Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is a comorbidity of childhood obesity.
Objective
We examined whole-body substrate metabolism and metabolic characteristics in obese adolescents with versus without NAFLD.
Subjects
Twelve obese (BMI≥95th) adolescents with and without NAFLD [intrahepatic triglyceride (IHTG) ≥5.0 % versus <5.0 %] were pair-matched for race, gender, age and % body fat.
Methods
Insulin sensitivity (IS) was assessed by a 3-hour hyperinsulinemic-euglycemic clamp and whole-body substrate oxidation by indirect calorimetry during fasting and insulin-stimulated conditions.
Results
Adolescents with NAFLD had increased (P<0.05) abdominal fat, lipids and liver enzymes compared with those without NAFLD. Fasting glucose concentration was not different between groups, but fasting insulin concentration was higher (P<0.05) in the NAFLD group compared with those without. Fasting hepatic glucose production and hepatic IS did not differ (P>0.1) between groups. Adolescents with NAFLD had higher (P<0.05) fasting glucose oxidation and a tendency for lower fat oxidation. Adolescents with NAFLD had lower (P<0.05) insulin-stimulated glucose disposal and lower peripheral IS compared with those without NAFLD. Although RQ increased significantly from fasting to insulin-stimulated conditions in both groups (main effect, P<0.001), the increase in RQ was lower in adolescents with NAFLD versus those without (interaction, P=0.037).
Conclusion
NAFLD in obese adolescents is associated with adverse cardiometabolic profile, peripheral insulin resistance and metabolic inflexibility.
Keywords: nonalcoholic fatty liver disease, visceral fat, insulin sensitivity, childhood obesity
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a serious comorbidity of childhood obesity, affecting ∼30% of obese children and adolescents (1-3). Previous studies have shown that independent of total fat, an increase in intrahepatic triglyceride (IHTG) is associated with metabolic syndrome (2, 4) and insulin resistance (2, 5, 6) in youth. Indeed, Wicklow et al. (2) observed that obese adolescents with NAFLD have 55% lower insulin sensitivity and a two-fold higher presence of metabolic syndrome compared with obese adolescents without NAFLD. Further, an increasing degree of fatty liver in obese adolescents, independent of total fat, is associated with the presence of prediabetes (e.g., impaired glucose tolerance and impaired fasting glucose), visceral obesity and anti-and proinflammatory markers (4).
In healthy, lean individuals, skeletal muscle displays substantial metabolic flexibility based on fuel availability and metabolic demand (7). It has the capacity to switch from predominantly fat oxidation during postabsorptive conditions to predominantly glucose oxidation, uptake and storage during insulin-stimulated conditions (7). Conversely, in insulin-resistant individuals, the ability to switch substrate oxidation is impaired, such that obese and type 2 diabetic patients have reduced fat oxidation during postabsorptive conditions and their ability to suppress fat oxidation and increase glucose oxidation and uptake is diminished during insulin-stimulated states known as “metabolic inflexibility” (7, 8). In support of this notion, Kelley et al. (8) demonstrated that fasting leg respiratory quotient (RQ) in obese adults was significantly elevated during fasting conditions and remained unchanged during the hyperinsulimic-euglycemic clamp in comparison to their lean counterparts. Similarly, in type 2 diabetic patients, postabsorptive leg RQ was higher and rates of lipid oxidation by skeletal muscle were lower, while glucose oxidation was increased compared with healthy controls (9).
Although previous studies have attempted to examine whole-body substrate oxidation and its relationship with metabolic characteristics in adults with NAFLD, the findings have been inconsistent to date (10-13). Croci et al. (10) reported that adults with NAFLD have significantly lower fasting whole-body fat oxidation and higher glucose oxidation, and the increases in RQ from fasting to insulin-stimulated conditions were reduced compared with their lean counterparts. By contrast, Bugianesi et al. (13) demonstrated that both fasting and insulin-stimulated whole-body fat oxidation is higher in adults with NAFLD compared with their BMI-matched controls. Others (11, 12) observed no differences in fasting carbohydrate and fat oxidation between adults with and without NAFLD.
