Abstract
Background
A standardized 4-hour adult-based gastric emptying scintigraphy (GES) protocol is increasingly being used in children to evaluate for gastroparesis. We sought to determine the effect of age, anthropometrics, and study duration on GES results using this protocol in children.
Methods
Retrospective review of children who underwent a 4-hour solid-meal GES study at a tertiary care center. GES results and anthropometric data (e.g., weight, stature, body surface area) were systematically captured.
Key Results
Of 216 children, 188 (87%) were able to complete the study meal. Children unable to complete the meal were younger and smaller. In multivariate analysis, only increasing body surface area (BSA) was identified as being positively associated with ability to complete the meal (odds ratio: 19.7; P<0.001). Of those completing the meal, 48 (26%) had delayed emptying (4-hour retention value >10%). These children were significantly younger and smaller than those with normal emptying. In multivariate analysis of those completing the meal, only increasing BSA (odds ratio: 0.26; P=0.006) was identified as being negatively associated with delayed emptying. There was a progressive increase in the positive predictive value for identification of delayed gastric emptying as the duration of the study increased (0.25, 0.60, and 0.71 at 1, 2, and 3 hr, respectively) using the 4-hr value as a comparator.
Conclusions and Inferences
Young children have more difficulty completing the GES meal. Childhood gastric retention is affected by age and anthropometric factors, primarily BSA. The standardized 4-hr GES protocol may need to take these factors into account in children.
Keywords: Children, Gastric Emptying, Gastroparesis, Nuclear Medicine, Motility, Dyspepsia
INTRODUCTION
Gastroparesis is a gastrointestinal (GI) motility disorder in which the emptying of the stomach is abnormally delayed in the absence of an anatomical obstruction. Estimates of the prevalence of gastroparesis in the adult population range widely, from 0.2 – 4% (1, 2). Females are more affected than males (3, 4). Prevalence rates of gastroparesis in the pediatric population are unknown.
Normal GI motility depends on the integrity of the “gut-brain axis” which is composed of the central, autonomic, and enteric nervous systems, along with the interstitial cells of Cajal and smooth muscle cells of the GI tract (5). Compromise of any of these components can potentially alter GI motility, causing such disorders as gastroparesis, intestinal pseudoobstruction, and intractable constipation (6–8). In the adult population, diabetes, postsurgical complications and Parkinson’s disease are common causes of gastroparesis, though idiopathic gastroparesis is the most common (35.6%) (4). In children, most cases (70%) are believed to be idiopathic (9). However, many so-called idiopathic cases of gastroparesis, in both adults and children, are thought to be postinfectious in nature (9, 10).
Gastric emptying scintigraphy (GES) provides an objective measure of gastric emptying (11). Until recently, the radiolabeled meal utilized for a GES study often differed between institutions. Currently, a low fat solid meal of 2 scrambled eggs (or egg substitute equivalent), 2 pieces of white toast, jam, and 120 mL of water is recommended as the standard meal for GES studies by both the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine (12). Meal standardization has allowed the development of normal adult values for gastric emptying as measured by GES in 123 healthy volunteers (13).
The use of the standardized meal and a 4-hour study has been validated for use in adults but there are few data regarding the utility of these test conditions in children. Although ethical concerns preclude carrying out studies with radiolabels in healthy children, it recently has been reported that the standard solid meal and adult normative values can be used for GES studies in children and that extending the GES study to 4 hours (as opposed to 2 hours) allows for increased sensitivity in detecting gastroparesis in children (14). However, the study population was small (n=71) and not all evaluated participants finished the meal. Other factors potentially affecting GES results were not evaluated.
We therefore sought to determine in children if age and anthropometric measures (weight, stature, body mass index, and/or body surface area) affect GES results. We anticipated that because the same size meal is used for all ages, younger, smaller children would have a more difficult time with meal completion and have slower gastric emptying than would older, larger children. We also aimed to determine in a larger group of patients than in the earlier study if extending the GES study to 4 hr alters the proportion of children identified with delayed gastric emptying and consequently, the predictive value of a gastric retention value obtained prior to the standard time of 4 hours (14). However, unlike the previous investigation, we only studied children able to ingest the entire meal as recommended (14).
