Abstract
Objective
To quantify the changes in regional dynamic loading patterns on tibial articular cartilage during simulated walking following medial meniscectomy and meniscal transplantation.
Methods
Seven fresh frozen human cadaveric knees were tested under multidirectional loads mimicking the activity of walking, while the contact stresses on the tibial plateau were synchronously recorded using an electronic sensor. Each knee was tested for three conditions: intact meniscus, medial meniscectomy, and meniscal transplantation. The loading profiles at different locations were assessed and common loading patterns were identified at different sites of the tibial plateau using an established numerical algorithm.
Results
Three regional patterns were found on the tibial plateau of intact knees. Following medial meniscectomy, the area of the first pattern which was located at the posterior aspect of the medial plateau was significantly reduced, while the magnitude of peak load was significantly increased by 120%. The second pattern which was located at the central-posterior aspects of the lateral plateau shifted anteriorly and laterally without changing its magnitude. The third pattern in the cartilage-to-cartilage contact region of the medial plateau was absent following meniscectomy. Meniscal transplantation largely restored the first pattern, but it did not restore the other two patterns.
Conclusion
There are site-dependent changes in regional loading patterns on both the medial and lateral tibial plateau following medial meniscectomy. Even when a meniscal autograft is used where the geometry and material properties are kept constant, the only region in which the loading pattern is restored is at posterior aspect of the medial plateau.
Keywords: Contact mechanics, cadaveric model, Tekscan, knee simulator, pressure sensor
INTRODUCTION
Over one million meniscal surgeries are performed annually in the United States,1 making it one of the most commonly performed orthopaedic procedures. For severe tears or degenerated menisci, total meniscectomy was historically performed,2 following which progressive cartilage loss and eventual joint degeneration was seen.3–5 It has been postulated that cartilage damage after total meniscectomy was caused in part by alterations in joint mechanics, specifically increased contact stresses, decreased contact area, and decreased joint stability.6–9 The early onset of degenerative changes in the ipsilateral knee compartment following meniscectomy is also felt to be due to alterations in contact location, resulting in cartilage regions being subjected to loads that they are not accustomed to.10 Meniscal allograft transplantation (MAT) is intended to restore ‘normal’ pre-injury mechanics to knees with deficient menisci2 – i.e., to distribute loads across articular cartilage and restore joint stability. While it has been shown in previous cadaveric studies that MAT does not completely restore contact mechanics to that of the intact knee,11–13 exactly how the subtle change in joint mechanics affects the long-term health of articular cartilage remains unknown.
We have previously developed a dynamic knee simulator which can apply synchronous multidirectional joint forces to mimic the activity of walking on human knees.14 In tests conducted on a series of 12 human cadaveric knees, we identified commonly occurring dynamic loading patterns on specific regions of tibial articular cartilage, regardless of knee-to-knee variability.15 If we could characterize how these loading patterns change in meniscectomized knee and then in the meniscal transplanted knee, the quantitative loading patterns could be used as inputs to bioreactors, so that the response of articular cartilage to such physiological loading patterns, and changes in those patterns, can be better understood.
The objective of this study was to quantify the regional dynamic loading patterns on tibial articular cartilage in the medial meniscectomized and meniscal transplanted knees. It was hypothesized that: (i) after medial meniscectomy, there will be a pronounced change in the commonly occurring knee-independent but regionally dependent loading patterns, and (ii) meniscal transplantation will fully restore the commonly occurring loading patterns to that of the intact joint.
