Abstract
Background
Eosinophilic gastroenteritis is a rare condition where eosinophilic inflammation occurs in the gastrointestinal tract in the absence of secondary causes. Little is known regarding aetiology, pathogenesis, or natural history.
Aims
To characterize the clinical, endoscopic, and histopathologic features of eosinophilic gastroenteritis and to summarize treatment outcomes.
Methods
Pathologic reports of all patients who had undergone upper endoscopy with biopsy between January 1, 2000 and June 20, 2013 were reviewed. Eosinophilic gastroenteritis was diagnosed if there were ≥20 eosinophils/hpf on either gastric of duodenal biopsy, symptoms attributable to the gastrointestinal tract, and no known secondary cause of eosinophilia. Descriptive statistics characterized patients diagnosed with eosinophilic gastroenteritis and bivariate analysis compared adults and children.
Results
There were 44 patients diagnosed with eosinophilic gastrointestinal disease. The most common symptoms were vomiting (71%) and abdominal pain (62%). Of the eosinophilic gastroenteritis cases, 12 (30%) had esophageal involvement, and 11 (28%) had colonic involvement. For treatment, 36 (80%) received corticosteroids. Overall, 27 (60%) had symptom resolution and 23 (51%) had endoscopic resolution. Cases underwent a mean of five endoscopic procedures per year.
Conclusion
Eosinophilic gastroenteritis presents with non-specific gastrointestinal symptoms and in almost one-third of cases has concomitant esophageal or colonic involvement. It remains difficult to treat, with high rates of endoscopic utilization.
Keywords: Eosinophilia, Eosinophilic gastroenteritis, Eosinophilic gastrointestinal disease
1. Introduction
Eosinophilic gastroenteritis (EoG) is a rare condition first described in 1937 [1]. It belongs to the family of eosinophilic gastrointestinal disorders (EGID) where eosinophilic inflammation occurs in the GI tract in the absence of secondary causes. Proposed secondary causes include adrenal insufficiency, medication hypersensitivity reactions, collagen vascular disease, malignancy, hypereosinophilic syndrome, or parasitic infection [2,3].
Though the aetiology and pathogenesis of the disease have yet to be fully elucidated, the condition is thought to be a polygenic allergic disorder on the spectrum between IgE mediated and delayed Th2 responses, but not fitting completely into either category [2,4]. Case reports suggest that EoG has no singular ethnic or age predilection. It may be more prevalent from the 3rd to 5th decades of life [5,6]. The disease has been associated with atopic conditions including food allergy, asthma, and atopic dermatitis [7]. As described by Klein and Talley, there can be eosinophilic infiltration throughout the different layers of the GI tract (mucosal, muscle layer, subserosal) [8,9]. This and the location of the eosinophilia in the GI tract impact the clinical presentation, and help to explain the diverse symptoms and signs that may be attributable to the condition. Endoscopic or full-thickness surgical biopsy demonstrating eosinophilic infiltration of the GI tract is necessary to make the diagnosis of EoG [10–12].
Because the current literature is limited to small case series and single case reports, there are limited epidemiologic, clinical, and histopathologic data describing the disease. This makes diagnosis and treatment challenging, as there are no consensus statements to guide the evidence-based management of this condition. The aim of this study was to characterize the clinical, endoscopic, and histopathologic features of a cohort of patients with EoG, and to summarize treatment outcomes and resource utilization.
2. Materials and methods
We conducted a retrospective cohort study at the University of North Carolina at Chapel Hill. Pathology reports of all patients who had undergone upper endoscopy with biopsy between January 1, 2000 and June 20, 2013 were obtained to identify patients with EoG. These reports were reviewed if the term “eosinophil” was mentioned anywhere in the report. Cases of EoG were defined by ≥20 eosinophils/hpf (hpf = 0.24 mm2) on either gastric or duodenal biopsy, symptoms attributable to the GI tract (i.e. abdominal pain, nausea, vomiting, weight loss, feeding intolerance, etc.), and no known secondary cause of eosinophilia. While there are no diagnostic guidelines published for EoG, this definition is consistent with what has been used in prior reports [9,13,14]. Colonoscopy reports from the same day as the index endoscopy were obtained to identify patients who also had colonic involvement.