METHODS
Study Design
We conducted a retrospective review of the electronic medical records of 216 children ranging from 4 – 18 years of age undergoing a GES study as part of their evaluation of upper GI symptoms at the nuclear medicine facility of Texas Children’s Hospital over an 18-month time period (February 2012 – July 2013). These evaluations were ordered at the discretion of the treating physician as part of routine clinical practice. The majority of children had dyspepsia (e.g., nausea) of a chronic nature for which the clinician was considering delayed gastric emptying as a potential etiology. Children with a known history of abdominal surgery (e.g., pyloric surgery, bowel resection, fundoplication, etc.) or any known organic GI disease (e.g., inflammatory bowel disease, celiac disease, H. pylori infection, etc.) were excluded. Patients who required a modification of the standard solid meal for GES (see below) were not included. Patient demographics, anthropometric data (height, weight, body mass index, and body surface area) and GES results were collected during the chart review process. The study was approved by the Baylor College of Medicine Institutional Review Board.
Gastric Emptying Scintigraphy
All GES studies utilized the recommended standard solid meal consisting of 2 pieces of white toast, 120 mL of scrambled egg substitute (equivalent of 2 large eggs), a 15 g packet of jelly, and 120 mL of water (13). Technetium-99-labeled sulfur colloid was utilized for all studies and was mixed in the egg substitute prior to cooking. Meals were consumed within a 10-minute period, after which a baseline scintigraphic image was obtained. Anterior and posterior images were acquired concurrently and a geometric mean value was calculated (12). Subsequent images were taken at 1-hour intervals over a 4-hour period. If a child was unable to consume the entire meal within a 10-minute period or if they vomited within the 4-hour duration of the GES study, he/she was excluded from the primary data analysis and labeled as “unable to complete GES.” Children who required a modification of the meal (e.g., gluten-free bread) were not included. GES results were reported as gastric retention values at the 1, 2, 3 and 4-hour time points of the study. Delayed gastric emptying was defined as a gastric retention value greater than 90%, 60%, 30%, and 10% at the respective 1-, 2-, 3-, and 4-hour time points of the GES study (12).
Data Analysis
Student t-test was used to compare mean age and anthropometric measures between children with and without abnormal GES studies, and between children with and without delayed gastric emptying based upon the recommended 4-hour time point. Age and anthropometric-based groups were created by dividing the range into quartiles. A binary logistic backward stepwise regression was completed to evaluate which anthropometric factors were most strongly associated with ability complete the meal and with the identification of delayed gastric emptying. Due to high correlation between anthropometric variables, only age, body mass index (BMI) and body surface area (BSA) were included in the regression models as independent variables. IBM statistical Package for the Social Sciences (Armonk, New York) version 19 was used for all statistical analysis. Unless otherwise specified, data are presented as mean ± standard deviation.
RESULTS
Over the 18-month study period, 216 children (135 females; mean age 12.0 ± 3.9 yrs., range 4.2–18.2 yrs.) were enrolled. 28 (13%) children were unable to complete the study (unable to consume or vomited the GES meal). Children unable to complete the study (n=28) were significantly younger and smaller in weight, stature, and BSA than those who were able to consume and tolerate the meal (n=188) (Table 1). BMI percentile (which takes into account normative values for age) did not differ between groups (Table 1). In multivariable analysis, only BSA remained in the final backward stepwise model suggesting larger children were more likely to complete the meal (Table 2).
Table 1.
