Abstract
Oral naltrexone could be a promising relapse prevention pharmacotherapy for recently detoxified opioid-dependent patients, however interventions are often needed to promote adherence with this treatment approach. We recently conducted a study to evaluate a 26-week employment-based reinforcement intervention of oral naltrexone in unemployed injection drug users (Dunn et al., 2013). Participants were randomly assigned into a Contingency (n=35) group required to ingest naltrexone under staff observation to gain entry into a therapeutic workplace, or a Prescription (n=32) group given a take-home supply of oral naltrexone and access to the workplace without observed ingestion. Monthly urine samples were collected and analyzed for evidence for naltrexone adherence, opioid use, and cocaine use. As previously reported, Contingency participants provided significantly more naltrexone-positive urine samples than Prescription participants during the 26-week intervention period. The goal of this current study is to report the 12-month outcomes, which occurred 6 months after the intervention ended. Results at the 12-month visit showed no between-group differences in naltrexone-positive, opioid-negative, or cocaine-negative urine samples, and no participant self-reported using naltrexone at the follow-up visit. These results show that even after a period of successfully reinforced oral naltrexone adherence longer-term naltrexone use is unlikely to be maintained after reinforcement contingencies are discontinued.
Keywords: naltrexone, opioids, therapeutic workplace, contingency management, cocaine
Introduction
Naltrexone is an opioid antagonist that has no abuse liability and can block the reinforcing, subjective, and physiological effects of opioids. These characteristics could make naltrexone a powerful relapse prevention medication for the treatment of opioid dependence. Naltrexone has been traditionally provided as an oral formulation; however several studies have indicated that, in the absence of formal monitoring, patients have very poor rates of adherence with oral naltrexone (Adi et al., 2007; Kirchmayer et al., 2002; San, Pomarol, Peri, Olle, & Cami, 1991; Sullivan et al., 2007). As a result, several different intervention styles have been developed to promote continued adherence on naltrexone. These include family monitoring (Anton, Hogan, Jalali, Riordan, & Kleber, 1981; Summers & Stone, 2002), intensive behavioral counseling (Nunes, Rothenberg, Sullivan, Carpenter, & Kleber, 2006; Rawson et al., 2001; Roozen, Kerkhof, & van den Brink, 2003), and provision of monetary-based incentives to encourage continued adherence (Carroll et al., 2001; Grabowski et al., 1979; Preston et al., 1999).
Our laboratory has been investigating a fourth potential option to promote naltrexone adherence, which is the use of workplaces as vehicles for arranging and maintaining long-term reinforcement of naltrexone adherence (Defulio et al., 2012; Dunn et al., 2013; Everly et al., 2011; Silverman, DeFulio, & Sigurdsson, 2012). Under this intervention, opioid-dependent patients are offered employment in a therapeutic workplace setting but are required to take scheduled doses of naltrexone to maintain access to the workplace and to earn the maximum pay rate. Through this model we have successfully reinforced adherence to both oral (Dunn et al., 2013) and extended-release naltrexone (DeFulio et al., 2012; Everly et al., 2011). In each of these studies, participants were randomly assigned to a Contingency group, which was required to adhere to a naltrexone regimen to enter a model workplace setting, or a Prescription group, which had access to naltrexone but was not required to show evidence of naltrexone adherence to enter the workplace. Naltrexone adherence was reinforced for a 26-week period and was assessed through direct urinalysis testing or confirmation of receipt of depot injections at monthly intervals. Results showed high rates of oral naltrexone (72% vs. 21%), and extended release naltrexone (81%-87% vs. 42%-52%) adherence in the Contingency vs. Prescription groups, respectively. However, it is not yet clear whether naltrexone adherence will be maintained and whether opioid use will increase following completion of the intervention and removal of adherence contingencies. This issue is critical; since opioid dependence has been conceptualized as a chronic, relapsing disorder (McLellan et al., 2000), it is important to understand whether an extended treatment with naltrexone is sufficient to prevent relapse, or whether naltrexone adherence should be reinforced for longer durations.