To our knowledge, we are aware of only one study wherein whole-body substrate oxidation was examined in youth with NAFLD. Perseghin et al. (3) have shown that obese adolescents with NAFLD (IHTG ≥ 5%) have significantly higher fasting RQ and blunted increases in RQ during an oral glucose challenge compared with obese adolescents without NAFLD (IHTG < 5%). Currently, no studies have comprehensively examined whole-body substrate oxidation during fasting and insulin-stimulated hyperinsulinemic-euglycemic clamp conditions in adolescents with NAFLD. In this study, we employed a hyperinsulinemic-euglycemic clamp in conjunction with stable isotope tracers to examine in vivo hepatic and peripheral insulin sensitivity, fasting hepatic glucose production and whole-body substrate metabolism in obese adolescents with and without NAFLD matched for race, gender, age and total adiposity.
Methods
Participants
Participants were recruited for lifestyle intervention studies (14, 15) via flyers in the public transportation system and posters placed on campus, and from the Weight Management and Wellness Center at Children's Hospital of Pittsburgh (CHP) of UPMC. To be eligible, subjects had to be obese (BMI ≥ 95th percentile for age and gender), 12-18 years of age, pubertal (Tanner Stages III-V), non-smokers, non-diabetic and physically inactive. Exclusion criteria included participation in structured exercise, significant weight change, endocrine disorders (e.g., diabetes, polycystic ovary syndrome), syndromic obesity, psychiatric disorders and use of medications (including oral and injectable contraceptives) known to affect glucose or fat metabolism and body composition. None of the subjects consumed alcoholic beverages nor had history of liver diseases.
Among 73 obese adolescents who had pre-intervention liver fat measurement by proton magnetic resonance spectroscopy (1H-MRS) as part of the intervention studies (14, 15), 12 obese adolescents (7 males and 5 females) had NAFLD [intrahepatic triglycerides (IHTG) ≥5.0 %] (3) assessed by proton magnetic resonance spectroscopy (1H-MRS). Twelve obese adolescents with NAFLD were pair matched to 12 obese adolescents without NAFLD (IHTG <5.0 %) for race, gender, age (within 2 years) and % body fat (within 4%). Parental informed consent and child assent were obtained from all participants before participation. The investigation was approved by the Institutional Review Board and performed during an overnight admission in the Pediatric Clinical and Translational Research Center (PCTRC) at CHP.
Anthropometrics
Body weight was measured to the nearest 0.1 kg and height was measured to the nearest 0.1 cm. Waist circumference was measured at the top of the iliac crest and the average of two measurements was used in the analyses.
Total and abdominal fat
Fat free mass and total % body fat were assessed by dual energy X-ray absorptiometry using lunar iDXA (GE Healthcare, Madison, WI, USA). MRI was obtained with a 3.0 Tesla MR scanner (Siemens, Magnetom TIM Trio) to quantify visceral and abdominal subcutaneous fat as shown previously (16).
Oral glucose tolerance test
Participants reported to the PCTRC after an overnight fast for a 2-hour oral glucose tolerance test (OGTT, 1.75 g/kg, max 75 g) as shown previously (14, 15). Blood samples were obtained at −15, 0, 15, 30, 60, 90 and 120 minutes for determination of glucose and insulin concentrations. Glucose and insulin area under the curve (AUC) was determined using a trapezoid model (17). The following morning subjects underwent the euglycemic clamp test.
Measurements of hepatic and peripheral insulin sensitivity and substrate oxidation
All participants had a 3-hr hyperinsulinemic (80 mU/m2/min)-euglycemic clamp after a 10-12 hr overnight fast, except one white boy with NAFLD whose clamp test was not completed due to difficulty with IV access. Fasting endogenous glucose production was measured with a primed (2.2 μmol/kg) constant-rate infusion of [6, 6-2H2]glucose (Isotech, Miamisburg, OH) from 0730–0930 h as reported previously (18). Blood was sampled at the start of the stable isotope infusion (−120 min) and every 10 min from −30 to 0 min (basal period) for determination of plasma glucose and insulin concentrations and isotopic enrichment of glucose. Fasting hepatic glucose production (HGP) was calculated during the last 30 min (−30 to time zero) of the basal 2-h infusion period. Fasting hepatic insulin sensitivity was calculated as the inverse of the product of hepatic glucose production and fasting plasma insulin concentration (1,000/HGP × fasting plasma inulin) as shown previously (18). After the 2-h baseline isotope infusion period, insulin-stimulated glucose uptake and insulin sensitivity were measured during a 3-h hyperinsulinemic-euglycemic clamp from 0930-1230 h. Intravenous crystalline insulin (Humulin; Lilly Indianapolis, IN) was infused at a constant rate of 80 mU/m2 per min, and plasma glucose was clamped at 100 mg/dl with a variable-rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min. Peripheral insulin sensitivity was calculated by dividing insulin-stimulated glucose disposal rate by the steady-state plasma insulin concentration during the last 30 min of the clamp. Indirect calorimetry was performed using a ventilated hood system (Parvo Medics, Salt Lake City, UT) for 30-minutes before starting (−30 to 0 min, fasting condition) and at the end of the euglycemic clamp (150-180 min, insulin-stimulated condition) and substrate oxidation was calculated based on Frayn formulas (19).
