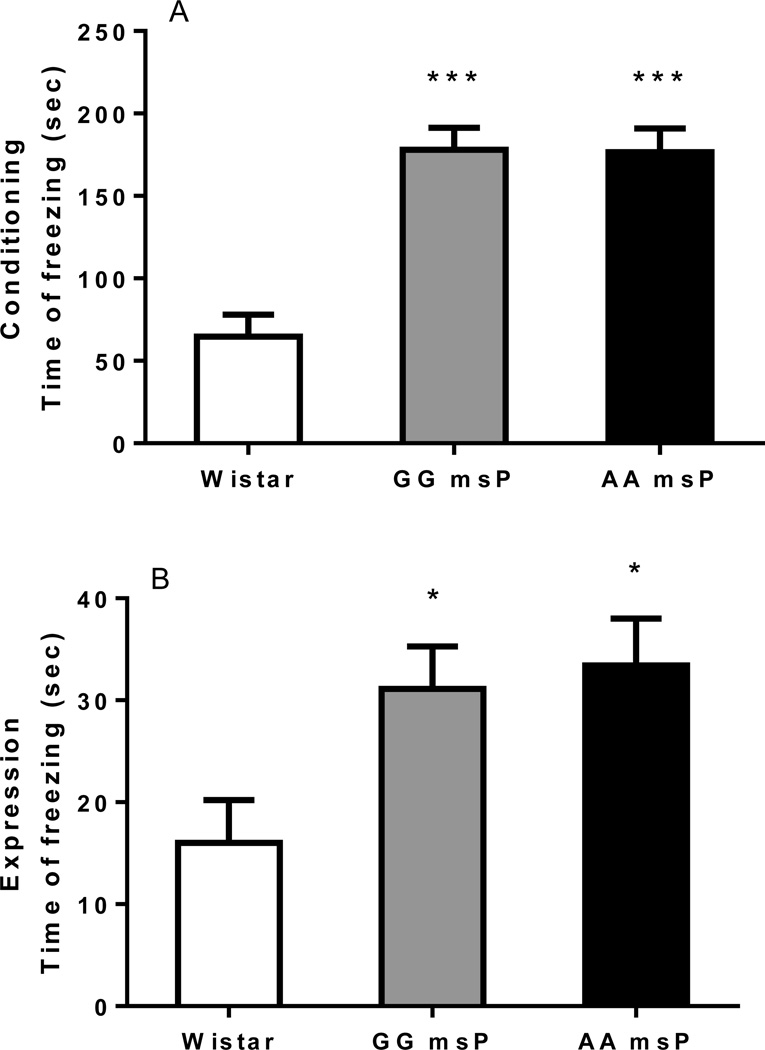
Freezing response in the acquisition and recall of fear conditioning
The fear conditioning test produced a pattern of freezing response that was characteristic of an anxiogenic-like phenotype for both the GG and the AA msP lines. Overall ANOVA showed a markedly higher level of freezing behavior during both conditioning [F(2,23)=21.4, p<0.001] and expression [F(2,23)=4.5, p<0.05] phases of the test. On post hoc analysis, fear reaction was increased in GG and AA msP rats as compared with the Wistar strain [acquisition: p<0.001 for both (Figure 2A), expression: p<0.05 for both (Figure 2B)].
Figure 2.
Increased time of freezing in both the lines GG and AA msPs during (A) conditioning (acquisition) and (B) expression (recall) sessions in a contextual fear conditioning paradigm. Data are the mean ± SEM seconds (sec) of freezing (Wistar N=8, GG msP N=9 and AA msP N=9) during six consecutive 5-min blocks of fear conditioning or 8-min re-exposure to context previously associated with foot-shock. *p<0.05, ***p<0.001, difference from the Wistar line. For detailed statistics, see Results.