The purpose of the present study is to report the 12-month outcomes of a randomized controlled trial in which employment-based reinforcement was used to promote the use of oral naltrexone in recently detoxified opioid-dependent injection drug users (Dunn et al., 2013). Opioid-dependent patients in this study were detoxified before being inducted onto oral naltrexone, and were invited to attend a model therapeutic workplace on a daily basis where they were paid hourly for achieving goals in computer training programs. Initially, all participants were required to ingest naltrexone under staff observation to enter the workplace. Following a 4-week induction period, participants were randomly assigned to one of two groups; a Contingency group that was required to continue ingesting oral naltrexone thrice weekly under staff observation to gain access to the workplace, and a Prescription group that received a monthly prescription of oral naltrexone but was able to enter the workplace independent of naltrexone consumption. Naltrexone adherence was assessed monthly via direct urinalysis testing of naltrexone and its metabolites. Opioid and cocaine abstinence were also assessed though monthly urinalysis testing. Results showed that Contingency participants provided significantly more urine samples that were positive for naltrexone compared to Prescription participants (72% vs. 21%, respectively, p<.01), however no effect of experimental group was observed on the percent opioid- (71% vs. 60%, respectively) or cocaine-negative (56% vs. 53%, respectively) urine samples (Dunn et al., 2013).
The data presented here assess the 12-month post-intervention outcomes of this randomized controlled trial, which was conducted 6 months after the intervention ended. Because naltrexone can be prescribed outside the context of a research study, this follow-up study evaluated whether participants continued to use oral naltrexone after the employment-based reinforcement intervention ended, whether participants were continuing to use opioids and cocaine, and whether participants had entered another substance abuse treatment program by the follow-up time point.
Methods
The full study methods and results of the original trial are available elsewhere (Dunn et al., 2013). The methods and results of the original trial are summarized here briefly, and only the methods relevant to the follow-up analyses are discussed here in detail.
Study Participants
Participants were recruited from detoxification programs and street outreach in Baltimore MD from May 2006 to September 2009. Eligibility criteria included ages 18-65; being unemployed (self-reporting not working and earning <$200 in taxable wages in the past 30 days), self-reporting injection drug use and having visible track marks, providing a urine sample that tested positive for opioids and cocaine at intake to detoxification treatment, meeting DSM-IV-TR criteria for opioid dependence, being medically eligible to take naltrexone, and living within a reasonable commuting distance from the workplace. Participants were excluded if they had evidence of an uncontrolled psychiatric disorder (e.g., hallucinations, delusions), were judged to be an immediate threat to harm themselves or others, were incarcerated or under constant monitoring, were pregnant or breastfeeding, had serum aminotransferase levels >3 times the upper limit of normal, required opioids for other medical problems, reported an interest in methadone maintenance treatment, had active tuberculosis, or had physical limitations that would prevent them from completing computer training programs. All participants provided voluntary informed consent to participate in the study and the Johns Hopkins Medicine Institutional Review Board approved the study.
Outcome Assessments
The outcome measures were collected at thirty-day intervals throughout the 26-week intervention period and at a 12-month outcome visit. Assessments consisted of a variety of self-report scales such as the Risk Assessment Battery measure of HIV risk behavior (Navaline et al., 1994), the Beck Depression Inventory of depressive symptoms (Beck, Steer, & Brown, 1996), and the Symptom Checklist-90 (Derogatis, 1977) measure of psychological functioning. Participants also completed the Addiction Severity Index (McLellan et al., 1985) with a staff member to evaluate changes in psychosocial functioning. Only assessments relevant to these analyses are described here; information on the full assessment battery is available in Dunn et al., (2013).
Urine samples from the thirty-day assessments collected within the 26-week intervention and from the 12-month visit were sent to a commercial CLIA-certified laboratory to be tested for evidence of recent opioids, cocaine, buprenorphine, methadone, amphetamine, and benzodiazepine use using EMIT qualitative processing (Friends Laboratory, MD). Buprenorphine testing was implemented midway through data collection and therefore was not completed for all participants. Urinalysis naltrexone levels were also analyzed at these time points using an Enzyme Linked Immunosorbent Assay (ELISA) procedure, and values <5ng/ml were considered negative for naltrexone (Friends Laboratory, MD).