IHTG content by 1H-MRS
1H-MRS spectra were acquired with a 3.0 Tesla MR system (Siemens, Tim Trio, Erlangen, Germany) using a body matrix coil and a spine matrix (Siemens, Erlangen, Germany) as shown previously (14, 15). Liver spectra were fitted using the AMARES algorithm in the Java-based magnetic resonance user interface (jMRUI) software package (20). The average of eight spectra was used for liver triglyceride calculation. NAFLD was defined as IHTG ≥5.0% as shown previously in adolescents (3).
Statistical analyses
Statistical procedures were performed using SPSS (Version 20; SPSS, Inc., Chicago, IL). Independent t-tests were used to compare physical and metabolic characteristics between obese adolescents with versus without NAFLD groups. A repeated-measures ANOVA was used to examine main effects (group, condition) and group interactions (group × condition) for substrate oxidation and insulin stimulated glucose disposal. Statistical significance was set at P<0.05. All data are presented as means ± SEM.
Results
Body composition and metabolic data are shown in Table 1. By design, IHTG (%) was significantly (P<0.001) higher in obese adolescents with versus without NAFLD with no differences in age, Tanner stage and total % body fat. Despite similar total % body fat, obese adolescents with NAFLD had increased waist circumference (P=0.003) and visceral fat (P=0.038) compared with obese adolescents without NAFLD. Additionally, total cholesterol (P=0.006), triglycerides (P=0.007) and VLDL (P=0.007) were higher in obese adolescents with versus without NAFLD. Liver enzymes [alanine aminotransferase (P=0.005) and aspartate aminotransferase (P=0.035)] were higher in obese adolescents with NAFLD than those without.
Table 1. Anthropometric and body composition in obese adolescents with (NAFLD +) and without NAFLD (NAFLD −).
| NAFLD − | NAFLD + | P | |
|---|---|---|---|
| n | 12 | 12 | |
| IHTG (%) | 1.5 ± 0.3 | 9.4 ± 0.9 | <0.001 |
| Male/female (n) | 7/5 | 7/5 | |
| Black/white (n) | 2/10 | 2/10 | |
| IGT (n)* | 2 | 4 | 0.640 |
| Age (yrs) | 14.6 ± 0.4 | 15.1 ± 0.4 | 0.340 |
| Tanner stage, 3/4/5 (n) | 3/1/8 | 0/2/10 | 0.169 |
| BMI (kg/m2) | 34.6 ± 1.1 | 38.1 ± 1.4 | 0.059 |
| Waist circumference (cm) | 108.5 ± 2.4 | 119.5 ± 2.2 | 0.003 |
| Body fat (%) | 44.0 ± 1.3 | 44.9 ± 1.4 | 0.652 |
| Fat free mass (kg) | 52.6 ± 2.8 | 58.8 ± 1.8 | 0.078 |
| Visceral AT (cm2) | 68.7 ± 5.5 | 90.1 ± 7.8 | 0.038 |
| Abdominal SAT (cm2) | 472.7 ± 43.1 | 586.2 ± 38.4 | 0.062 |
| Cholesterol (mg/dl) | 133.2 ± 6.8 | 165.4 ± 8.2 | 0.006 |
| Triglycerides (mg/dl) | 75.7 ± 7.4 | 135.6 ± 18.5 | 0.007 |
| HDL (mg/dl) | 37.4 ± 1.9 | 40.2 ± 2.6 | 0.391 |
| LDL (mg/dl) | 80.7 ± 6.2 | 98.1 ± 7.3 | 0.082 |
| VLDL (mg/dl) | 15.1 ± 1.5 | 27.1 ± 3.7 | 0.007 |
| ALT (IU/l)** | 18.9 ± 1.8 | 35.4 ± 4.6 | 0.005 |
| AST (IU/l)** | 21.4 ± 1.3 | 28.8 ± 2.8 | 0.035 |
| OGTT variables | |||
| Fasting glucose (mg/dl) | 89.0 ± 1.4 | 90.0 ± 2.1 | 0.691 |
| Fasting insulin (μU/ml) | 22.3 ± 2.7 | 36.9 ± 4.9 | 0.016 |
| Glucose at 120 min (mg/dl) | 116.3 ± 5.1 | 137.1 ± 5.8 | 0.014 |
| Insulin at 120 min (μU/ml) | 77.6 ± 9.3 | 244.1 ± 52.5 | 0.005 |
| Glucose AUC (mg·min/dl) | 15125.4 ± 481.3 | 16818.8 ± 623.7 | 0.043 |
| Insulin AUC (μU·min/ml) | 12382.6 ± 1142.1 | 24157.9 ± 3843.4 | 0.008 |
| Insulinogenic Index | 2.6 ± 0.5 | 3.4 ± 0.7 | 0.376 |
| OGTT-Disposition Index | 0.12 ± 0.01 | 0.10 ± 0.02 | 0.499 |
Mean ± SEM. IHTG, intrahepatic triglyceride; IGT, impaired glucose tolerance; SAT, subcutaneous adipose tissue; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the curve.