Demographic and Anthropometric Measures of Children Able vs Unable to Complete the GES Study
| Completed GES (n=188) | Unable to Complete GES* (n=28) | P-value | |
|---|---|---|---|
| Gender | 120 F (64%) | 15 F (54%) | 0.296 |
| Age (yrs.) | 12.5 ± 3.7 | 8.6 ± 4.2 | < 0.001 |
| Weight (kg) | 46.4 ± 18.7 | 29.7 ± 19.6 | < 0.001 |
| Height (cm) | 149.2 ± 19.2 | 126.5 ± 23.6 | < 0.001 |
| BMI# (kg/m2) | 20.0 ± 4.7 | 16.8 ± 3.8 | 0.001 |
| BMI (percentile) | 56.4 ± 32.1 | 45.3 ± 35.1 | 0.11 |
|
| |||
| BSA^ (m2) | 1.4 ± 0.4 | 1.0 ± 0.4 | < 0.001 |
Refused to eat standard meal, ate eggs only, or vomited during the study. Data presented as Mean ± SD.
BMI = Body Mass Index
BSA = Body Surface Area
Table 2.
Backward stepwise logistic regression multivariate analysis of factors associated with ability to complete the meal
| Model 1 | Model 2 | Final Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | OR | 95% CI | P- Value | Factor | OR | 95% CI | P- value | Factor | OR | 95% CI | P- value |
| BMI* | 0.97 | 0.77 – 1.2 | 0.81 | Age | 1.15 | 0.89 – 1.49 | 0.29 | BSA | 19.7 | 5.1 – 75.7 | <0.001 |
| Age | 1.13 | 0.83 – 1.5 | 0.44 | BSA | 4.97 | 0.3 – 82.0 | 0.26 | ||||
| BSA# | 7.88 | 0.08 – 815.3 | 0.38 |
BMI = Body Mass Index
BSA = Body Surface Area
Each step of the analysis is shown with the final model only including BSA after both BMI and age have been removed.
Of the 188 children who ate and tolerated the meal and underwent GES, 48 (26%) had delayed gastric emptying based upon the 4-hour time point (Table 3). Children with delayed gastric emptying were significantly younger, with lower body weight, height, and BSA than those with normal gastric emptying (Table 3). BMI percentile did not differ between groups (Table 3). Gender was not associated with an increased likelihood of having delayed gastric emptying (Table 3). In multivariable analysis, only BSA remained in the final backward stepwise model and was negatively associated with having delayed gastric emptying suggesting larger children were less likely to be identified with gastroparesis (Table 4).
Table 3.
Demographic and Anthropometric Measures of Children with Normal versus Delayed Gastric Emptying
| Normal Emptying (n=140) | Delayed Gastric Emptying (n=48) | P-value | |
|---|---|---|---|
| Gender | 86 F (61%) | 34 F (71%) | 0.242 |
| Age (yrs.) | 12.9 ± 3.5 | 11.3 ± 3.8 | 0.011 |
| Weight (kg) | 48.5 ± 18.4 | 40.4 ± 18.8 | 0.010 |
| Height (cm) | 151.6 ± 18.3 | 142.2 ± 20.2 | 0.003 |
| BMI* (kg/m2) | 20.3 ± 4.7 | 18.8 ± 4.7 | 0.066 |
| BMI (percentile) | 58.6 ± 31.4 | 50.0 ± 33.6 | 0.135 |
| BSA# (m2) | 1.41 ± 0.35 | 1.25 ± 0.37 | 0.005 |
Mean ± SD
BMI = Body Mass Index
BSA = Body Surface Area
Table 4.
Backward stepwise logistic regression multivariate analysis of factors associated with identification of delayed gastric emptying
| Model 1 | Model 2 | Final Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | OR | 95% CI | P- Value | Factor | OR | 95% CI | P- value | Factor | OR | 95% CI | P- value |
| BMI* | 1.0 | 091 – 1.2 | 0.5 | BMI | 1.0 | 0.92 – 1.2 | 0.5 | BSA | 0.26 | 0.10 – 0.68 | 0.006 |
| Age | 1.0 | 0.83 – 1.2 | 0.89 | BSA | 0.17 | 0.03 – 0.85 | 0.03 | ||||
| BSA# | 0.14 | 0.01 – 2.59 | 0.19 |
BMI = Body Mass Index
BSA = Body Surface Area
Each step of the analysis is shown with the final model only including BSA after both BMI and age have been removed.