Once cases were identified, additional data were extracted from the electronic medical record. These included patient demographics, symptoms, co-morbidities, habits (tobacco and alcohol abuse), medications, endoscopic findings, age specific BMI, and treatments. Symptoms were from patient or caregiver self-report. Comorbid conditions or disease complications required a diagnosis by a provider. Protein-losing enteropathy was defined by an albumin level less then 2.6 without hepatic dysfunction or proteinuria; ascites required a radiographic diagnosis. For patients with follow-up data available in our system, we assessed treatment outcomes including symptomatic, endoscopic, and histologic response. Interval changes in patient symptoms were also from self-report. All follow-up evaluations were done at the discretion of the physician to detail treatment response or to clarify recurrent symptoms. Endoscopic and histopathologic findings after treatment were compared with pre-treatment findings, but endoscopic response was only assessed for those patients with abnormal endoscopic findings at baseline. The total number of endoscopic procedures performed on patients during the follow-up period was also recorded.
For analysis, patients were characterized with descriptive statistics. Bivariate analysis was performed to compare adults (≥18 years) and children using t-tests for means and chi-square for proportions. We also calculated the mean number of endoscopic procedures performed per patient during the follow-up time frame. This study was approved by the UNC Institutional Review Board.
3. Results
There were 44 patients diagnosed with EGID over the study time frame. Four of these patients were diagnosed with isolated eosinophilic colitis (EoC) without involvement of the stomach or bowel. The mean age was 16 years (range 0.4–83), 58% were male, and 58% were white. The most common presenting symptoms were vomiting (71%) and abdominal pain (62%) (Table 1). While the mean BMI at diagnosis was normal (20 ± 7), the median BMI was in the underweight category (17, IQR: 16–23). Food allergies were noted in 42%, 64% had a family history of atopic disease, and both the serum total IgE levels and peripheral eosinophil counts were elevated (Table 2).
Table 1.
Characteristics of subjects with eosinophilic gastrointestinal disorders.
| Number of patients studied | 44 |
| Age at diagnosis, Mean ± SD; range | 16.0 ± 19.1; 0.42–82.8 |
| Length of sx before biopsy, Mean ± SD; range | 4.9 ± 9.4; 0.08–55.7 |
| Adult, ≥18; N (%) | 11 (24) |
| Male, N (%) | 26 (58) |
| Race, N (%) | |
| White | 31 (58) |
| Black | 7 (16) |
| Asian | 0 (0) |
| Hispanic | 2 (4) |
| Unknown | 5 (11) |
| Symptoms, N (%) | |
| Dysphagia | 12 (27) |
| Heartburn | 8 (18) |
| Abdominal pain | 28 (62) |
| Nausea | 17 (38) |
| Vomiting | 32 (71) |
| Chest pain | 3 (7) |
| Bloating | 8 (18) |
| Diarrhoea | 14 (31) |
| Constipation | 15 (33) |
| Comorbid conditions, N (%) | |
| Food allergy | 19 (42) |
| Asthma | 12 (27) |
| Allergic rhinitis | 17 (38) |
| Drug allergy | 14 (31) |
| Eczema | 1 (16) |
| Complications, N (%) | |
| Family history of atopic disease | 29 (64) |
| Anaemia at diagnosis | 4 (9) |
| Failure to thrive | 14 (31) |
| Ascites | 1 (2) |
| Small bowel obstruction | 1 (2) |
| Food impaction | 5 (11) |
| Weight loss >4 pounds | 12 (27) |
| Protein losing enteropathy | 3 (7) |
| Steatorrhea | 1 (2) |
Table 2.