2-hr post-OGTT glucose of ≥140–199 mg/dl according to ADA criteria (21).
n=10 (NAFLD−).
Oral glucose tolerance
Four subjects with NAFLD (1 male, 3 females) and two subjects without NAFLD (1 male, 1 female) had impaired glucose tolerance during the OGTT based on the 2-hr post-OGTT glucose level of ≥140–199 mg/dl according to ADA criteria (21). The 2-hr glucose concentrations (P=0.014) and glucose AUC (P=0.043) were significantly higher in obese adolescents with NAFLD versus without NAFLD (Table 1). Further, obese adolescents with NAFLD had higher fasting insulin (P=0.016), 2-hr insulin (P=0.005) and insulin AUC (P=0.008) than those without NAFLD.
Hepatic and peripheral insulin sensitivity
During the postabsorptive period, fasting glucose concentration was not different between obese adolescents with versus without NAFLD, but insulin concentration was significantly (P=0.023) higher in the NAFLD group (Table 2). Fasting hepatic glucose production and hepatic insulin sensitivity did not differ (P>0.1) between groups. During the final 30 min of the 3-h hyperinsulinemic-euglycemic clamp, steady-state plasma glucose and insulin concentrations did not differ (P>0.1) between groups. However, obese adolescents with NAFLD had significantly lower peripheral insulin sensitivity (Table 2) and insulin-stimulated glucose disposal (9.6 ± 0.9 versus 13.6 ± 0.8 mg/kgFFM/min, P=0.004, Figure 1) compared with those without NAFLD. The difference in insulin-stimulated glucose disposal between groups was attributed to the lower non-oxidative glucose disposal (5.1 ± 0.9 versus 9.5 ± 0.9 mg/kgFFM/min, P=0.002) in obese adolescents with NAFLD (Figure 1).
Table 2. Metabolic parameters in the basal postabsorptive state and during the hyperinsulinemic-euglycemic clamp in obese adolescents with (NAFLD +) and without NAFLD (NAFLD −).
| NAFLD − | NAFLD + | P | |
|---|---|---|---|
| Basal postabsorptive period | |||
| Glucose (mg/dl) | 92.5 ± 1.0 | 93.3 ± 1.9 | 0.709 |
| Insulin (μU/ml) | 22.7 ± 2.3 | 35.0 ± 4.5 | 0.023 |
| HGP (mg/kg/min) | 2.2 ± 0.2 | 2.0 ± 0.1 | 0.403 |
| Hepatic insulin sensitivity (mg/kgFFM/min per μU/ml)−1 | 43.9 ± 5.1 | 33.1 ± 5.6 | 0.167 |
| Energy expenditure (kcal/24h/kgFFM) | 36.7 ± 1.3 | 38.1 ± 1.3 | 0.485 |
| Respiratory quotient | 0.78 ± 0.02 | 0.82 ± 0.01 | 0.045 |
| Fat oxidation (mg/kgFFM/min) | 2.0 ± 0.2 | 1.5 ± 0.1 | 0.133 |
| Glucose oxidation (mg/kgFFM/min) | 1.4 ± 0.4 | 2.7 ± 0.4 | 0.019 |
| Final 30 min of the 3-h hyperinsulinemic-euglycemic clamp | |||
| Glucose (mg/dl) | 100.8 ± 0.6 | 100.5 ± 0.5 | 0.676 |
| Insulin (μU/ml) | 249.8 ± 18.1 | 323.9 ± 51.8 | 0.176 |
| Peripheral insulin sensitivity (mg/kgFFM/min per μU/ml) | 5.8 ± 0.6 | 3.8 ± 0.8 | 0.043 |
| Energy expenditure (kcal/24h/kgFFM) | 40.5 ± 1.5 | 39.6 ± 1.5 | 0.674 |
| Respiratory quotient | 0.87 ± 0.02 | 0.89 ± 0.02 | 0.575 |
| Fat oxidation (mg/kgFFM/min) | 1.1 ± 0.2 | 0.8 ± 0.2 | 0.383 |
| Glucose oxidation (mg/kgFFM/min) | 4.2 ± 0.4 | 4.5 ± 0.5 | 0.672 |
| Non-oxidative glucose disposal (mg/kgFFM/min) | 9.5 ± 0.9 | 5.1 ± 0.9 | 0.002 |
Mean ± SEM. HGP, hepatic glucose production. FFM, fat free mass.