MATERIAL AND METHODS
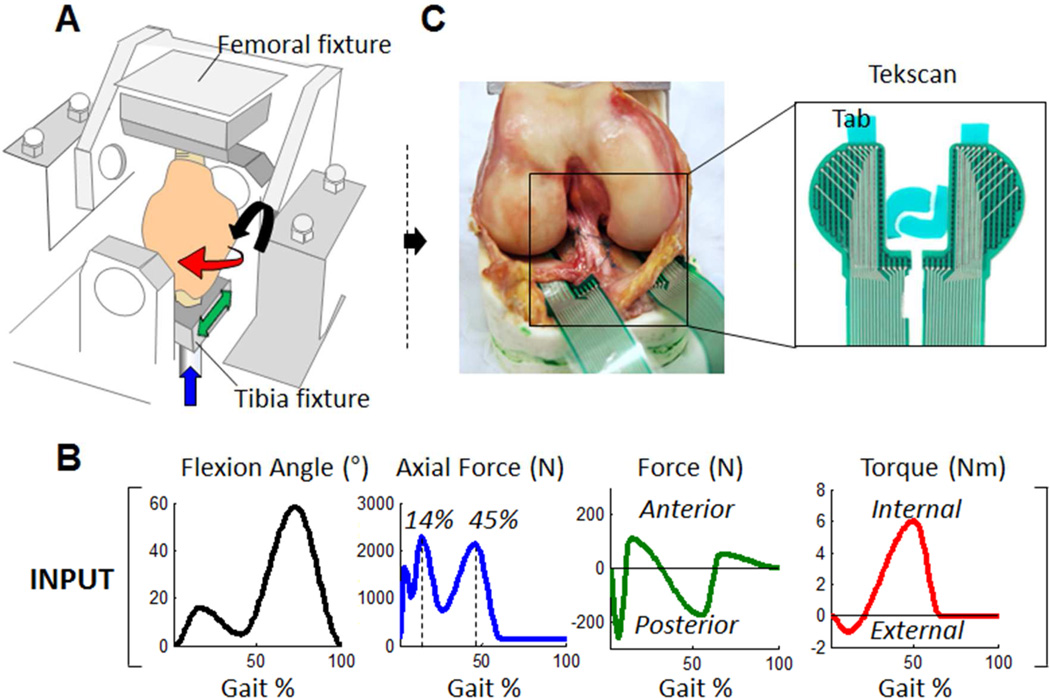
For this study, we retrospectively analyzed contact mechanics data that were generated from seven fresh frozen human cadaveric knees, subjected to loads that mimic the activity of walking; the knees were tested in the intact, medial meniscectomized and medial meniscal transplanted conditions.13 For the total meniscal transplantation condition, we selected bone plug suture fixation since it has been previously shown that it restores joint contact mechanics closest to that of the intact knee versus the suture-only fixation condition. Briefly, the specimens were mounted to a modified load-controlled Stanmore Knee Simulator (University College London, Middlesex, UK) after stripping the surrounding soft tissue (fat, musculature) (Fig. 1A). Each knee was subjected to a prescribed, simultaneously controlled cyclic femoral flexion/extension angle and axial force, anterior-posterior force and internal/external torque applied to the tibia to mimic the activity of walking (Fig. 1B).15–17 Contact stresses normal to the tibial plateau surface were recorded in real-time (100 Hz) using a thin electronic sensor (model: 4010N, Tekscan Inc., MA), Fig. 1C, which was inserted under both menisci of the medial and lateral plateau. Plastic tabs were place along the edges of the Tekscan sensors to allow for suture fixation. Tegaderm™ (3M, St. Paul, MN) adhesive film was then placed on the top and bottom surface of each Tekscan sensor. Approximately 1 cm incisions were made in the meniscotibial ligaments and joint capsule anteriorly and posteriorly of both menisci. The sensor was curled at its edges and passed underneath the menisci from anterior to posterior by pulling the posterior tab via a posterior incision with minimal disruption of the meniscocapsular attachments. After adjusting its position, the tabs were sutured in place using 3-0 Ethibond sutures which attached to the tibial insertion of the anterior cruciate ligament (ACL) and the posteroinferior capsule.13, 18
Figure 1.
Experiment setup. (A) The femur and tibia were potted into fixtures with PMMA bone cement. The forces and torque were applied on the tibia, and the flexion and extension was applied on the femur, while the varus/valgus rotation and superior/inferior translation were not controlled. (B) The knee simulator applies flexion/extension rotation, anterior/posterior force, axial force and internal/external torque to mimic the dynamic multidirectional loads during gait. (C) The contact stresses on the tibial plateaus were collected in real-time using a Tekscan sensor.