General Workplace Procedures
The original study was conducted in a model workplace setting. All participants were eligible to attend the workplace daily Monday – Friday for 4 hours per day (with a 1-hour break for lunch). Participants earned monetary-based vouchers for their attendance and performance on computerized typing and keypad tasks. Participants earned $8.00 per hour in base pay and could earn up to $2.00 more per hour based on their productivity in the computerized training programs. Detailed descriptions of the therapeutic workplace, the web-based training programs, the staffing requirements, and the cost of the intervention can be found elsewhere (DeFulio, Donlin, Wong, & Silverman, 2009; Donlin, Knealing, Needham, Wong, & Silverman, 2008; Knealing, Roebuck, Wong, & Silverman, 2008; Silverman et al., 2007).
Naltrexone Administration
All participants completed an opioid detoxification before being invited to attend the workplace for induction onto oral naltrexone (Depade®; Mallinckrodt Inc.). All participants completed a 4-week induction period during which they were required to ingest scheduled doses of oral naltrexone to gain entry to the workplace. Only participants who completed the 4-week induction period were eligible for randomization. Throughout the study, naltrexone was offered to participants at no cost to them. All participants were offered assistance with identifying and contacting a physician who would continue their naltrexone prescription upon leaving the study, but did not receive any additional naltrexone from the study once the primary intervention ended.
Experimental Groups
Participants were stratified and randomized in a 1:1 ratio into one of two groups at the end of the induction period using an urn randomization procedure (Wei & Lachin, 1988). The stratification variables were: attending the workplace on ≥85% of days during the 4-week induction period (Y/N), providing ≥1 opioid-positive urine sample during the final 2 weeks of the induction period (Y/N), and providing ≥75% cocaine-positive urine samples during the 4-week induction period (Y/N). All participants were then invited to continue attending the workplace for 26-weeks.
Participants assigned to the Contingency group were required to continue ingesting oral naltrexone under staff observation in order to access the workplace, where they could continue earning vouchers for base and productivity pay. Contingency participants who did not take a naltrexone dose were not permitted to access the workplace on that day and had their base pay earnings reset to $1.00/hour. Once a reset had occurred, the participant’s base pay increased by $1.00/hour with each subsequent day that he/she ingested naltrexone and gained access to the workplace until it reached the initial starting value of $8.00/hour. Participants assigned to the Prescription group received a medication bottle that contained a 30-day supply of oral naltrexone every 30-days, but were no longer required to ingest naltrexone to gain access to the workplace. Prescription participants were able to continue earning base and productivity pay vouchers and were not subject to resets.
Data Analysis
The primary outcome measures at the 12-month visit were the percentage of urine samples that tested positive for naltrexone, negative for opioids, and negative for cocaine. Point-prevalence urinalysis test results for naltrexone, opioids, and cocaine at 12-months were compared as a function of experimental group using chi-square analyses. Experimental groups were also compared on several measures that were hypothesized to contribute to point-prevalent abstinence at 12-months, including visit attendance, self-reported engagement in treatment, and self-reported naltrexone use. Groups were compared on each of these items using t-tests for continuous variables and Fisher’s Exact test for dichotomous variables. Since providing a methadone or buprenorphine-positive urine sample may indicate the participant had entered agonist maintenance treatment between the end of the intervention and the 12-month outcome assessment, Pearson product correlations were conducted to assess whether providing a methadone or buprenorphine-positive urine sample was significantly associated with self-report of treatment in the past 30 days.
For all analyses, missing urine samples were analyzed in two ways; by treating the missing sample as missing (missing-missing), or by converting missing opioid and cocaine samples to positive (missing-positive) and missing naltrexone samples to negative (missing-negative). Two-tailed tests were used and results were considered statistically significant if P≤.05. Statistical analyses were conducted using SPSS software version 21.
Results
A full list of demographic and drug use characteristics have been previously reported (Dunn et al., 2013). Participants were, on average, 39% female, 86% African American, and 45 years old. All participants met DSM-IV-TR criteria for opioid dependence, and 92% met criteria for cocaine dependence. The two groups did not significantly differ on any demographic or drug use characteristics except the amount of money spent on drugs in the past 30 days, with the Prescription group reporting significantly less money spent compared to the Contingency group ($956 vs. $2,253, respectively; t(65)=−2.22, p=.03)).