Laboratory and histologic findings of eosinophilic gastrointestinal disorder patients.
| Lab results | Mean ± SD | Median | IQR |
|---|---|---|---|
| Serum IgE level | 418 ± 722 | 188 | 24–467 |
| Absolute eosinophil count | 1.53 ± 2.64 | 0.55 | 0.2–1.8 |
| ESR | 9.9 ± 11.3 | ||
| CRP | 2.9 ± 4.0 | 0.5 | 15.6–23.1 |
| BMI | 20.1 ± 6.9 | 16.7 | 15.6–23.1 |
| Cases of EGID | N = 44 |
|---|---|
| EoG predominant, N (%) | 40 (91) |
| Gastric & Duodenal eosinophilia | 12 (30) |
| Gastric eosinophilia | 18 (45) |
| Duodenal eosinophilia | 10 (25) |
| EoE also present | 12 (30) |
| EoC also present | 11 (28) |
| EoC predominant, N (%) | 4 (9) |
| Eosinophil count (#/HPF) | Mean ± SD | Median | IQR |
|---|---|---|---|
| Gastric | 61 ± 70 | 37 | 25–60 |
| Duodenal | 55 ± 23 | 50 | 40–75 |
| Esophageal | 56 ± 62 | 42 | 9–100 |
| Colonic eosinophil count | 84 ± 73 | 68 | 50–80 |
EoG, eosinophilic gastroenteritis; EoE, eosinophilic esophagitis; EoC, eosinophilic colitis.
Serum IgE level (IU/L); Absolute eosinophil count (n * 109/l); ESR (mm/h); CRP (mg/dl); BMI (kg/m2).
Eosinophilic infiltration of the gastrointestinal tract varied for this population. Of the EoG cases, 12 (30%) had both gastric and duodenal involvement, 18 (45%) had gastric involvement only, and 10 had duodenal involvement only. In addition, we found that 12 (30%) had esophageal involvement (eosinophilic esophagitis [EoE]) and 11 (28%) had colonic involvement (EoC). The average of the peak eosinophil counts were 61, 55, 56, and 84 for the stomach, duodenum, oesophagus, and colon respectively (Table 2).
Endoscopic exams were normal in 21 (47%) of patients. Esophageal white plaques and esophagitis were found in 4 (9%) of those studied and these patients all had eosinophilic infiltration of the oesophagus. The most common gastric and duodenal finding was mucosal erythema. For diagnosis and treatment monitoring, EGID cases underwent a mean of 5 ± 4 endoscopic procedures per year (Table 3).
Table 3.
Endoscopic data of subjects with eosinophilic gastrointestinal disorders.
| Mean ± SD | Range | |
|---|---|---|
| Endoscopies performed | 4 ± 5 | 1–23 |
| Colonoscopies performed | 0.8 ± 0.9 | 0–3 |
| Average endoscopies and colonoscopies per year | 5.4 ± 4.4 | 0.68–20 |
| EGD findings | N (%) |
|---|---|
| Normal | 21 (47) |
| Rings | 4 (1) |
| Stricture | 1 (2) |
| Narrowing | 2 (4) |
| Furrows | 3 (7) |
| Crêpe-paper | 0 (0) |
| White plaques/exudates | 4 (9) |
| Decreased vascularity | 3 (7) |
| Erosive esophagitis | 0 (0) |
| Esophagitis | 4 (9) |
| Erythema | 0 (0) |
| Ulcers | 0 (0) |
For treatment, 36 (80%) received corticosteroids, 21 (47%) had dietary restriction, and 34 (76%) required >1 treatment modality. Budesonide, either in viscous slurry or enteral release formulations, was prescribed for 30 patients with doses ranging from 0.25 mg twice daily to 9 mg once a day. Prednisone was prescribed for 10 patients with doses ranging from 5 to 40 mg daily. Fluticasone was also prescribed for 3 patients at either 110 mcg or 220 mcg daily. Twenty-one patients were treated with dietary elimination; 13 were placed on an elemental formula; 7 received an elimination diet targeted after allergy testing; 1 patient’s dietary modification was not specified. Detailed clinical, endoscopic, histologic, and treatment information for each patient is presented in Supplementary Table S1.