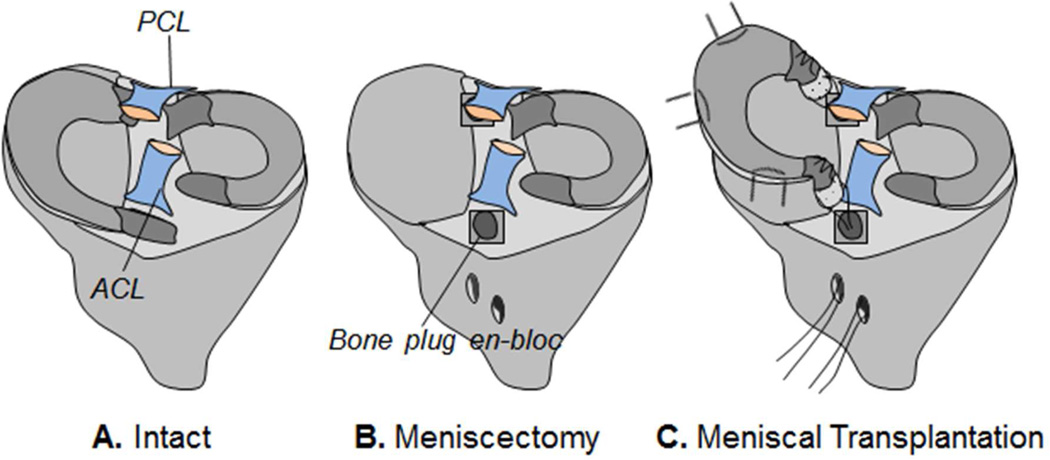
Contact forces applied to each individual sensel across the tibial plateau throughout simulated gait were recorded for three conditions (Fig. 2): 1) intact, 2) meniscectomy, where the medial meniscus was resected with bone plugs en-bloc, and 3) meniscal transplantation, where the bone plugs of the medial meniscus were reduced back to their respective insertions. In order to minimize the factor of graft size, geometry and material properties, a ‘remove-replace’ method was performed where the native medial meniscus was excised out of the knee in its entirety, and then fixed back in place using attachment techniques similar to that used clinically. First, the medial meniscus was circumferentially released at the meniscocapsular junction using a number-15 blade. The anterior and posterior horn attachment sites were then osteotomized to preserve bone plugs. Second, the removed meniscus was prepared by placing number 5 Ethibond sutures through a 2.7 mm tunnel through the center of each bone plug and a separate set of sutures through the anterior and posterior horns of the meniscus using a modified-Kessler configuration. Then, the bone plugs were reduced back to their respective insertions using the bone plug sutures.
Figure 2.
Schematic diagrams of three conditions tested: (A) intact, (B) medial meniscectomy, (C) medial meniscal transplantation using a “remove and replace” autograft with bone-plug fixation.
In addition, the meniscus was repaired back to the capsule using 2-0 Ethibond sutures placed in horizontal mattress configuration spaced evenly into the anterior horn, body and posterior horn of the meniscus.13 To avoid damaging the sensor during manipulation of the joint, the sensor was removed and then replaced in between conditions. A custom scribe was used to check the position of the Tekscan to make sure it was placed consistently for each condition.15
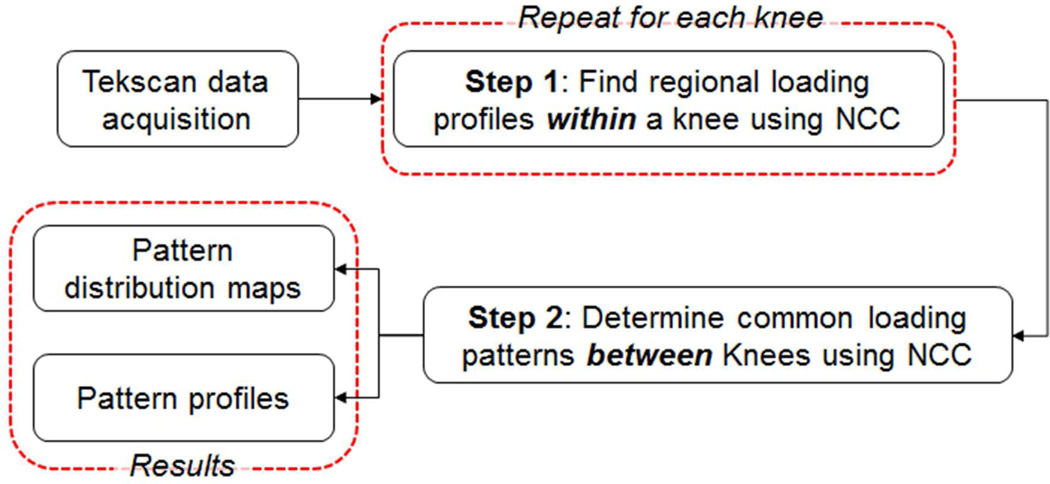
For each specimen and each test condition, the raw contact stress data from each individual sensel across the tibial plateau was low-pass filtered (4th order zero-lag low-pass Butterworth) with a cut-off frequency of 6 Hz to remove high-frequency noises. Within each condition (intact, meniscectomy or transplantation), the common loading patterns were determined using a Normalized Cross Correlation (NCC) algorithm following a published protocol.15 Briefly, the process consists of two steps (Fig. 3): the first step is to find regional loading patterns within each knee (Step 1); the second step is to determine common loading patterns across knees (Step 2). For Step 1, the loading profile at each sensel across the tibial plateau of each knee was compared using NCC – a metric of similarity between two profiles (0 ≤ NCC ≤ 1, 0-no match, 1-identical), and only those with NCC greater than a pre-selected threshold (0.93) were selected. The average loading profiles of sensels with NCC greater than the threshold were calculated as the knee specific regional loading pattern. For Step 2, the common loading patterns between knees were identified by combining profiles shared by a majority (≥75%) of the knees. A custom written program (MATLAB, MathWorks Inc., Natick, MA) was used for data analysis.15 After these two steps, a distribution map of the common loading profiles (Fig. 4) was created for the intact, meniscectomized and meniscal transplanted conditions.