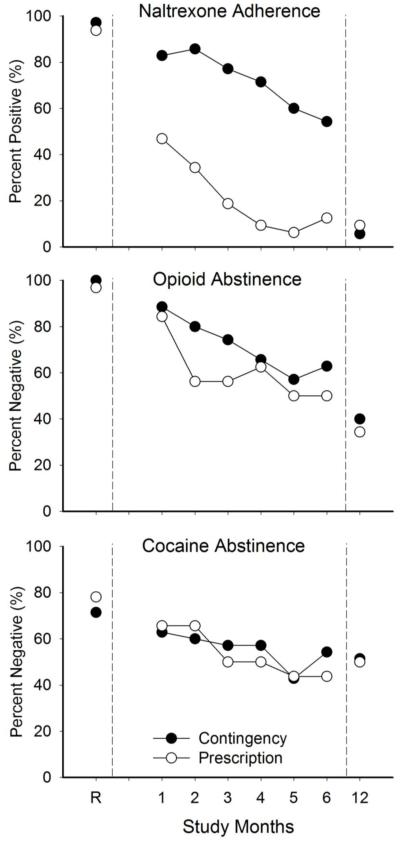
Although participants in the Contingency group took naltrexone significantly more than Prescription participants during the 26-week intervention period (72% vs. 21% of 30-day urine samples positive for naltrexone, respectively, p<.01; see Dunn et al., 2013), only 3 and 2 participants in the Contingency and Prescriptions groups, respectively, provided naltrexone-positive urine samples at the 12-month outcome visit. No participant from either group self-reported taking naltrexone in the 30 days prior to the 12-month outcome visit. Overall there were no significant between-group differences in the percent of urine samples that tested positive for naltrexone in either the missing-missing or missing-positive analyses (Table 1). There were also no significant between-group differences in the percent of participants providing a urine sample that tested negative for opioids or cocaine in either the missing-missing or missing-positive analyses. This is further illustrated by Figure 1, which presents the percent of samples testing positive for naltrexone and testing negative for opioids and cocaine throughout the active intervention (as previously reported in Dunn et al., 2013) and at 12-months.
Table 1.
Urinalysis and Drug Treatment Characteristics at 12-Months
| Prescription (n=32) |
Contingency (n=35) |
pa | |
|---|---|---|---|
| Urinalysis Test Results (%) | |||
| Naltrexone-Positive | |||
| Missing-Missing | 10 | 6 | 0.66 |
| Missing-Negative | 9 | 6 | 0.66 |
| Opioid-Negative | |||
| Missing-Missing | 42 | 44 | 0.99 |
| Missing-Positive | 34 | 40 | 0.80 |
| Cocaine-Negative | |||
| Missing-Missing | 59 | 56 | 0.99 |
| Missing-Positive | 50 | 51 | 0.99 |
| Methadone Positive (%) | 8 | 14 | 0.42 |
| Buprenorphine Positiveb (#/n) | 2/10 | 2/9 | 0.66 |
| Receiving Drug Treatment (%) | 23 | 17 | 0.45 |
| Self-report Naltrexone Use (%) | 0 | 0 | -- |
| Attendance at Visit (%) | 95 | 91 | 0.61 |
P-values based on Fisher's Exact tests for categorical and t-tests for continuous variables, two-tailed, alpha of .05.
Buprenorphine testing was implemented mid-way through the study, therefore only the # positive /# collected are presented.
Figure 1. Urinalysis Test Results.
Urinalysis-testing results. The X-axis is study visits. Visits include results from the end of the induction period, immediately prior to randomization (R), results from 6 monthly assessments during the 26-week intervention, and results from the 12-month outcomes after the intervention ended. The data on the Y-axis are percent naltrexone-positive samples (top panel), representing naltrexone-adherence; and percent opioid-(middle panel) and cocaine-(bottom panel) negative samples, representing opioid and cocaine abstinence. Contingency participants are designated by filled symbols, and Prescription participants are designated by open symbols. Missing samples have been treated as negative for naltrexone, and positive for opioids and cocaine. Data is adapted from Dunn et al., 2013 with permission.