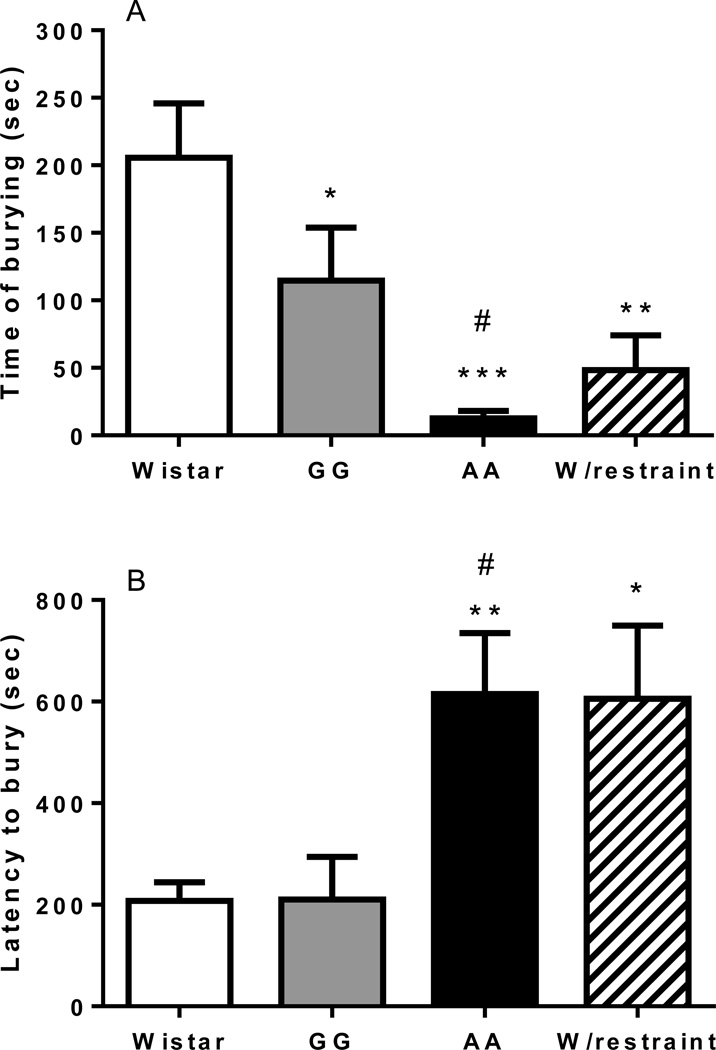
Defensive burying response in AA, GG, Wistar and previously restrained Wistar rats
Overall ANOVA comparing Wistar, GG, AA msPs, and Wistar rats previously exposed to 60-min restraint stress revealed a significant difference in the total time spent burying [F(3,28)=7.4, p<0.001]. Pairwise comparisons indicated higher burying time in Wistar rats compared to GG (p<0.05) and AA (p<0.001). Noteworthy, the burying time of AA was also lower (p<0.05) compared to that of GG rats. Wistar rats subjected to restraint, showed decreased burying, compared to non-restrained Wistars (p<0.01), suggesting that exposure to stress decreases this stress-coping behavior (Figure 3A). When the latency to start burying was evaluated (Figure 3B) overall ANOVA showed increased latency to start burying [F(3,28)=4.9, p<0.01]. Post hoc analysis revealed a significant higher latency to bury in the AA rats compared to GG or non-restrained Wistars (p<0.05 and p<0.01, respectively). A significant difference between W/restraint and non-restrained Wistar groups was also observed (p<0.05).
Figure 3.
15-min defensive burying (DB) performance of Wistar (N=8), GG msP (N=8), AA msP (N=8) and restrained Wistar (W/Restraint, N=8) rat lines. The AA line exhibits (A) decreased time of burying and (B) increased latency to bury as compared to the GG msP line and unselected Wistar rats. W/Restraint group performs in a similar way than AA. Time of burying and latency to bury values are expressed in mean (±S.E.M.) seconds (sec). *p<0.05, **p<0.01, ***p<0.001, significant difference from the Wistar line. #p<0.05, difference between the AA and the GG line. For detailed statistics, see Results.