Figure 3.
Flowchart showing our method of determining common loading patterns between different knees. NCC – normalized cross correlation.
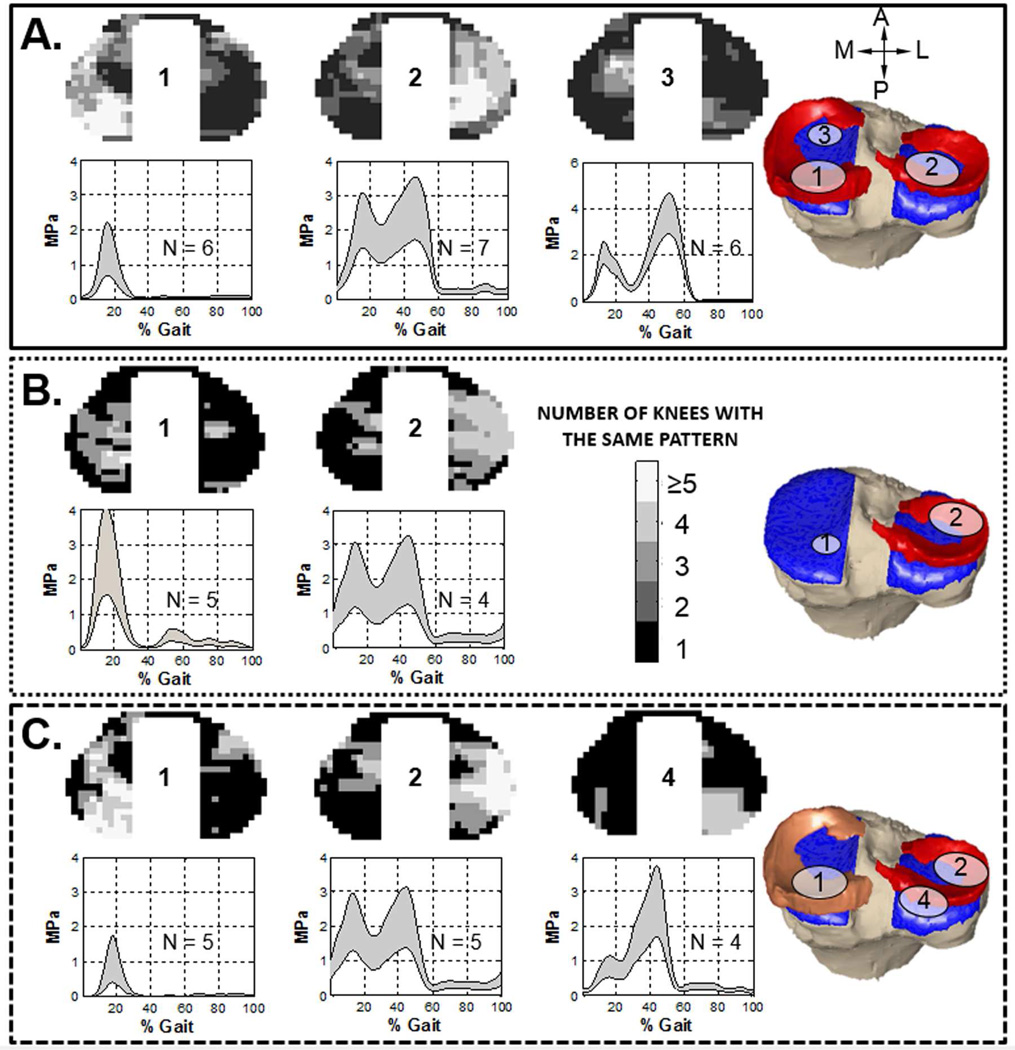
Figure 4.
The pattern distribution maps and loading profiles on the tibial plateau for the intact (A), medial meniscectomy (B), and meniscal transplanted (C) conditions. For the intact condition, three loading patterns were found. The greyscale color in pattern distribution map represented the number of knees that had the pattern in each area. The right-most illustration signified the region in which more than 75% of knees share this type of loading profile. The loading profile represented the weighted average (mean ± SD) of the contact stress within the pattern region during a gait cycle. The number of knees with each pattern was specified on the plot (i.e. N = 6). Pattern-3 was absent in both meniscectomy and meniscal transplanted conditions, while a new pattern (pattern-4) was found in meniscal transplanted condition.