Figure 1.
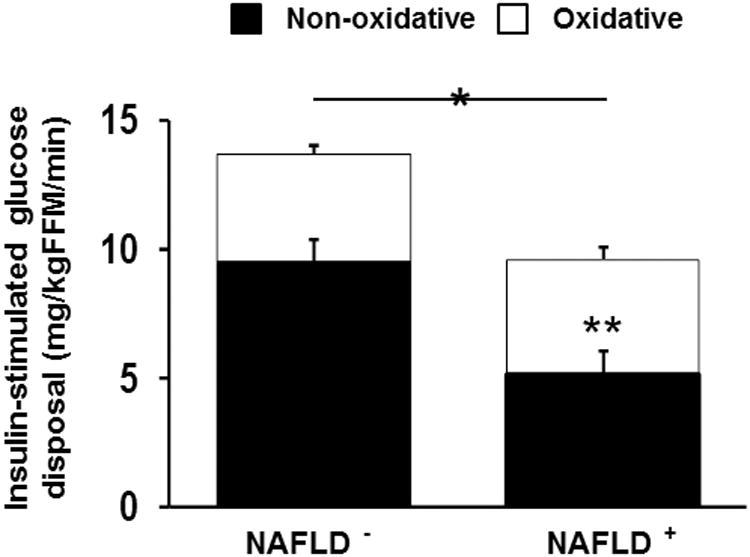
Insulin-stimulated glucose disposal during the last 30 min of the hyperinsulinemic-euglycemic clamp. Solid bars represent non-oxidative glucose disposal and open bars oxidative glucose disposal. * Insulin-stimulated glucose disposal is lower in obese adolescents with NAFLD compared with obese adolescents without NAFLD (P<0.05). ** Non-oxidative glucose disposal is lower in obese adolescents with NAFLD compared with obese adolescents without NAFLD (P<0.05).
Substrate oxidation during the postabsorptive period
After adjusting for FFM, total energy expenditure did not differ (P>0.1) between groups. However, the proportion of energy expenditure derived from glucose and fat was different between groups, such that obese adolescents with NAFLD had significantly higher fasting glucose oxidation (2.7 ± 0.4 versus 1.4 ± 0.4 mg/kgFFM/min, P=0.019) and tendency for lower fat oxidation (1.5 ± 0.1 versus 2.0 ± 0.2 mg/kgFFM/min, P=0.133) compared with those without NAFLD (Table 2). Accordingly, the fasting RQ was significantly higher in adolescents with NAFLD than those without NAFLD (0.82 ± 0.01 versus 0.78 ± 0.02, P=0.045). Fasting RQ was positively correlated with IHTG (%) (r=0.50, P=0.016) in the two groups combined.
Substrate oxidation during hyperinsulinemic-euglycemic clamp
During the insulin-stimulated conditions, there was no difference (P>0.1) in total energy expenditure between groups (Table 2). However, a switch in substrate oxidation was noted, such that independent of group, there was a significant reduction in fat oxidation (Figure 2A) and increase in glucose oxidation (Figure 2B) compared to the postabsorptive period (main effect, P<0.001 for both). Obese adolescents with NAFLD increased glucose oxidation to a lesser degree than those without NAFLD (Figure 2B: condition × group, P=0.015). There was a significant increase in RQ from fasting to insulin-stimulated conditions in both groups (main effect, P<0.001). However, the increase in RQ was significantly lower in obese adolescents with NAFLD (Δ 0.07 ± 0.015) compared with obese adolescents without NAFLD (Δ 0.102 ± 0.007) (condition × group, P=0.037). In both groups, energy expenditure (kcal/24h/kgFFM) increased during insulin-stimulated conditions (main effect, P<0.001), and the increase in energy expenditure was lower in adolescents with NAFLD compared with those without NAFLD (condition × group, P=0.05).