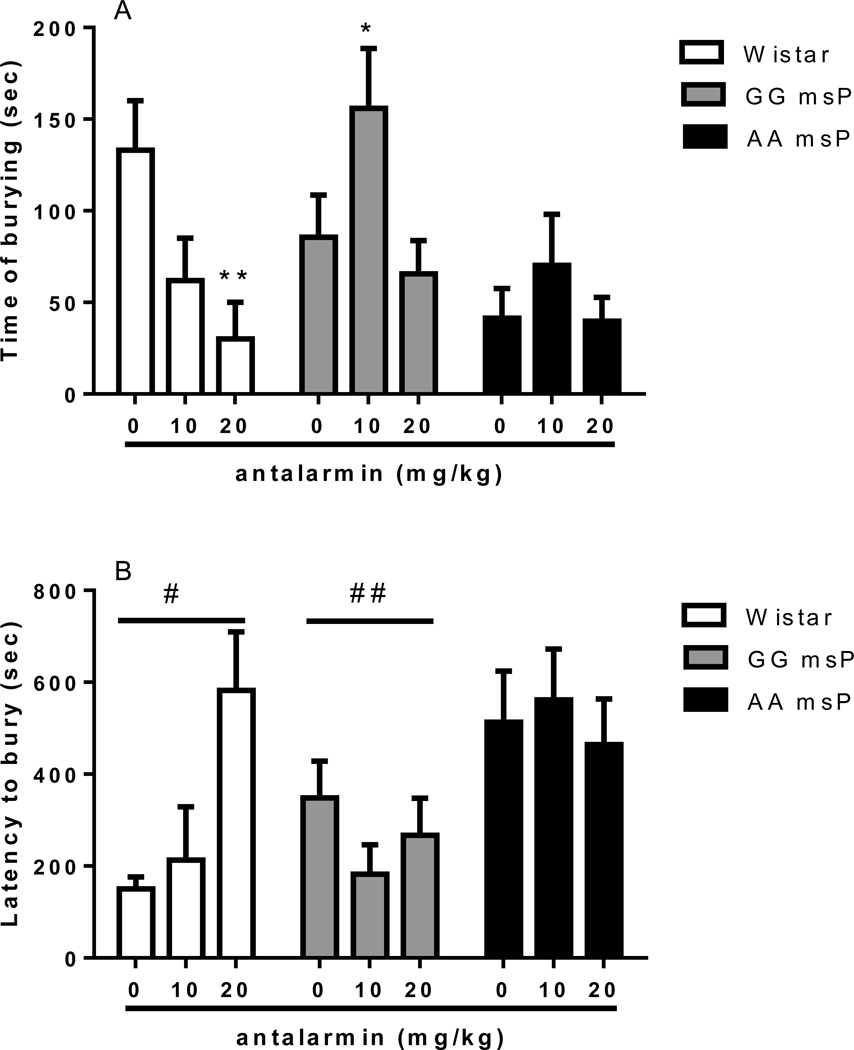
Defensive burying response in AA, GG and Wistar rats following antalarmin treatment
Overall ANOVA revealed changes in the total duration of defensive burying behavior with a significant main effect of “treatment” [F(2,101)=3.8, p<0.05], significant main effect of “line” [F(2,101)=4.3, p<0.05] and “treatment x line” interaction [F(4,101)=2.6, p<0.05]. In Wistar rats post hoc analysis indicated that, compared to vehicles, 20 mg/kg of antalarmin significantly (p<0.01) reduced the duration of burying (Figure 4A). In the GG line 10 mg/kg of antalarmin significantly increased the burying time (p<0.05) compared to vehicles. At the higher dose (20 mg/kg) antalarmin did not appear to evoke significant effects. In the AA line antalarmin showed a trend to an increase in burying time but statistical difference was not reached.
Figure 4.
Effect of the selective CRF1-R antagonist antalarmin (0, 10, 20 mg/kg, i.p.) in the DB performance of Wistar (N=23), GG (N=47) and AA msP (N=40) rats. (A) Antalarmin dose-dependently decreases the total time of burying during the 15-min test in unselected Wistars and increases this variable in the GG line when injected at a dose of 10 mg/kg. (B) Latency to initiate burying is increased in the AA msP animals. Time of burying and latency to bury values are expressed in mean (±S.E.M.) seconds (sec); *p<0.05, **p<0.01, difference from antalarmin vehicle (0 mg/kg); #p<0.05, ##p<0.01, difference from the AA msP line. For detailed statistics, see Results.