To assess treatment outcomes, patients had a mean follow-up of 26.2 months, which encompassed an average of 6.6 Gastroenterology clinic encounters over the study period. In addition, patients underwent an average of 5 endoscopies per year (Fig. 1). In regards to treatment response, 27 (60%) had symptom resolution and 23 (51%) had endoscopic resolution on repeat endoscopic evaluation. When repeat biopsies were obtained, 20/29 (69%) had histologic resolution with treatment. Ongoing eosinophilic inflammation with ≥20 eos/hpf was found in 5/29 (17%) patients and non-specific changes were reported in 4/29 patients (14%) patients. The degree of continued eosinophilic infiltration demonstrated a wide range from 68 to 500 eos/hpf. Of patients with histologic resolution, 63% had symptomatic resolution and 95% had endoscopic resolution; 27% had resolution in all three areas.
Fig. 1.
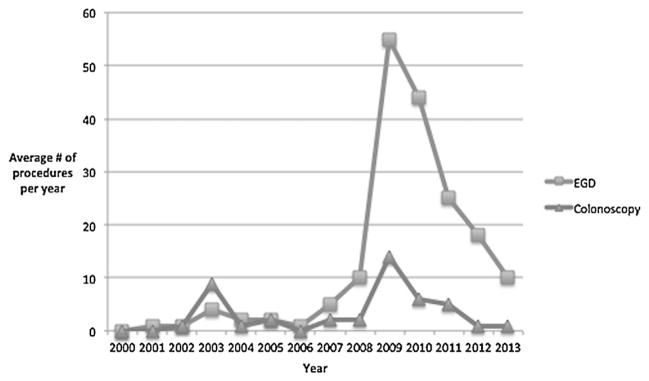
Trends over time in endoscopic utilization in patients with eosinophilic gastrointestinal diseases. EGD, esophagogastroduodenoscopy.
Higher response rates were noted for oral steroids than for diet modification, leukotriene antagonists, H2 blockers, or mast-cell inhibitors. Twenty-two of the 36 patients receiving corticosteroids reported symptom resolution. Twelve of the 21 patients who received dietary modification noted symptom resolution. However, 15 of these 21 patients also received corticosteroids during their treatment course (Table 4).
Table 4.
Therapeutic data of subjects with eosinophilic gastrointestinal disorders.
| Pharmacologic therapy, N (%) | |
| Diet modification | 21 (47) |
| Mast cell inhibitor | 2 (4) |
| H-2 antagonist | 18 (40) |
| Leukotriene receptor antagonist | 6 (13) |
| Greater than 1 therapy | 34 (76) |
| Oral corticosteroids | 36 (80) |
| Systemic steroids | 9 (25) |
| Topical steroids | 27 (75) |
| Repeat endoscopy performed | 29 (66) |
| Response rate, N (%) | |
| Endoscopic resolution | 23 (51) |
| Symptom resolution | 27 (61) |
| Histologic resolution | 20 (69) |
Repeat endoscopy – esophagogastroduodenoscopy or colonoscopy.
Symptom resolution – 22/36 patients on steroids, 12/21 patients placed on dietary modification, 15/21 patients received both steroids and dietary modification.
When adult and children with EoG were compared, the two groups had similar laboratory findings, endoscopic findings, treatment modalities, and symptom resolution per treatment responses. Demographic data suggested that the prevalence of EGID is greater in male children and female adults (Table 5). When those with symptomatic resolution (n = 15; 44%) were compared to non-symptom responders, no statistically significant differences in clinical or endoscopic features were found (Supplementary Table S2).
Table 5.