For statistical analysis, the peak loads of each pattern and the area over which the pattern occurred were compared between intact, meniscectomized, and MAT conditions using the Kruskal-Wallis test. For the Kruskal-Wallis test with a significant statistic, post hoc analysis was performed by using the Dunn multiple comparison test. The level of significance was set at P < .05.
RESULTS
Three common regional loading patterns were found for the intact condition (Fig. 4A). The first pattern was located at the posterior aspect of the medial plateau (pattern-1). Pattern-1 consisted of a single peak at 14% of the gait cycle with an average magnitude of 1.30 ± 0.75 MPa (mean +/− standard deviation). Another pattern was found in central-posterior aspect of the lateral plateau (pattern-2). Pattern-2 had two peaks which occurred at 14% and 45% of the gait cycle; the magnitudes of the first and second peaks were 2.2 ± 0.71 MPa and 2.55 ± 0.85 MPa, respectively. Finally, a third pattern was found at the anterior aspect of the cartilage-to-cartilage contact region of the medial plateau (pattern-3), consisting of two peaks with the second peak (3.69 ± 0.92 MPa) significantly higher (P < .01) than the first peak (2.05 ± 0.57 MPa).
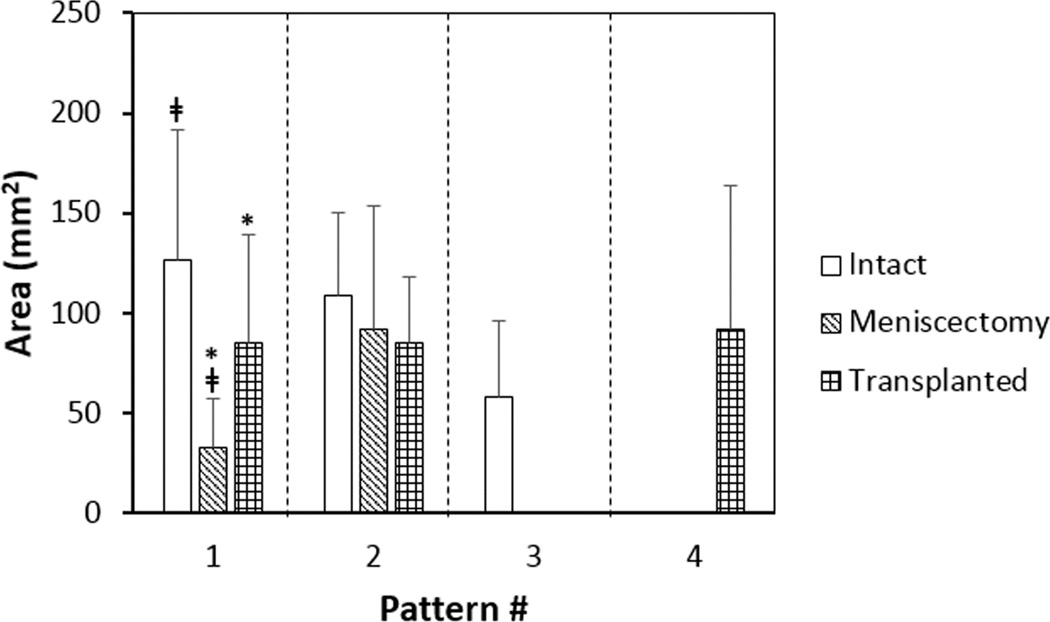
Following medial meniscectomy, the area in which pattern-1 occurred was significantly reduced (intact 126.3 ± 92.8 mm2, meniscectomy 32.7 ± 30.1 mm2, p < .01, Fig. 5), but the magnitude of the peak contact stress was significantly increased to 2.85 ± 1.11 MPa (P < .01, Fig. 4B). Pattern-3 at the anterior aspect of the cartilage-to-cartilage contact region of the medial tibial plateau was absent completely. On the lateral plateau, pattern-2 shifted anteriorly and laterally to the external region while both the magnitudes and the pattern area remained unchanged.
Figure 5.
The area (mean ± 95% confidence interval) of the region for each individual loading pattern for all three conditions. The area of pattern-1 was significantly reduced (‡p < 0.05) at meniscectomy condition, while no significant change was observed in the area of pattern-2. After meniscal transplantation, the area of pattern-1 was significantly increased (*p < 0.05), but still slightly smaller than the intact condition. The number of knees (N) with each pattern was shown in Fig. 4.