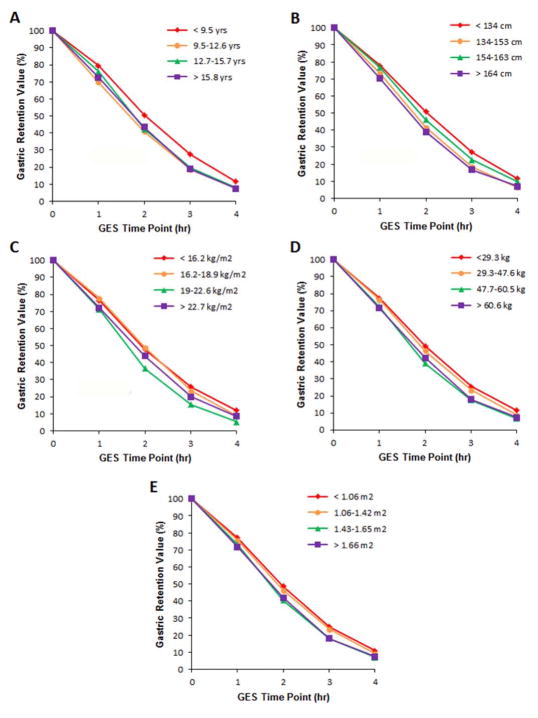
In order to evaluate further the relationship between gastric emptying vs age and anthropometric factors, we compared gastric retention values to these variables with the population broken down by quartile groups to facilitate visualization (Figure). In all cases the group with the lowest quartile value had increased gastric retention, particularly noted from the 2-hour time point onward.
FIGURE. Gastric Retention Values over Time between Age- and Anthropometric-based Groups.
Gastric retention values over time between children (n=188) when divided into quartiles by (A) age, (B) height, (C) body mass index, (D) weight, and (E) body surface area.
Increasing the duration of the GES study improved the positive predictive value of the test as compared to the 4-hour time point (Table 5). In contrast, the negative predictive value did not change significantly as a function of study duration (Table 5).
Table 5.
Number of Gastric Emptying Scintigraphy Studies Demonstrating Normal or Delayed Emptying at 1, 2, and 3 hr vs Results of the 4-hr Study
| 1 hr | 2 hrs. | 3 hrs. | ||||
|---|---|---|---|---|---|---|
| Normal | Delayed | Normal | Delayed | Normal | Delayed | |
| Normal at 4 hrs. | 129 | 11 | 122 | 18 | 130 | 10 |
| Delayed at 4 hrs. | 36 | 12 | 19 | 29 | 14 | 34 |
|
| ||||||
| Positive predictive value | 0.25 | 0.60 | 0.71 | |||
| Negative predictive value | 0.92 | 0.87 | 0.93 | |||
DISCUSSION
GES is the gold standard utilized in evaluating gastric retention and making the diagnosis of delayed gastric emptying. The current recommendations for GES require patients to consume a standard meal within a relatively short period of time. Although it has been suggested that the standard GES protocol used in adults can be adopted for use in the pediatric population, our results suggest age and anthropometric factors should be taken into account (14). First, a sizable proportion of children (13%) who tended to be younger, could not consume or tolerate (i.e., vomited) the standard meal within the required time. This could compromise study results. Second, of the children who were able to complete the GES study, those with delayed gastric emptying were found to be significantly younger and smaller than those without delayed gastric emptying. Future studies may help elucidate better standards for children while taking into account age and anthropometric factors.