When the latency to start burying was evaluated (Figure 4B), ANOVA revealed no significant overall effect of treatment [F(2,101)=0.8, NS] although a significant effect of rat “line” was observed [F(2,101)=6.3, p<0.01]. Finally, no “treatment x line” interaction was displayed [F(4,101)=1.6, NS]. Post hoc comparisons revealed increased latency to initiate burying in AA rats compared to Wistars (p<0.05) and GG msPs (p<0.01).
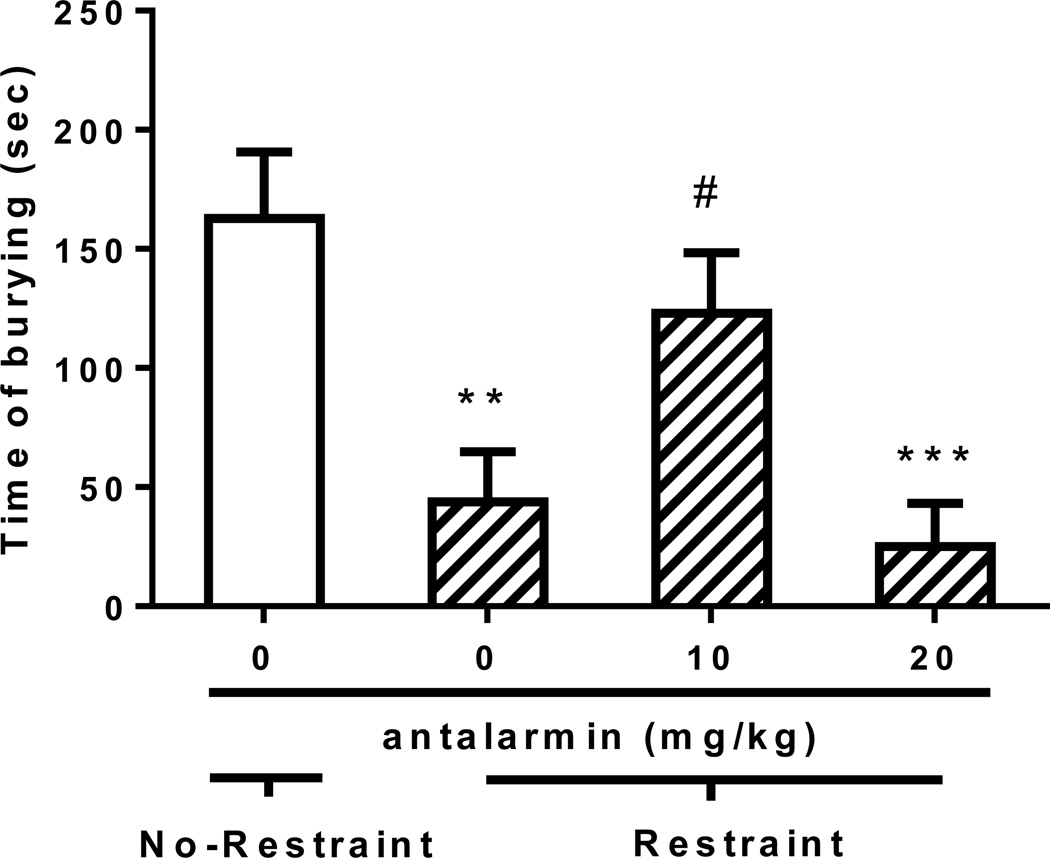
Defensive burying response in Wistar rats exposed to restraint following antalarmin treatment
One-way ANOVA, conducted in unselected Wistar rats exposed to restraint following antalarmin treatment and a group of Wistars receiving the vehicle of antalarmin and not exposed to restraint, revealed changes in the total duration of defensive burying behavior [F(3,25)=8.1, p<0.001]. Post hoc analysis indicated an expected reduction in time of burying of the restrained group non-pretreated with antalarmin as compared to the group not exposed to stress (p<0.01). In a manner similar to the effects of antalarmin observed in the msP lines, group comparisons also indicated that treatment with 10 mg/kg antalarmin prior to restraint significantly increased the burying time (p<0.05) compared to the restrained group receiving antalarmin 0 mg/kg. At the higher dose (20 mg/kg) antalarmin did not appear to evoke significant effects (Figure 5).