Following meniscal transplantation, the area over which pattern-1 occurred was increased to a level close to that of the intact condition (intact 126.3 ± 92.8 mm2, transplanted 85.3 ± 69.4 mm2, p >.05, Fig. 5). The magnitude of the single peak was 1.02 ± 0.71 MPa, which was slightly lower than that of the intact condition (P > .05, Fig. 4C). No further change was found in the location or profile magnitude of pattern-2 compared to the meniscectomy condition; however, a new commonly occurring loading pattern (pattern-4) was observed at the posterior aspect of the lateral tibial plateau. Pattern-4 profile has skewed double peaks – with the second peak (2.71 ± 1.0 MPa) significantly higher than the first (0.83 ± 0.31 MPa) (P < .01).
DISCUSSION
By way of a dynamic cadaveric test that mimics the activity of walking, we quantified the regional loading patterns on the tibial plateau in the medial meniscectomized and medial meniscal transplanted knee. For meniscectomized knees, we found significantly changes in the location and magnitude of loading profiles that commonly occur in intact knees, leading us to accept our first hypothesis. Upon meniscal transplantation, the commonly occurring loading profiles of the intact knee were not fully restored, leading us to reject our second hypothesis.
The effect of meniscectomy and MAT on joint contact mechanics has been extensively studied in static and quasi-static models11, 19–22 and more recently in dynamic23 and physiological cadaveric models.13, 24 Each study has quantified reduced contact area and increased contact stresses across the tibial plateau in the meniscectomized knee, and recently medial meniscectomy changes in contact mechanics have been localized to occur in the early-to-mid phase of stance13, 24. But, it remains unclear how changes in joint contact forces affect the health of the articular cartilage. It has been hypothesized that as joint contact mechanics change, chondrocytes cannot adapt to the alteration in forces. To explore this hypothesis, the effect of a single injurious compression at a high strain rate25, 26 or dynamic cyclic compressive loading has been investigated using in vitro tests.27 According to these studies, cartilage explants subjected to high load magnitudes and high strain rates exhibited a decrease in cell viability, decrease in biosynthesis of extracellular matrix, increase in chondrocyte apoptosis and increased level of glycosaminoglycan release. The effect of cyclic loading on collagen and proteoglycan content has also been tested in vivo using a rabbit model.28 In that study, cyclical joint loading with moderate magnitude (1~2 MPa) stimulated the proteoglycan synthesis in deep zone of the articular cartilage. More recently, Ko et al29 studied the effect of in vivo cyclic compression on joint degeneration. It was found that mechanical loading (4.5 N and 9 N) induced cartilage damage in both young and adult mice, and the severity of joint damage increased with the duration of the loading.29 However, for these experiments the simplified loads applied are not necessarily representative of the loads that articular cartilage experiences in vivo; thus, the clinical relevance of the data generated is still unclear.
In the meniscectomized knees, significant changes in the magnitude and location of regional loading patterns were quantified. At the posterior aspect of the medial plateau, the magnitude of regional loading profile, pattern-1, was significantly increased by 120%. This increased loading magnitude could damage the articular cartilage in this zone which is thinner compared to that of the central cartilage-to-cartilage contact region (1.8 ± 0.3 mm vs. 2.5 ± 0.6 mm).30 At the anterior aspect of the cartilage-to-cartilage contact region of the medial plateau, pattern-3 completely disappeared, suggesting the consistent loading within this region could shift to the different locations in the meniscectomized knees. This disrupted loading could endanger the cartilage in the adjacent regions where again, the cartilage is thinner30, 31 and considered not to be conditioned to withstand such high loads.17 In addition to the changes in the affected medial compartment, we also found accompanying changes in the lateral compartment, where pattern-2 shifted anterior-laterally. This may be due to the increased joint rotational laxity associated with meniscal deficiency.32 Although the loading magnitude was sustained, this shift in location could also be deleterious to cartilage at the new location, given it is much thinner than cartilage in the central and posterior regions (2.1 ± 0.4 mm vs 4.0 ± 0.9 mm and 3.1 ± 0.5 mm)30 where the loading pattern used to reside.
Meniscal allograft transplantation is the only therapeutic option for total meniscal replacement2, thus we tested the ability of MAT to restore intact knee joint loading patterns. Indeed, we saw a restored loading pattern at the posterior aspect of the medial tibial plateau after meniscal transplantation. However, the loading patterns at the other sites were not restored – which provides a potential explanation to a moderate rate (42% to 75%) of further cartilage degeneration following MAT according to previous clinical studies.33, 34 To the best of our knowledge, no study has investigated the cartilage loss following meniscal transplantation at a regional level. Thus, to better understand the clinical relevance of the current finding of incompletely restored regional loading patterns, the location of continued cartilage degeneration on the articular surface should be specified in future longitudinal study.