We questioned whether there was a time by age and/or anthropometry effect on the GES results. We divided the entire population into quartiles based on age and anthropometric factors In order to evaluate the effect of these variables on gastric residual values over time. The youngest children had higher gastric retention values when compared to the other age groups (Figure). Not surprisingly, because age, weight, stature, body mass index, and body surface area are highly correlated, generally parallel results were seen when dividing the cohort into quartiles according to anthropometric factors (Figure). These findings suggest that younger and smaller children are likely to have increased gastric retention values as compared to older children using the standard 4-hour GES protocol. We note however, that the differences were most pronounced beginning at the 2 hour time point. Drubach et al. compared gastric retention values at 1 hour in children < 8 yrs. of age vs older children (n=65) and in those < 30 kg vs > 30 kg (n=65) and found no differences between groups (15). Although the meal differed between their study and ours in that only labeled eggs or labeled cheese was used in the Drubach study, our findings fit with theirs in that differences among quartiles in our study were not apparent at the 1-hour time point (Figure).
In adults it has been identified that gender plays a role in gastric emptying with women having more gastric retention compared to men as measured by half-time gastric emptying (16). However, in our study a gender difference was not seen when examining the amount of gastric retention at 4 hours. Future prospective studies evaluating the role of gender in children undergoing GES are needed to confirm our findings.
Gastric weight (and presumably size) increases linearly in relationship to age from approximately 3 months of age until adulthood (17). The same linear relationship can be found to anthropometric measures such as BMI with other visceral organs such as the liver through adulthood (18, 19). However in adults, gastric size based on mucosal surface area does not appear to be related to age, weight, or height (20). Some children in the youngest age group have a gastric size less than one third the size of an adult (17). Therefore, we hypothesize that the increased gastric retention in the young children may be related more to the ratio of meal size to gastric size than abnormal gastric emptying per se. The fact that 13% of younger children could not tolerate the GES meal and that increasing BSA was negatively associated with delayed gastric emptying in those who could complete the meal supports this speculation. Given the nature of our study, we can only show an association and as such, it is possible that children with delayed gastric emptying or dyspepsia may have secondary effects on their anthropometric measures (e.g., weight) due to the underlying pathologic process. Nevertheless our results, combined with previous work evaluating gastric size and visceral organ size correlations with body surface area lead us to believe that the currently recommended meal for GES studies in adults may not be appropriate for all children, particularly in those less than ten years of age given the current normal gastric retention values.
Alternatively, normal gastric retention values would need to be defined for young children vs older children if the same meal was used for all groups. Future studies might employ simultaneous use of single photon emission computed tomography (SPECT) and GES. SPECT has been utilized to measure gastric capacity during fasting and during the postprandial state and can be performed in tandem with GES (21, 22). However, SPECT radiation exposure may limit its feasibility in children. In an attempt to minimize radiation exposure, 13C gastric emptying breath testing using various meal sizes may be used (23) as can ultrasound evaluations of liquid gastric emptying (24). In addition, satiety testing in normal children and those with dyspepsia may provide insight into appropriate meal sizes (25). Data from such studies may help adjust meal size to gastric size in children. Additionally, it may afford the possibility of assessing the presence of gastric fundus accommodation dysfunction as well as delayed gastric emptying as an etiology of symptoms (21, 22).
Our data also suggest that the positive predictive value of GES increases with a longer duration of study, using the 4-hour time point as a reference (Table 5). Similar findings have been reported in adults (12). However, our results differ from those of Chogle et al. who studied a smaller number of children (n=71) and found a higher positive predictive value at 2 hours of 0.89 using the value at 4 hours as a comparator (14). This may be due in part to the fact that in their study children who finished ≥ 25% of the meal were included. Additionally, the studied population may have differed between our two studies given the frequency of delayed gastric emptying was much greater in their study than in ours (66% vs 28%, respectively based on the gastric residual value at the 4-hour time point) (14). Our data suggest that, although the positive predictive value increases with time, the negative predictive value at 1, 2, and 3 hours is high and remains relatively stable (0.92, 0.87, and 0.93, respectively) (Table 5).