Figure 5.
Effect of the selective CRF1-R antagonist antalarmin (0, 10, 20 mg/kg, i.p.) in the DB performance of Wistar rats (N=29, 6–8 per group) previously exposed to 60 min restraint stress. Antalarmin or vehicle (0 mg/kg) was administered 30 min prior to restraint that preceded the 15 min DB performance. Vehicle of antalarmin was injected to a rat group not exposed to restraint that served as control. Time of burying values are expressed in mean (±S.E.M.) seconds (sec); **p<0.01, ***p<0.001 difference from control; #p<0.05 difference from rats exposed to restraint following antalarmin 0 mg/kg treatment. For detailed statistics, see Results.
Latency to initiate burying was also changed [F(3,25)=4.7, p<0.01] with the antalarmin 20 mg/kg group being substantially increased as compared to the other treatment groups. Mean±SEM latency to start burying for the 4 groups were 287.4±61.9 (antalarmin vehicle/No-Restraint), 358.9±141.3 (antalarmin vehicle/Restraint), 116.3±18.1 (antalarmin 10/Restraint) and 611.4±96.5 seconds (antalarmin 20/Restraint).
DISCUSSION
The gene expression analysis reported here indicated significant CRF1-R over-expression in the amygdala of both AA and GG rats compared to the progenitor Wistar line. Although binding data on brain CRF1-R protein levels in AA and GG rats are not provided here, this elevated CRF1-R expression may reflect increased density of CRF1-R sites in numerous brain regions, including different portions of the amygdala, as we previously demonstrated in the original msP line from which the AA and GG lines were derived (Hansson et al. 2006). Increased CRF function in the CeA has been linked to excessive anxiety-like behavior in the EPM (Ciccocioppo et al. 2014; Ji et al. 2007). In excellent agreement with this evidence, in the EPM test we found that the AA and GG lines express lower exploratory behavior and spent less time in the open arm of the maze compared to heterogeneous Wistar rats. Closed arm entries were also reduced in AA and GG rats compared to Wistars. This is consistent with previous published data showing that exploratory behavior in msP rats is reduced under condition of novelty (Hansson et al. 2006). On the other hand, the two examined polymorphisms at CRF1-R locus did not seem to determine the innate anxiogenic phenotype of our two lines of alcohol preferring animals. In fact, no differences between AA and GG rats were observed in the EPM test. Previous studies have shown that msP rats from which the AA and the GG were derived are highly sensitive to stress exposure (Ciccocioppo et al. 2006; Hansson et al. 2006). Hence, we decided to expand our EPM study by looking at whether the observed point mutations in the CRF1-R gene would play a role in enhanced anxiety resulting from exposure to a stressful stimulus. The test was therefore replicated in GG, AA and unselected Wistars previously subjected to 1 hour restraint stress. As expected, stress exposure elicited a considerable anxiogenic-like response in Wistar rats that showed levels of anxiety comparable to those seen in the AA and GG lines without restraint. A trend towards increased anxiety-like behavior was also observed in AA and GG rats that showed further decreased time spent in the open arms and entries onto the open arms of the maze. Although the AA line appeared to be more responsive to the restraint stress than the GG line, statistical analysis did not show significant line difference. We argued that possible floor effects may have attenuated the possibility to detect potential differences between the lines. Therefore, to better address this issue we studied the behavior of AA and GG rats in the fear conditioning model which reflects fear-like responses generated by exposure to stressful environmental conditions rather than generalized anxiety disorders. Results showed that both AA and GG lines subjected to contextual fear conditioning showed higher time of freezing (complete absence of somatic motility except for respiratory movements) as compared to Wistar rats during both the acquisition and recall sessions with freezing levels being indistinguishable between the two lines. It is known that neuronal processing in the amygdala is important for classical fear conditioning to contextual as well as explicit conditioned stimuli (Goosens and Maren 2001; LeDoux 2000) and work carried out using electric shock stress has suggested that the central portion of the amygdala is predominantly involved in the expression of passive behavioral coping (Legradi et al. 2007; Roozendaal et al. 1997). On the other hand, the basolateral amygdala has been shown to be implicated in the acquisition of fear-related behaviors (Bijlsma et al. 2011). Hence, although the longer acquisition latency observed in both AA and GG lines compared to the Wistar is an important evidence of spontaneously increased inhibitory/passive response to stress in msP rats, the negative finding in the fear conditioning test (no differences between AA and GG rats) nicely correlates with the over expression of the CRF1-R gene found in the amygdala of both msP rat lines compared to Wistars and suggests the lack of a functional role for the examined polymorphisms in passive fear response.