As shown in Table 1, 91% of Contingency and 95% of Prescription participants completed the 12-month visit and this difference was not statistically significant (p=.61). Few participants in either group reported attending treatment for drug abuse, which was not operationally defined and could consist of pharmacological, psychosocial, or self-help groups, in the 30 days prior to the 12-month visit, and there was no significant between-group difference. Overall, 11% and 24% of the urine samples analyzed at 12 months tested positive for methadone and buprenorphine, respectively, and this did not differ significantly as a function of experimental group (Table 1). Only a small percentage of participants who provided a methadone or buprenorphine-positive urine sample reported receiving any drug treatment in the past 30 days (29% and 25%, respectively), and providing a methadone (r(59)=.07, p=.57) or buprenorphine (r(17)=.02, p =.94) -positive urine sample was not significantly correlated with reporting drug treatment in the past 30 days, suggesting that positive urine samples were likely related to non-prescribed use of these drugs.
Discussion
This study presents the 12-month outcomes of participants who had recently detoxified from opioids and cocaine and were offered oral naltrexone therapy for a 26-week period. As previously reported (Dunn et al., 2013), Contingency participants who were required to take naltrexone to work and maintain maximum pay during the active treatment intervention consumed naltrexone consistently and significantly more often than Prescription participants who were not required to take naltrexone to work. Specifically, 49% (17/35) of Contingency participants tested positive for naltrexone ≥80% of the time during the active intervention (and while the employment-based reinforcement contingency for oral naltrexone adherence was in effect), although a downward trend in naltrexone adherence was evident across the 26-week study (Figure 1). Results at 12-months reveal that, despite this prior high rate of adherence, no Contingency participant reported taking oral naltrexone and only three participants (9%) provided naltrexone-positive urine samples at 12 months. Thus, naltrexone use was not maintained in the absence of an adherence contingency.
The reason for the discrepancy between self-reported naltrexone use and provision of a naltrexone-positive urine sample in this study is not well-understood. It is possible that the naltrexone-positive samples occurred among patients who were taking buprenorphine/naloxone (Suboxone), which has been shown via thin layer chromatography testing to test positive for naloxone (Pal Singh Balhara & Jain, 2012). Buprenorphine testing was implemented midway through treatment, and was therefore not available for 4 of the 5 naltrexone-positive urine samples, though the final sample did test positive for buprenorphine. The product insert for the naltrexone urinalysis test does confirm cross-reactivity with low levels of naloxone, which may explain this discrepancy. In addition, closer inspection of our data suggests a consistent discrepancy between self-reported naltrexone use and naltrexone urinalysis results. At the 12-month visit, 8% (5/60) of the participants self-reported no naltrexone but tested positive. In the primary trial, 10% (13/129) of the samples provided by participants who self-reported no naltrexone tested positive. The consistency between these results suggests this may be error from the urinalysis test itself. Due to the limited reliance on direct urinalysis testing for naltrexone, the potential for cross-reactivity with buprenorphine/naloxone and the general standard error rate for naltrexone-urinalysis testing have not yet been reported by other naltrexone-based studies. More research into the limitations of urinalysis testing for naltrexone in outpatient studies would help refine the use of this novel approach more broadly.
Of primary interest is whether participants stopped using naltrexone because they enrolled in another form of drug treatment, such as maintenance in methadone or buprenorphine maintenance, which might contraindicate or negate the need for naltrexone. This could lead to the incorrect assumption that a naltrexone-negative urine sample was indicative of poor functioning and continued opioid abuse, when in fact these participants may have been successfully participating in another treatment. Such alternative treatment enrollment does not appear to explain the non-continuation of naltrexone. Only 14% of Contingency participants and 8% of Prescription participants provided methadone-positive urine samples, and only 17% and 23% of participants in the two groups, respectively, reported receiving any kind of drug treatment in the 30 days before the 12-month assessment. Taken together, these results provide additional support for the use of sustained interventions for this population. If naltrexone is going to be of long-term value to this population, methods will be needed to promote long-term naltrexone adherence. Employment-based reinforcement of naltrexone adherence could potentially serve this role.
Poor medication adherence has been recognized as a major problem throughout all clinical conditions and populations (Iuga & McGuire, 2014; Osterberg & Blaschke, 2005) and generally results from poor medication adherence, in general, is reported to result from patient forgetfulness, having other priorities, intentionally omitting doses, being uninformed about medication dosing schedules, and emotional distress (Iuga & McGuire, 2014; see also Osterberg & Blacschke, 2005). Naltrexone has additional unique features that may further complicate adherence. First, as noted in a recent review of medication adherence, naltrexone’s blockade of opioid-induced effects may threaten adherence because extinguishing a highly reinforced behavior can be aversive (see DeFulio & Silverman, 2012). Second, the antagonistic properties of naltrexone make it difficult to resume adherence following a relapse to opioids; thus, even patients who are interested in resuming naltrexone may be reluctant to do so after experiencing a relapse to opioids. Overall, the data presented here further support the results of previous studies that suggest oral naltrexone adherence is not likely to be maintained in the absence of a continuous adherence intervention.