There are some limitations of our study. First the study was retrospective, though we note that all captured measures were objective and readily available in the electronic medical record and as such, a prospective design would be unlikely to demonstrate significantly different results. Second, the study was conducted at a single center, and therefore the results may not be generalizable to all centers.
Our study has several strengths. First, to our knowledge, it is the largest study to date to evaluate GES in children. Second, the results are clinically relevant and potentially more generalizable to other pediatric practices given that all studies were completed as part of routine clinical practice. Third, the population studied was diverse in nature with a wide range of ages and body sizes represented. Finally, the results were standardized with the data regarding gastric residual values being based only on children who consumed and tolerated the entire meal.
In conclusion, in children, GES results following a standardized 4-hour protocol can be affected by patient age, anthropometric factors such as height and body mass index, and study duration. Extending the duration of GES beyond one hour appears to accentuate the effects of anthropometric factors while increasing the positive predictive value of results at time points prior to 4 hours. Further studies are needed to establish an appropriate meal size and/or normative GES values for use in pediatric patients, particularly young children.
KEY MESSAGES.
We determined the effect of age, anthropometrics, and study duration on gastric emptying as measured by up to 4 hours of gastric emptying scintigraphy (GES) in 188 children in a retrospective study.
Age, weight, height, and body surface area were less in those who were unable to finish the GES meal compared with those who ingested all of the meal.
In those children who ate all of the meal, younger age and lower weight, height, and body surface area were associated with slower gastric emptying. The differences were magnified by increasing duration of the GES.
Acknowledgments
We would like to thank O’Brian Smith for his help with the statistical analysis. We also thank Victor Seghers for providing the GES data.
FUNDING
This study was supported in part by R01 NR05337 and NR013497 from the National Institutes of Health, the Daffy’s Foundation, the USDA/ARS under Cooperative Agreement No. 6250-51000-043, and P30 DK56338 which funds the Texas Medical Center Digestive Disease Center. Funding was also provided by the NASPGHAN Foundation/Nestle Nutrition Young Investigator Development Award and K23 DK101688 (B.P.C.).
Abbreviations
- GES
gastric emptying scintigraphy
Footnotes
CONFLICTS OF INTEREST
The authors do not have conflicts of interest to report.
AUTHOR CONTRIBUTION
GKW, RJS, and BPC designed the research study, analyzed the data and wrote the paper. GKW collected the data. All authors have read, edited, and approved the submitted version of the manuscript.
References
- 1.Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18(4):263–83. doi: 10.1111/j.1365-2982.2006.00760.x. Epub 2006/03/24. [DOI] [PubMed] [Google Scholar]
- 2.Jung HK, Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Szarka LA, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–33. doi: 10.1053/j.gastro.2008.12.047. Epub 2009/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJ, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–15. doi: 10.1053/j.gastro.2010.10.015. Epub 2010/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43(11):2398–404. doi: 10.1023/a:1026665728213. Epub 1998/11/21. [DOI] [PubMed] [Google Scholar]