An important difference in CRF1-R expression was found in BNST, where AA rats showed significantly lower expression levels of the transcript than the Wistar group. The BNST, is an important structure implicated in the integration and processing of stress responses, plays a role pathological anxiety (Hammack et al. 2004; Sparta et al. 2013) and is a critical neuroanatomical substrate for stress-induced reinstatement of drug seeking, where CRF plays a major role (Erb and Stewart 1999; Silberman and Winder 2013). This suggests that CRF neurotransmission in the BNST is involved in triggering active reactions to stress. Moreover, activation of the CRF system in the BNST seems to contribute to the expression of defensive behavior and CRF antagonists directly injected into this nucleus attenuate it (Jasnow et al. 2004). Finally, it has been reported that administration of a CRF antagonist into the BNST did not attenuate phasic but blocked sustained fear behaviors (Davis et al. 2010), indicating that this structure may be recruited to regulate forms of anxiety associated to a more long-lasting state of apprehension rather than transient fear. Based on this background, we postulated that the different organization of the CRF system in the BNST of AA rats would influence active stress coping responses in this rat line.
To test this hypothesis we used the shock-probe DB model originally described by Pinel and Treit (1978). This model seems to be particularly appropriate to examine whether the observed SNPs in the CRF1-R gene would have functional relevance as it requires rats to engage in an active behavioral response to stress (to bury an electrified probe) and is highly dependent on the extrahypothalamic CRF system (Basso et al. 1999; De Boer and Koolhaas 2003). For instance, CRF administration increases DB in rats (Diamant et al. 1992), and CRF antagonists block this response (Basso et al. 1999; Richardson et al. 2008). In the DB test we found that the AA line had the lowest total time of burying in a single 15-min trial as well as the highest latency to start burying followed by the GG line and then the unselected Wistar rats. This result is indicative of the fact that the examined polymorphisms in the CRF1-R gene could play a role in regulating active forms of stress avoidance behavior. To some extent the decreased burying response observed in AA rats, and also partly in the GG line, contradicts the common notion that higher stress sensitivity and increased anxiety positively correlates with enhanced burying response DB test (De Boer and Koolhaas 2003; Korte et al. 1994). To reconcile this apparent paradox, we subjected Wistar rats to restraint stress prior to the DB and we found that after this stress manipulation, the burying behavior of Wistar rats was dramatically reduced and similar to that observed in the AA line. These data suggest that when animals are in a state of excessive stress, either innate as in AA and GG rats or evoked following physical restraint, they lose the ability to engage in active reactions to stress. Evidence linking these behavioral responses to over-function of the CRF system also exists. For example, it has been documented that CRF1-R agonist stressin1-A elicited burying reactions at low doses but increased freezing at 25-fold higher doses switching the behavior of rats from active to passive (Zhao et al. 2007). Another study showed that cortagine, a selective CRF1-R agonist, administered bilaterally into the cerebral ventricles of rodents evoked anxiogenic-like effects in a model of defensive behaviors by dose-dependently enhancing passive avoidance and freezing, while burying was decreased (Tovote et al. 2010).
To prove that DB response was under the control of the CRF1-R system we administered antalarmin. Interestingly, blockade of CRF1-R resulted in opposite responses. Confirming previously published data in Wistar rats it reduced the burying behavior (Heinrichs et al. 2002; Richardson et al. 2008). On the other hand, antalarmin increased the burying response in GG and AA rats at the low dose, an effect that disappeared at higher dosages. This reversal of a previously inhibited burying behavior could be attributed to the ability of antalarmin to contrast the abnormally heightened anxiety/stress state of msP rats bringing it back to normal levels thus enabling re-gain of active stress coping response (i.e., increase in burying behavior). Additional evidence that a low antalarmin dose administered to unselected Wistar animals exposed to acute restraint stress produced a similar defensive burying response than observed in the msP lines strengthens this view. In agreement, a more complete blockade of the CRF1-Rs as that obtained by administering antalarmin at high doses led to a full anxiolytic effect that in turn resulted in burying reduction.