Comparison of adult and child population.
| Adult N = 11 | Child N = 34 | P value | |
|---|---|---|---|
| Age at diagnosis, Mean (SD) | 41.2 (18.9) | 7.9 (9.9) | |
| Male, N (%) | 2 (18) | 24 (71) | 0.002 |
| White, N (%) | 10 (91) | 21 (62) | 0.3 |
| Symptoms, N (%) | |||
| Dysphagia | 4 (36) | 8 (24) | 0.47 |
| Heartburn | 2 (18) | 6 (18) | 0.97 |
| Chest pain | 3 (27) | 0 (0) | 0.03 |
| Abdominal pain | 10 (91) | 18 (53) | 0.05 |
| Nausea | 10 (91) | 7 (21) | <0.001 |
| Vomiting | 7 (64) | 25 (74) | 0.62 |
| Bloating | 6 (55) | 2 (6) | 0.001 |
| Diarrhoea | 4 (37) | 10 (29) | 0.79 |
| Constipation | 2 (18) | 13 (38) | 0.12 |
| Serum IgE level, Mean (SD) | 302 (195) | 451 (817) | 0.73 |
| Absolute eosinophil count, Mean (SD) | 2.3 (2.7) | 1.3 (2.3) | 0.35 |
| BMI, Mean (SD) | 24.8 (7.2) | 18.6 (6.1) | 0.008 |
| Comorbid conditions, N (%) | |||
| Food allergies | 7 (64) | 12 (35) | 0.25 |
| Asthma | 3 (27) | 9 (26) | 0.96 |
| Allergic rhinitis | 5 (45) | 12 (35) | 0.55 |
| Drug allergy | 4 (36) | 10 (29) | 0.67 |
| Eczema | 0 (0) | 7 (21) | 0.1 |
| Endoscopic resolution, N (%) | 3 (27) | 20 (59) | 0.04 |
| Symptom resolution, N (%) | 4 (36) | 23 (68) | 0.18 |
| Symptom resolution per treatment modality, N (%) | |||
| Diet modification | 2 (50) | 11 (48) | 0.94 |
| Mast cell inhibitor | 0 (0) | 1 (4) | 0.67 |
| H-2 antagonist | 1 (25) | 11 (48) | 0.4 |
| Leukotriene receptor antagonist | 2 (50) | 2 (9) | 0.03 |
| Oral corticosteroids | 4 (100) | 18 (78) | 0.3 |
| Greater than 1 therapy prescribed | 3 (75) | 18 (78) | 0.89 |
| Endoscopies performed, Mean (SD) | 5.2 (5.9) | 3.5 (4.7) | 0.35 |
| Colonoscopies performed, Mean (SD) | 0.7 (0.9) | 0.8 (0.9) | 0.91 |
Comorbid conditions required diagnosis by a provider.
Symptom resolution per treatment modality done for the proportion of subjects (n = 27) who had symptom resolution, as stratified by adult/kids status).
Determination of endoscopic resolution required repeat endoscopic evaluation.
4. Discussion
EoG is a rare condition, and the epidemiology, clinical presentation, treatments, and outcomes have not been extensively described. The goal of this study was to present a large cohort of well characterized patients with EoG. There were several key findings. First, symptoms of EoG were non-specific. Second, there was frequent GI tract eosinophilia in other locations such as the oesophagus and colon, even though the majority of patients had a normal appearing mucosa. Because of this, it is important to have a high degree of suspicion of an EGID; if biopsies are not obtained from multiple areas of the GI tract, an accurate diagnosis cannot be made. Finally, EoG was difficult to treat, with just over half having a treatment response, and many patients required multiple endoscopies.
These findings extend what has previously been reported in the literature [8,9,14,15]. In seminal papers by Klein et al. and Talley et al. [8,9] non-specific symptoms such as nausea and abdominal pain were cardinal features, and a system classifying disease involvement of the bowel wall (mucosal, muscular, or subserosal) was described. Chang et al. extended the data published by Talley et al. in 2010 [9,14]. They described 59 adult patients with eosinophilic gastroenteritis reporting abdominal pain, nausea, and vomiting predominantly of the mucosal variant. A recently published series of 28 children with EoG also found that abdominal pain and vomiting were common, as were atopic diseases including asthma and food allergies [15].