Several limitations need to be acknowledged. First, to test the function of MAT, we performed a “remove-replace” procedure, which represents a meniscal autograft condition, in which geometry and material properties are maintained, but fixation is not. While it is unlikely to find allograft meniscal tissue with such perfectly matched properties clinically, in this experimental study we chose this approach to isolate the effect of fixation technique on the outcome of the MAT procedure by using the specimens’ own meniscus, thus variation in graft sizing and material properties will not factor into differences noted in the study. Second, the sample size is relative small. Third, the knees were driven under the same load inputs, due to limited demographic information of the donors. Moreover, sensor degradation due to exposure to an aqueous environment was not accounted for. However, Jasson et al35 noted only a loss in sensor output of 4.6% per hour when exposed to an aqueous environment. Since the knees are tested in the following sequence: intact, repairs, and meniscectomized, the increase in loading seen in the meniscectomy condition can be attributed to meniscectomy and not due to sensor degradation. Additionally, in this study a normalized cross-correlation based algorithm was used to identify the common loading patterns - this method matches shapes and not magnitudes of load and therefore is not affected by sensor degradation.
In summary, all the three loading profiles which commonly occur in intact knees were altered following medial meniscectomy, and only the profile located at the posterior aspect of the medial plateau was restored by medial meniscal transplantation. The finding of alterations in regional loading patterns on articular cartilage following meniscectomy and transplantation helps better understand the effect of the subtle change in joint mechanics on the long-term health of articular cartilage.
Acknowledgements
Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number R01 AR057343. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also received from the Clark & Kirby Foundations and the Russell Warren Chair in Tissue Engineering. We would like to thank Dr. Peter Torzilli for advice on manuscript preparation.
Role of the funding source
The funding sources had no involvement in the study design, collection, analysis or interpretation of data, in the writing of the manuscript or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
All authors were involved in the conception and design of the study and the subsequent acquisition, analysis and interpretation of the data. All authors were involved in the drafting and critical revision for important intellectual content and final approval of the article. H. Wang takes full responsibility for the integrity of the work as a whole.
Conflict of interest
Authors have no conflict of interest.
REFERENCES
- 1.Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39:774–782. doi: 10.1177/0363546511398040. [DOI] [PubMed] [Google Scholar]
- 2.Rodeo SA. Meniscal allografts--where do we stand? Am J Sports Med. 2001;29:246–261. doi: 10.1177/03635465010290022401. [DOI] [PubMed] [Google Scholar]
- 3.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Fairbank TJ. Knee joint changes after meniscectomy. J Bone Joint Surg Br. 1948;30B:664–670. [PubMed] [Google Scholar]
- 5.Cicuttini FM, Forbes A, Yuanyuan W, Rush G, Stuckey SL. Rate of knee cartilage loss after partial meniscectomy. J Rheumatol. 2002;29:1954–1956. [PubMed] [Google Scholar]
- 6.McDermott I. Meniscal tears, repairs and replacement: their relevance to osteoarthritis of the knee. British journal of sports medicine. 2011;45:292–297. doi: 10.1136/bjsm.2010.081257. [DOI] [PubMed] [Google Scholar]
- 7.McCann L, Ingham E, Jin Z, Fisher J. Influence of the meniscus on friction and degradation of cartilage in the natural knee joint. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17:995–1000. doi: 10.1016/j.joca.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Arno S, Hadley S, Campbell KA, Bell CP, Hall M, Beltran LS, et al. The effect of arthroscopic partial medial meniscectomy on tibiofemoral stability. Am J Sports Med. 2013;41:73–79. doi: 10.1177/0363546512464482. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Maher SA, Spilker RL. Biphasic finite element contact analysis of the knee joint using an augmented Lagrangian method. Medical Engineering & Physics. 2013;35:1313–1320. doi: 10.1016/j.medengphy.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 11.Dienst M, Greis PE, Ellis BJ, Bachus KN, Burks RT. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35:34–42. doi: 10.1177/0363546506291404. [DOI] [PubMed] [Google Scholar]