At present it is unclear how to reconcile the hypersensitivity to stress observed in AA and GG rats with reduced expression of CRF1-R transcript in the BNST. It is possible that downregulation of the transcript is part of a compensatory change in CRF1-R expression aimed at balancing the over-function of the CRF system in the CeA of msP rats (Hansson et al. 2006; Herman et al. 2013). In this respect, the more pronounced reduction in CRF1-R transcript associated with the polymorphisms found in AA rats may be viewed as an additional compensatory mechanism occurring in this rat line. For instance, it may be argued that the low CRF1-R transcript level in AA rats may reflect changes at neurocircuitry levels (i.e., reduction in the number of CRF1-R positive neurons in the BNST). Several evidences, in fact, indicate profound reorganization of this nucleus as a result of exposure to stress. For instance, it has been shown that in the BNST, stress can have a major impact on dendritic/synaptic remodeling (Pego et al. 2008), blunts neuronal plasticity (Conrad et al. 2011) and can change GABAergic and glutamergic innervation of the nucleus (Ventura-Silva et al. 2012). Additional studies will have to be performed to understand the impact of CRF system over-activation and CRF1-R transcript polymorphisms occurring in the AA line on the neuroanatomical and functional organization of the BNST.
In conclusion, two major findings are outlined here. First, we show that two previously identified point mutations at the CRF1-R gene locus do not seem to play a major role in basal anxiety or in passive behavioral responses to stress. However, they appear to contribute to a reduced capacity to actively react to stress. Secondly, these findings may have important pharmacogenetic implications because they support the notion that polymorphisms at CRF1-R locus correlate with stress hypersensitivity, and possibly with specific forms of anxiety.
ACKNOWLEDGEMENTS
We are thankful to Rina Righi and Mariangela Fiorelli for animal care and Marino Cucculelli and Alfredo Fiorelli for technical support. This work was supported by the National Institutes of Health, grant RO1 AA017447, and RO1 AA014351 from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
DISCLOSURES/CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCE LIST
- Ayanwuyi LO, Carvajal F, Lerma-Cabrera JM, Domi E, Bjork K, Ubaldi M, Heilig M, Roberto M, Ciccocioppo R, Cippitelli A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Frontiers in psychiatry. 2013;4:23. doi: 10.3389/fpsyt.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Vendruscolo LF, Schlosburg JE, Edwards S, Juergens N, Park PE, Misra KK, Cheng K, Rice KC, Schank J, Schulteis G, Koob GF, Heilig M. The NK1 receptor antagonist l822429 reduces heroin reinforcement. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:976–984. doi: 10.1038/npp.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology. 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bijlsma EY, van Leeuwen ML, Westphal KG, Olivier B, Groenink L. Local repeated corticotropin-releasing factor infusion exacerbates anxiety- and fear-related behavior: differential involvement of the basolateral amygdala and medial prefrontal cortex. Neuroscience. 2011;173:82–92. doi: 10.1016/j.neuroscience.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & therapeutics. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R. Genetically selected alcohol preferring rats to model human alcoholism. Current topics in behavioral neurosciences. 2013;13:251–269. doi: 10.1007/7854_2012_199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, Roberto M. Restraint Stress Alters Nociceptin/Orphanin FQ and CRF Systems in the Rat Central Amygdala: Significance for Anxiety-Like Behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:363–372. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction biology. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de Guglielmo G, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology. 1999;144:151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, Kallupi M, Sagratini G, Rodriguez de Fonseca F, Piomelli D, Ciccocioppo R. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PloS one. 2011a;6:e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Singley E, Thorsell A, Ciccocioppo R, Eskay RL, Heilig M. Pharmacological blockade of corticotropin-releasing hormone receptor 1 (CRH1R) reduces voluntary consumption of high alcohol concentrations in non-dependent Wistar rats. Pharmacology, biochemistry, and behavior. 2012;100:522–529. doi: 10.1016/j.pbb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Rezvani AH, Robinson JE, Eisenberg L, Levin ED, Bonaventure P, Motley ST, Lovenberg TW, Heilig M, Thorsell A. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011b;45:567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Louderback KM, Gessner CP, Winder DG. Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiology & behavior. 