Eosinophilic involvement of the gastrointestinal tract occurred in multiple locations in our population. Eosinophils were equally likely to be found in the stomach, duodenum, or both. In addition, one-third of patients had concurrent involvement of the oesophagus or colon. Laboratory data had relatively little utility in characterizing our patient population. Though markers associated with non-specific inflammation and allergy were consistently elevated, there was a high degree of variability in these values. Analysis of endoscopic features suggested that approximately one-half of patients have normal findings. The abnormal findings were also variable with no consistent feature noted.
Because we included patients of all ages in this study, we were able to compare the disease presentation in children to that in adults. Surprisingly, we found few differences in the two populations. Our data suggests that EGID may be more prevalent in male children and female adults. This is quite different than what has been reported for EoE, the best studied EGID [16–18].
Our data also show that treatment of EoG is difficult. Despite using a number of modalities, only one-half of our patients had symptomatic resolution over the study period, including those treated with corticosteroids. This is in contrast to other studies where steroid response rates ranged from 80% to 100% [6,14,15,19]. The lack of universal response to corticosteroids in our cohort of EoG patients may bring into question the true utility of this medication, especially in light of potential adverse effects, or may suggest non-allergic or non-immune mediated pathogenic mechanisms in some patients. We also did not observe the high rates of response to dietary elimination therapy that has also been recently reported [15]. In the context of this relatively unsuccessful treatment, management of EoG is also extremely resource intensive. Our patients required multiple endoscopies per year for diagnosis and monitoring of the disease. This emphasizes that in addition to more effective treatment, less invasive options for monitoring are needed.
The natural history and clinical course of EGID have yet to be fully characterized. A French study described the course of 43 adult patients with EGID over a mean follow-up period of 13 years [20]. Three distinct patterns were observed: a single disease flare that lasted less than three months; a relapsing and remitting course; and a progressive disease course. It is difficult to infer natural history data from our study cohort as patients were treated rather than observed. However, only one third remained in long-term remission, so many of our patients had a persistent or progressive disease course.
There are some limitations to this study. First, it has a retrospective design, which potentially introduces recall bias and limits the data that are available. For example, we do not have uniform data on depth of involvement (mucosal, muscular, or serosal) as full thickness biopsies are not part of routine practice and cross-sectional imaging is not performed in all subjects. In addition, symptom outcomes were subjective and must be interpreted with caution. However, we were able to use documented endoscopy and histologic findings to provide more objective outcome measures. Second, the study was from a single centre, so results may not be generalizable. However, we performed a comprehensive case-finding strategy that captured every case seen at our institution that had endoscopy and biopsy with active disease. We also cannot estimate the true prevalence of EoG based on this single centre study, but accounting for the total number of endoscopies performed over the study period (>144,000), we can calculate that EoG is seen in approximately 30/100,000 procedures. This is similar to a published prevalence estimate of 28/100,000 [21]. While this is one of the largest series yet reported, the numbers are still relatively small so the study may be underpowered to detect differences between subgroups. Nevertheless, because of the cohort study design, we are able to provide important information about treatments, patient response, and resource utilization.
In conclusion, EoG presents with non-specific GI symptoms, and in almost one-third of cases involvement of the GI with eosinophils includes either the oesophagus or colon. Therefore, a high level of clinical suspicion with biopsies from all levels of the GI tract is required to make an accurate diagnosis. Once the diagnosis is made, EoG remains difficult to treat, with only just over half of patients in our cohort responding to treatment. Finally, management of EoG is resource intensive, with high rates of endoscopic utilization presenting a financial burden for many patients with EoG and their families.
Supplementary Material
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dld.2014.11.009.
Footnotes
Conflict of interest
None declared.