Figure 2.
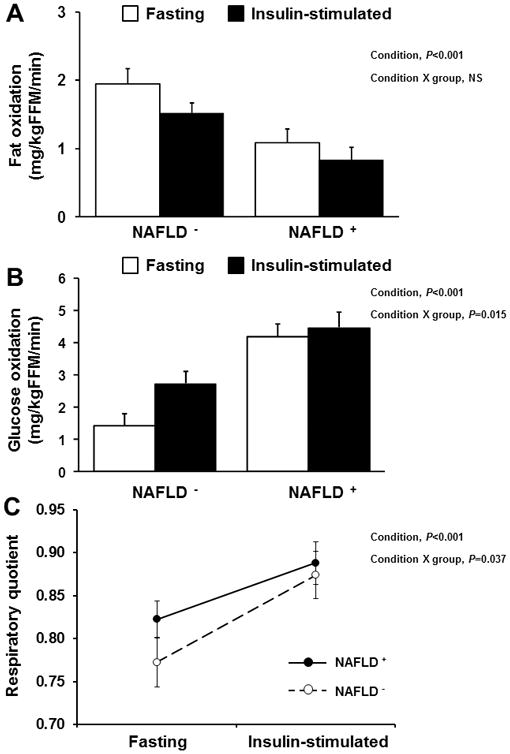
Fat oxidation (A), glucose oxidation (B) and respiratory quotient (C) during fasting and insulin-stimulated conditions in obese adolescents with verses without NAFLD.
Discussion
In this study, we compared whole-body energy metabolism and metabolic characteristics in obese adolescents with and without NAFLD as determined by 1H-MRS. With the use of the hyperinsulinemic-euglycemic clamp combined with stable isotope tracers and indirect calorimetry, we found that NAFLD in adolescents is associated with fasting hyperinsulinemia, decreased peripheral insulin sensitivity and metabolic inflexibility manifested by a blunted increase in RQ from the fasting to insulin-stimulated conditions when compared with pair-matched obese adolescents without NAFLD.
Previously, Wicklow et al. (2) demonstrated that IHTG is significantly associated with insulin sensitivity measured by an intravenous glucose tolerance test in obese adolescents, and obese adolescents with NAFLD have 55% lower insulin sensitivity compared with their obese healthy controls. Using the hyperinsulinemic-euglycemic clamp, Deivanayagam et al. (22) have shown that obese adolescents with NAFLD have a significantly lower peripheral glucose uptake compared with BMI-matched obese healthy controls. By combining the hyperinsulinemic-euglycemic clamp with indirect calorimetry and stable isotopes, our data extends the previous observations in adolescents (2, 22) and demonstrates that NAFLD is associated with reductions in peripheral insulin-stimulated glucose uptake. Our finding of significantly lower non-oxidative glucose disposal in obese adolescents with NAFLD (∼30%) compared with those without, may suggest a defect in skeletal muscle glycogen synthesis (23) in the former group. Although the mechanisms responsible for the association between elevated IHTG and skeletal muscle insulin resistance are unclear, it appears that skeletal muscle insulin resistance may occur earlier than NAFLD (24). Petersen et al. (24) demonstrated that insulin resistance in skeletal muscle, due to decreased skeletal muscle glycogen synthesis, promotes atherogenic dyslipidemia by promoting the conversion of energy derived from ingested carbohydrate into hepatic de novo lipogenesis, resulting in hypertriglyceridemia and NAFLD. However, our data are countered by Deivanayagam et al. (22) who demonstrated that obese adolescents with NAFLD also have lower hepatic insulin sensitivity compared with obese healthy controls. Although we cannot be certain, it is possible that the different findings could be attributed to the differences in the cutoffs used to define NAFLD between studies. In Deivanayagam et al.'s study (22), NAFLD is defined as IHTG ≥10% (means ± SEM: 28.4 ± 3.6%) compared with the ≥5.0% cutoff used in this present study, which is the similar criteria used in adults (25), resulting in a threefold greater IHTG content compared to our obese adolescents with NAFLD (means ± SEM: 9.4 ± 0.9%). Indeed, a previous study demonstrated that the increasing severity of NAFLD is associated with the degree of glucose dysregulation and inflammatory markers in obese adolescents (4).