These data also reveal the Contingency and Prescription groups did not differ in the rate of opioid and cocaine-negative urinalyses provided at 12-months. Based on the missing-positive analyses, 37% and 51% of participants in this study tested negative for opioids and cocaine, respectively, at 12-months. Though there have been few long-term follow-up evaluations of naltrexone adherence and illicit drug use following removal of a structured adherence intervention, the results of this study are consistent with the results of two other studies that promoted naltrexone adherence for 6 months and then conducted a 12-month visit. The first study used Community Reinforcement Therapy to promote oral naltrexone adherence and reported that 55% (12/22) of participants who received naltrexone were abstinent at 12 months (6 months after oral naltrexone treatment ended) (Roozen et al., 2003). A second study that administered naltrexone or placebo to detoxified patients for a 6-month period reported no significant between-group differences in the number of patients who were rated as a “therapeutic success” (32% vs. 46%, respectively) at 12 months (San et al., 1991).
The rate of naltrexone-positive samples at the 12-month visit was very low overall and no participant self-reported taking naltrexone, therefore it is not surprising that opioid and cocaine use were not associated with naltrexone adherence at that visit. These results are also consistent with the primary intervention, which also did not detect significant effects of naltrexone consumption on opioid and cocaine urine testing (Dunn et al., 2013). The continued use of opioids in conjunction with naltrexone also has been reported by other naltrexone studies (Kunoe et al., 2010; Sullivan et al., 2007). The reason that naltrexone adherence did not result in greater opioid abstinence among the Contingency participants is not completely understood, but there was a high rate of concordance between opioid use and cocaine use noted in the primary intervention. That is, the majority of urine samples that tested positive for opioids also tested positive for cocaine (Figure 4 of Dunn et al., 2013). This result is consistent with other research from our laboratory (Everly et al., 2011; DeFulio et al., 2012), as well as other researchers who have reported that cocaine use was a robust predictor of relapse to opioids following opioid detoxification (Broers, Giner, Dumont, & Mino, 2000; Gossop, Stewart, Browne, & Marsden, 2002). Since naltrexone is only indicated as a treatment for opioid use, the enrollment of dual opioid and cocaine abusers may have impacted the drug abuse outcomes by leaving cocaine use essentially untreated. Therefore, the results from the present study further strengthen the assertion made in the primary trial report (Dunn et al., 2013) that treatment for opioid polysubstance abusers should evaluate additional treatments that target concurrent drug use directly and in conjunction with naltrexone treatment for opioid relapse prevention.
This study has some important limitations. The first is that participants may not have continued naltrexone use because they did not have access to a continued supply of the medication once the intervention ended. All participants in this study were offered assistance in identifying a provider to maintain their naltrexone dosing upon completion of the study, though the number of participants who attempted to continue this prescription is unknown. However, the fact that no participant reported taking naltrexone at the 12-month visit is consistent with other studies on oral naltrexone that suggest participants do not adhere to oral naltrexone without an intensive monitoring intervention (Adi et al., 2007; Kirchmayer et al., 2002). Second, the study did not enroll the intended sample size during the intervention and therefore may have been underpowered to detect an effect at 12-months, despite the high level of attendance achieved at that visit. Third, a limited number of data were collected at the 12-month visit regarding why the participant had stopped naltrexone use, which prevented more detailed analyses from being conducted. Fourth, due to the cost of the procedure, naltrexone urinalysis testing was only conducted on a monthly basis; more frequent testing throughout the active intervention would have enabled more sensitive analytic modeling and enabled more predictive analyses of continued drug use at the 12-month visit. Finally, additional follow-up visits (e.g., 7 month, 9 month, 24-month) would have provided a more thorough opportunity to evaluate the natural history of naltrexone adherence in the absence of an adherence contingency.