- 12.Alhalki MM, Howell SM, Hull ML. How three methods for fixing a medial meniscal autograft affect tibial contact mechanics. Am J Sports Med. 1999;27:320–328. doi: 10.1177/03635465990270030901. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Gee AO, Hutchinson ID, Stoner K, Warren RF, Chen TO, et al. Bone Plug Versus Suture-Only Fixation of Meniscal Grafts: Effect on Joint Contact Mechanics During Simulated Gait. Am J Sports Med. 2014 doi: 10.1177/0363546514530867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottrell JM, Scholten P, Wanich T, Warren RF, Wright TM, Maher SA. A new technique to measure the dynamic contact pressures on the Tibial Plateau. J Biomech. 2008;41:2324–2329. doi: 10.1016/j.jbiomech.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Chen T, Torzilli P, Warren R, Maher S. Dynamic contact stress patterns on the tibial plateaus during simulated gait: A novel application of normalized cross correlation. J Biomech. 2014;47:568–574. doi: 10.1016/j.jbiomech.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedi A, Kelly NH, Baad M, Fox AJ, Brophy RH, Warren RF, et al. Dynamic contact mechanics of the medial meniscus as a function of radial tear, repair, and partial meniscectomy. J Bone Joint Surg Am. 2010;92:1398–1408. doi: 10.2106/JBJS.I.00539. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert S, Chen T, Hutchinson ID, Choi D, Voigt C, Warren RF, et al. Dynamic contact mechanics on the tibial plateau of the human knee during activities of daily living. J Biomech. 2013 doi: 10.1016/j.jbiomech.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Chen T, Koff MF, Hutchinson ID, Gilbert S, Choi D, et al. Image based weighted center of proximity versus directly measured knee contact location during simulated gait. J Biomech. 2014 doi: 10.1016/j.jbiomech.2014.04.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhalki MM, Hull ML, Howell SM. Contact mechanics of the medial tibial plateau after implantation of a medial meniscal allograft. A human cadaveric study. Am J Sports Med. 2000;28:370–376. doi: 10.1177/03635465000280031501. [DOI] [PubMed] [Google Scholar]
- 20.Ihn JC, Kim SJ, Park IH. In vitro study of contact area and pressure distribution in the human knee after partial and total meniscectomy. Int Orthop. 1993;17:214–218. doi: 10.1007/BF00194181. [DOI] [PubMed] [Google Scholar]
- 21.Kim JG, Lee YS, Bae TS, Ha JK, Lee DH, Kim YJ, et al. Tibiofemoral contact mechanics following posterior root of medial meniscus tear, repair, meniscectomy, and allograft transplantation. Knee Surg Sports Traumatol Arthrosc. 2013;21:2121–2125. doi: 10.1007/s00167-012-2182-4. [DOI] [PubMed] [Google Scholar]
- 22.McDermott ID, Lie DT, Edwards A, Bull AM, Amis AA. The effects of lateral meniscal allograft transplantation techniques on tibio-femoral contact pressures. Knee Surg Sports Traumatol Arthrosc. 2008;16:553–560. doi: 10.1007/s00167-008-0503-4. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Greve JM, Carter DR, Giori NJ. Meniscectomy alters the dynamic deformational behavior and cumulative strain of tibial articular cartilage in knee joints subjected to cyclic loads. Osteoarthritis Cartilage. 2008;16:1545–1554. doi: 10.1016/j.joca.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Chen T, Gee A, Hutchinson ID, Stoner K, Warren R, et al. Meniscal Allografts: Biomechanical Consequences of Different Methods of Fixation; ASME 2013 Summer Bioengineering Conference: American Society of Mechanical Engineers; 2013. pp. V01BT55A018–V001BT055A018. [Google Scholar]
- 25.Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 26.D'Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 27.Thibault M, Poole AR, Buschmann MD. Cyclic compression of cartilage/ bone explants in vitro leads to physical weakening, mechanical breakdown of collagen and release of matrix fragments. J Orthop Res. 2002;20:1265–1273. doi: 10.1016/S0736-0266(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 28.Saadat E, Lan H, Majumdar S, Rempel DM, King KB. Long-term cyclical in vivo loading increases cartilage proteoglycan content in a spatially specific manner: an infrared microspectroscopic imaging and polarized light microscopy study. Arthritis Res Ther. 2006;8:R147. doi: 10.1186/ar2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko FC, Dragomir C, Plumb DA, Goldring SR, Wright TM, Goldring MB, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, et al. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech (Bristol, Avon) 2005;20:736–744. doi: 10.1016/j.clinbiomech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. 2008;90:1922–1931. doi: 10.2106/JBJS.G.00748. [DOI] [PubMed] [Google Scholar]
- 33.Verdonk PC, Verstraete KL, Almqvist KF, De Cuyper K, Veys EM, Verbruggen G, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694–706. doi: 10.1007/s00167-005-0033-2. [DOI] [PubMed] [Google Scholar]
- 34.Vundelinckx B, Bellemans J, Vanlauwe J. Arthroscopically assisted meniscal allograft transplantation in the knee: a medium-term subjective, clinical, and radiographical outcome evaluation. Am J Sports Med. 2010;38:2240–2247. doi: 10.1177/0363546510375399. [DOI] [PubMed] [Google Scholar]
- 35.Jansson KS, Michalski MP, Smith SD, LaPrade RF, Wijdicks CA. Tekscan pressure sensor output changes in the presence of liquid exposure. J Biomech. 2013;46:612–614. doi: 10.1016/j.jbiomech.2012.09.033. [DOI] [PubMed] [Google Scholar]