2011;104:248–256. doi: 10.1016/j.physbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Gold LH. Targeted mutations of the corticotropin-releasing factor system: effects on physiology and behavior. Neuropeptides. 2002;36:103–116. doi: 10.1054/npep.2002.0899. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. European journal of pharmacology. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Diamant M, Croiset G, de Wied D. The effect of corticotropin-releasing factor (CRF) on autonomic and behavioral responses during shock-prod burying test in rats. Peptides. 1992;13:1149–1158. doi: 10.1016/0196-9781(92)90022-u. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo [1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learning & memory. 2001;8:148–155. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral neuroscience. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addiction biology. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regulatory peptides. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, Ciccocioppo R, Roberto M. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology. 2013;67:337–348. doi: 10.1016/j.neuropharm.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikichi T, Akiyoshi J, Yamamoto Y, Tsutsumi T, Isogawa K, Nagayama H. Suppression of conditioned fear by administration of CRF receptor antagonist CP-154,526. Pharmacopsychiatry. 2000;33:189–193. doi: 10.1055/s-2000-7587. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral neuroscience. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Molecular pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Korte-Bouws GA, Bohus B, Koob GF. Effect of corticotropin-releasing factor antagonist on behavioral and neuroendocrine responses during exposure to defensive burying paradigm in rats. Physiology & behavior. 1994;56:115–120. doi: 10.1016/0031-9384(94)90268-2. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural plasticity. 2007;2007:79102. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MB, Wurst W. Getting closer to affective disorders: the role of CRH receptor systems. Trends in molecular medicine. 2004;10:409–415. doi: 10.1016/j.molmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nature neuroscience. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. The Journal of clinical psychiatry. 2005;7(66 Suppl):5–13. [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacology, biochemistry, and behavior. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacology, biochemistry, and behavior. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. The European journal of neuroscience. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of neuroscience methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pinel JPJ, Treit D. Burying as a defensive response in rats. J Comp Physiol Psychol. 1978:1992. [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacology, biochemistry, and behavior. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biological psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Koolhaas JM, Bohus B. The role of the central amygdala in stress and adaption. Acta physiologica Scandinavica Supplementum. 1997;640:51–54. [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological reviews. 2001;53:209–243. [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, 3rd, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Frontiers in psychiatry. 2013;4:42. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skutella T, Probst JC, Renner U, Holsboer F, Behl C. Corticotropin-releasing hormone receptor (type I) antisense targeting reduces anxiety. Neuroscience. 1998;85:795–805. doi: 10.1016/s0306-4522(97)00682-9. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Jennings JH, Ung RL, Stuber GD. Optogenetic strategies to investigate neural circuitry engaged by stress. Behavioural brain research. 2013;255:19–25. doi: 10.1016/j.bbr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature genetics. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Tovote P, Farrokhi CB, Gonzales RM, Schnitzbauer U, Blanchard DC, Blanchard RJ, Spiess J. Activation of central CRF receptor 1 by cortagine results in enhanced passive coping with a naturalistic threat in mice. Psychoneuroendocrinology. 2010;35:887–895. doi: 10.1016/j.psyneuen.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Molecular psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Ventura-Silva AP, Pego JM, Sousa JC, Marques AR, Rodrigues AJ, Marques F, Cerqueira JJ, Almeida OF, Sousa N. Stress shifts the response of the bed nucleus of the stria terminalis to an anxiogenic mode. The European journal of neuroscience. 2012;36:3396–3406. doi: 10.1111/j.1460-9568.2012.08262.x. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, Weiss F, Zorrilla EP. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. The Journal of pharmacology and experimental therapeutics. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert opinion on investigational drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]