References
- 1.Kaijser R. Zur kenntnis der allegischen affecktioner desima verdauungskanal von standpunkt desima chirurgen aus. Arch Klin Chir. 1937;188:36–64. [Google Scholar]
- 2.Rothenberg ME. Eosiniphilic gastrointestinal disorders (EGID) Journal of Allergy and Clinical Immunology. 2004;113:11–28. doi: 10.1016/j.jaci.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Khan S, Orenstein SR. Eosinophilic gastroenteritis. Gastroenterology Clinics of North America. 2008;37:333–48. doi: 10.1016/j.gtc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Oh HE, Chetty R. Eosinophilic gastroenteritis: a review. Journal of Gastroenterology. 2008;43:741–50. doi: 10.1007/s00535-008-2230-5. [DOI] [PubMed] [Google Scholar]
- 5.Yun MY, Cho Yu, Park IS, et al. Eosinophilic gastroenteritis presenting as a small bowel obstruction: a case report and review of the literature. World Journal of Gastroenterology. 2007;13:1758–60. doi: 10.3748/wjg.v13.i11.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MJ, Chu CH, Lin SC, et al. Eosinophilic gastroenteritis: clinical experience with 15 patients. World Journal of Gastroenterology. 2003;9:2813–6. doi: 10.3748/wjg.v9.i12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez-Sanchez N, Chavez-Tapia NC, Vazquez-Elizondo G, et al. Eosinophilic gastroenteritis: a review. Digestive Diseases and Sciences. 2007;52:2904–11. doi: 10.1007/s10620-005-9011-2. [DOI] [PubMed] [Google Scholar]
- 8.Klein NC, Hargrove RL, Sleisenger MH, et al. Eosinophilic gastroenteritis. Medicine. 1970;49:299–319. doi: 10.1097/00005792-197007000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Talley NS, Shorter RG, Phillips SF, et al. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissue. Gut. 1990;31:54–8. doi: 10.1136/gut.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naylor AR. Eosinophilic gastroenteritis. Scottish Medical Journal. 1990;35:163–5. doi: 10.1177/003693309003500601. [DOI] [PubMed] [Google Scholar]
- 11.Daneshjoo RJ, Talley N. Eosinophilic gastroenteritis. Current Gastroenterology Reports. 2002;4:366–72. doi: 10.1007/s11894-002-0006-2. [DOI] [PubMed] [Google Scholar]
- 12.Khan S. Eosinophilic gastroenteritis. Best Practice and Research Clinical Gastroenterology. 2005;19:177–98. doi: 10.1016/j.bpg.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Hurrell JM, Genta RM, Melton SD. Histopathologic diagnosis of eosinophilic conditions in the gastrointestinal tract. Advances in Anatomic Pathology. 2011;18:335–48. doi: 10.1097/PAP.0b013e318229bfe2. [DOI] [PubMed] [Google Scholar]
- 14.Chang JY, Choung RS, Lee RM, et al. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clinical Gastroenterology and Hepatology. 2010;8:669–75. doi: 10.1016/j.cgh.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Ko HM, Morotti RA, Yershow O, et al. Eosinophilic gastritis in children: clinocopathological correlation, disease course, and response to therapy. American Journal of Gastroenterology. 2014 doi: 10.1038/ajg.2014.166. http://dx.doi.org/10.1038/ajg.2014.166. [DOI] [PubMed]
- 16.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. Journal of Allergy and Clinical Immunology. 2011;128:3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Dellon ES, Gibbs WB, Fritchie KJ, et al. Clinical, endoscopic, and histologic findings distinguish eosinophilic esophagitis from gastroesophageal reflux disease. Clinical Gastroenterology and Hepatology. 2009;7:1305–13. doi: 10.1016/j.cgh.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonsalves N. Distinct features in the clinical presentations of eosinophilic esophagitis in children and adults: is this the same disease? Digestive Diseases. 2014;32:89–92. doi: 10.1159/000357078. [DOI] [PubMed] [Google Scholar]
- 19.Lee CM, Changchien CS, Chen PC, et al. Eosinophilic gastroenteritis: 10 years experience. American Journal of Gastroenterology. 1993;88:70–4. [PubMed] [Google Scholar]
- 20.de Chambrun GP, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clinical Gastroenterology and Hepatology. 2011;9:950–6. e1. doi: 10.1016/j.cgh.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Spergel JM, Book WM, Mays E, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. Journal of Pediatric Gastroenterology and Nutrition. 2011;52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