During postabsorptive conditions, lipids are the primary substrate oxidized by skeletal muscle in healthy individuals, explaining ∼80% of oxygen consumption (26). It is suggested that reduced postabsorptive fat oxidation is one of the contributing factors leading to positive energy balance and weight gain (27). Kelley et al. (7, 8) showed that obese adults manifested less lipid oxidation during postabsorptive conditions and greater lipid oxidation during insulin-stimulated conditions compared with their lean counterparts, thus displaying metabolic inflexibility. Similarly, we found that obese adolescents with NAFLD showed a significantly higher fasting RQ compared with their obese counterparts without NAFLD, indicating that a higher proportion of whole-body resting energy expenditure is derived from carbohydrate and not from lipid. Additionally, combined across both groups, we observed that IHTG (%) was positively correlated with fasting RQ (r=0.50, P<0.05), which remained true after accounting for total and visceral fat (r=0.54, P=0.01). Further, our finding that the RQ increased less during the insulin-stimulated steady states in adolescents with NAFLD versus those without NAFLD is novel and extends the previous findings by Perseghin et al. (3) who examined the effect of NAFLD on whole-body energy metabolism in response to a 3-hour OGTT in obese adolescents. In that study (3), a significantly higher fasting RQ was observed in obese adolescents with NAFLD, and the increase in RQ in response to a 75-gram OGTT was lower in adolescents with NAFLD compared with those without NAFLD. Taken together, these observations suggest that NAFLD in obese youth is associated with altered basal and insulin-stimulated whole-body substrate metabolism.
Our study suggests that despite similar % body fat, obese adolescents with NAFLD have increased cardiometabolic risk factors compared with their matched controls. We demonstrated that obese adolescents with NAFLD had increased abdominal obesity, in particular visceral fat, and had adverse lipid profiles, findings similar to previous studies in adults (10, 28) and adolescents (4, 22). Given the strong associations between visceral fat and IHTG in obese adolescents (4, 14), it is important to develop intervention strategies to target both fat depots to improve obesity-related health risks in youth. Currently, diet and exercise are the first line of approach to treat youth with NAFLD since they do not carry side effects and confer multiple cardiometabolic health benefits (29, 30). Moderate diet-induced weight loss (8% of initial body weight) was associated with significant reductions in IHTG (62%) and improvements in both hepatic (56%) and peripheral (97%) insulin sensitivity in obese adolescents (31). Further, we recently demonstrated that engaging in aerobic exercise, independent of calorie restriction, is also an effective strategy to reduce visceral fat and IHTG in previously sedentary, obese adolescents (14, 15).
In this study, we examined fasting hepatic and insulin-stimulated peripheral insulin sensitivity and whole-body substrate metabolism in pair matched obese adolescents with versus without NAFLD using state-of-the-art methodologies including hyperinsulinemic-euglycemic clamp combined with stable isotopes, 1H-MRS and indirect calorimetry. However, our findings are based on cross-sectional observations and thus do not allow us to infer a causal relationship. Although participants were pair-matched based on total % body fat measured by DEXA, BMI tended to be higher in subjects with NAFLD than those without NAFLD. Further, the lack of a normal-weight control group is a limitation in this study.
In summary, NAFLD in obese adolescents is associated with metabolic inflexibility, with an increased resting RQ and a blunted increase in RQ during insulin-stimulated conditions, together with increased cardiometabolic risk factors and peripheral insulin resistance compared with obese peers without NAFLD. Altered basal and insulin-stimulated whole-body substrate oxidation may contribute to defects in skeletal muscle glucose uptake in obese adolescents with NAFLD. A previous adult study demonstrated a significant improvement in postabsorptive fat oxidation in obese, insulin-resistant individuals in response to moderate-intensity physical activity combined with caloric reduction (32). Whether regular exercise is associated with improvement in postabsorptive fat oxidation and metabolic flexibility in obese adolescents with NAFLD is unknown and warrants investigation.
Acknowledgments
The authors express their gratitude to the study participants and their parents, to Nancy Guerra (Nurse practitioner), Resa Stauffer (Laboratory research technician) and the PCTRC nursing staff. This research was funded by the American Diabetes Association (S.L.,7-08-JF-27), Department of Defense (S.L., FA7014-02-2-001), National Institutes of Health through Grant Numbers 1R21DK083654-01A1 (S.L.), UL1 RR024153, and Cochrane-Weber Foundation at Children's Hospital of Pittsburgh (S.L.).