- 5.Huizinga JD. Neural injury, repair, and adaptation in the GI tract. IV. Pathophysiology of GI motility related to interstitial cells of Cajal. The American journal of physiology. 1998;275(3 Pt 1):G381–6. doi: 10.1152/ajpgi.1998.275.3.G381. Epub 1998/09/02. [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Seminars in neurology. 2003;23(4):365–72. doi: 10.1055/s-2004-817720. Epub 2004/04/17. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler D, Schadewaldt P, Pour Mirza A, Piolot R, Schommartz B, Reinhardt M, et al. [13C]octanoic acid breath test for non-invasive assessment of gastric emptying in diabetic patients: validation and relationship to gastric symptoms and cardiovascular autonomic function. Diabetologia. 1996;39(7):823–30. doi: 10.1007/s001250050516. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 8.Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49(10):1731–9. doi: 10.2337/diabetes.49.10.1731. Epub 2000/10/04. [DOI] [PubMed] [Google Scholar]
- 9.Chumpitazi B, Nurko S. Pediatric gastrointestinal motility disorders: challenges and a clinical update. Gastroenterology & hepatology. 2008;4(2):140–8. Epub 2008/02/01. [PMC free article] [PubMed] [Google Scholar]
- 10.Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92(9):1501–4. Epub 1997/10/08. [PubMed] [Google Scholar]
- 11.Camilleri M, Hasler WL, Parkman HP, Quigley EM, Soffer E. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115(3):747–62. doi: 10.1016/s0016-5085(98)70155-6. Epub 1998/08/28. [DOI] [PubMed] [Google Scholar]
- 12.Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AH, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103(3):753–63. doi: 10.1111/j.1572-0241.2007.01636.x. Epub 2007/11/22. [DOI] [PubMed] [Google Scholar]
- 13.Tougas G, Eaker EY, Abell TL, Abrahamsson H, Boivin M, Chen J, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95(6):1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. Epub 2000/07/14. [DOI] [PubMed] [Google Scholar]
- 14.Chogle A, Saps M. Gastroparesis in children: the benefit of conducting 4-hour scintigraphic gastric-emptying studies. J Pediatr Gastroenterol Nutr. 2013;56(4):439–42. doi: 10.1097/MPG.0b013e31827a789c. Epub 2012/11/01. [DOI] [PubMed] [Google Scholar]
- 15.Drubach LA, Kourmouzi V, Cao X, Zurakowski D, Fahey FH. Gastric emptying in children: what is the best acquisition method? J Pediatr Gastroenterol Nutr. 2012;55(2):191–3. doi: 10.1097/MPG.0b013e31824ee2fa. Epub 2012/02/22. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil. 2012;24(12):1076–e562. doi: 10.1111/j.1365-2982.2012.01972.x. Epub 2012/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scammon RE. Some graphs and tables illustrating the growth of the human stomach. Am J Dis Child. 1919;17:395–422. [Google Scholar]
- 18.DeLand FH, North WA. Relationship between liver size and body size. Radiology. 1968;91(6):1195–8. doi: 10.1148/91.6.1195. Epub 1968/12/01. [DOI] [PubMed] [Google Scholar]
- 19.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2002;8(3):233–40. doi: 10.1053/jlts.2002.31654. Epub 2002/03/23. [DOI] [PubMed] [Google Scholar]
- 20.Cox AJ. Variations in size of human stomach. California and western medicine. 1945;63:267. Epub 1945/12/01. [PubMed] [Google Scholar]
- 21.Bredenoord AJ, Chial HJ, Camilleri M, Mullan BP, Murray JA. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1(4):264–72. Epub 2004/03/16. [PubMed] [Google Scholar]
- 22.Simonian HP, Maurer AH, Knight LC, Kantor S, Kontos D, Megalooikonomou V, et al. Simultaneous assessment of gastric accommodation and emptying: studies with liquid and solid meals. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2004;45(7):1155–60. Epub 2004/07/06. [PubMed] [Google Scholar]
- 23.Bharucha AE, Camilleri M, Veil E, Burton D, Zinsmeister AR. Comprehensive assessment of gastric emptying with a stable isotope breath test. Neurogastroenterol Motil. 2013;25(1):e60–9. doi: 10.1111/nmo.12054. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devanarayana NM, Rajindrajith S, Rathnamalala N, Samaraweera S, Benninga MA. Delayed gastric emptying rates and impaired antral motility in children fulfilling Rome III criteria for functional abdominal pain. Neurogastroenterol Motil. 2012;24(5):420–5. e207. doi: 10.1111/j.1365-2982.2011.01871.x. Epub 2012/01/26. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman I, Tack J. Assessment of gastric motor function in childhood functional dyspepsia and obesity. Neurogastroenterol Motil. 2012;24(2):108–12. e81. doi: 10.1111/j.1365-2982.2011.01813.x. Epub 2011/11/23. [DOI] [PubMed] [Google Scholar]