Footnotes
The authors' contributions were as follows. S.L. designed the study, obtained funding, researched data and wrote the manuscript. C.B. assisted MRS data analyses and acquisition and reviewed the manuscript. H.A assisted with data maintenance and analyses and reviewed the manuscript. M.R. and I.L. researched data and reviewed the manuscript. S.L. is the guarantor of this work, had full access to all the data and takes full responsibility for the integrity of data and the accuracy of data analysis.
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Wicklow BA, Wittmeier KD, MacIntosh AC, Sellers EA, Ryner L, Serrai H, et al. Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Diabetes Care. 2012;35:905–10. doi: 10.2337/dc11-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perseghin G, Bonfanti R, Magni S, Lattuada G, De Cobelli F, Canu T, et al. Insulin resistance and whole body energy homeostasis in obese adolescents with fatty liver disease. American journal of physiology Endocrinology and metabolism. 2006;291:E697–703. doi: 10.1152/ajpendo.00017.2006. [DOI] [PubMed] [Google Scholar]
- 4.Cali AM, De Oliveira AM, Kim H, Chen S, Reyes-Mugica M, Escalera S, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896–903. doi: 10.1002/hep.22858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett B, Larson-Meyer DE, Ravussin E, Volaufova J, Soros A, Cefalu WT, et al. Impaired insulin sensitivity and elevated ectopic fat in healthy obese vs. nonobese prepubertal children. Obesity (Silver Spring) 2011;20:371–5. doi: 10.1038/oby.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittmeier KD, Wicklow BA, MacIntosh AC, Sellers EA, Ryner LN, Serrai H, et al. Hepatic steatosis and low cardiorespiratory fitness in youth with type 2 diabetes. Obesity (Silver Spring) 2012;20:1034–40. doi: 10.1038/oby.2011.379. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–83. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 8.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. The American journal of physiology. 1999;277:E1130–41. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 9.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. The Journal of clinical investigation. 1994;94:2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croci I, Byrne NM, Choquette S, Hills AP, Chachay VS, Clouston AD, et al. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut. 2013;62:1625–33. doi: 10.1136/gutjnl-2012-302789. [DOI] [PubMed] [Google Scholar]
- 11.Kotronen A, Seppala-Lindroos A, Vehkavaara S, Bergholm R, Frayn KN, Fielding BA, et al. Liver fat and lipid oxidation in humans. Liver international : official journal of the International Association for the Study of the Liver. 2009;29:1439–46. doi: 10.1111/j.1478-3231.2009.02076.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 13.Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–42. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61:2787–95. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Deldin AR, White D, Kim Y, Libman I, Rivera-Vega M, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. American journal of physiology Endocrinology and metabolism. 2013;305:E1222–9. doi: 10.1152/ajpendo.00285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Kim Y, Kuk JL, Boada FE, Arslanian S. Whole-body MRI and ethnic differences in adipose tissue and skeletal muscle distribution in overweight black and white adolescent boys. Journal of obesity. 2011;2011:159373. doi: 10.1155/2011/159373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes care. 1995;18:245–50. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 18.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes care. 2004;27:547–52. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 19.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 20.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, et al. Java-based graphical user interface for the MRUI quantitation package. Magma. 2001;12:141–52. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 21.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care. 2003;25(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 22.Deivanayagam S, Mohammed BS, Vitola BE, Naguib GH, Keshen TH, Kirk EP, et al. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr. 2008;88:257–62. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. The New England journal of medicine. 1990;322:223–8. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 24.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–94. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 26.Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. The Journal of clinical investigation. 1976;58:421–31. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. The American journal of physiology. 1990;259:E650–7. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 28.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 29.Lindback SM, Gabbert C, Johnson BL, Smorodinsky E, Sirlin CB, Garcia N, et al. Pediatric nonalcoholic fatty liver disease: a comprehensive review. Advances in pediatrics. 2010;57:85–140. doi: 10.1016/j.yapd.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Alisi A, Nobili V. Non-alcoholic fatty liver disease in children now: lifestyle changes and pharmacologic treatments. Nutrition. 2012;28:722–6. doi: 10.1016/j.nut.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Vitola BE, Deivanayagam S, Stein RI, Mohammed BS, Magkos F, Kirk EP, et al. Weight loss reduces liver fat and improves hepatic and skeletal muscle insulin sensitivity in obese adolescents. Obesity. 2009;17:1744–8. doi: 10.1038/oby.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–7. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 33.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. The Journal of pediatrics. 2012;161:51–7. doi: 10.1016/j.jpeds.2011.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