The primary conclusions to be drawn from these data are that, despite 6-months of directly observed and urinalysis-confirmed adherence to oral naltrexone, participants in this study did not continue to take oral naltrexone once the adherence intervention ended. These data are consistent with the larger literature on oral naltrexone that suggests overall poor adherence in the absence of any structured intervention. The results of this long-term follow-up provide additional support for the continued integration of medication adherence contingencies into workplace environments. There may be some environments in which the frequent dosing required for oral naltrexone maintenance may make this treatment approach too difficult to implement (e.g., difficulty finding staff to observe dosing). Extended release naltrexone, which can provide up to a 4-week naltrexone blockade after a single injection, is a promising alternative to oral naltrexone in these circumstances because it can reduce the compliance burden on the participant while still providing a continuous and clinically meaningful blockade for relapse prevention. The benefits of using extended release naltrexone in an employment-based setting have been previously evaluated and supported in two randomized controlled trials (Defulio et al., 2012; Everly et al., 2011).
Future research should focus on methods to incorporate naltrexone contingencies into real-world employment settings to enable long-term reinforcement of adherence (Silverman et al., 2012). It will also be important to more thoroughly evaluate the utility of using extended release naltrexone as a relapse prevention tool, particularly among patients who show early signs of poor adherence with oral naltrexone. Finally, additional emerging adherence intervention strategies, including electronic reminders and electronic medical record monitoring, have shown promise in meta-analytic reviews for medication adherence (Demonceau et al., 2013; Vervloet et al., 2012). These approaches have not yet been applied to naltrexone adherence, but represent intriguing and potentially cost-effective methods of promoting oral naltrexone adherence.
Acknowledgements
The project described was supported by Award Numbers R01DA019386 and T32DA07209 from the National Institute on Drug Abuse
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT 00149669
Disclosures
All authors provided substantive contributions to the manuscript and have read and approved the final manuscript.
In recent years George Bigelow has received research support from Pain Therapeutics, Inc.,Titan Pharmaceuticals, and Orexo Pharmaceuticals, as well as consulting payments from Abbott Laboratories, Acura Pharmaceuticals, GW Pharmaceuticals, Pfizer, Teva Pharmaceuticals, and Transcept Pharmaceuticals. Paul A. Nuzzo has been paid as a statistical consultant / project coordinator for the NIDA Clinical Trials Network (CTN), NIDA Clinical Coordinating Center, and Yaupon Therapeutics, Inc. The remaining authors report no conflicts of interest or financial disclosures. This research was conducted while Dr Will Aklin was affiliated with Johns Hopkins University. Dr Aklin is now at the National Institute on Drug Abuse, Bethesda Maryland. Dr. Aklin does not have personal affiliations or financial relationships with any commercial interest to disclose. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse, the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
The authors would like to acknowledge the hard work of the numerous Center for Learning and Health and the Behavioral Pharmacology Research Unit employees who assisted in the administration of this study, and the research assistants who participated within them, without whom this research would not have been possible.
References
- Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: A systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2007;11(6):iii–iv. doi: 10.3310/hta11060. 1-85. [DOI] [PubMed] [Google Scholar]
- Anton RF, Hogan I, Jalali B, Riordan CE, Kleber HD. Multiple family therapy and naltrexone in the treatment of opiate dependence. Drug and Alcohol Dependence. 1981;8(2):157–168. doi: 10.1016/0376-8716(81)90110-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for beck depression inventory II (BDI II) Psychology Corporation; San Antonio, TX: 1996. [Google Scholar]
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in geneva: Follow-up at 1 and 6 months. Drug and Alcohol Dependence. 2000;58(1-2):85–92. doi: 10.1016/s0376-8716(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Archives of General Psychiatry. 2001;58(8):755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: A randomized controlled trial. Addiction (Abingdon, England) 2009;104(9):1530–1538. doi: 10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defulio A, Everly JJ, Leoutsakos JM, Umbricht A, Fingerhood M, Bigelow GE, et al. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: A randomized controlled trial. Drug and Alcohol Dependence. 2012;120(1-3):48–54. doi: 10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio A, Silverman K. The use of incentives to reinforce medication adherence. Preventive Medicine. 2012;55:S46–53. doi: 10.1016/j.ypmed.2012.04.017. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: A systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90: Administration, scoring & procedures manual for the revised version. Clinical Psychometric Research; Baltimore, MD: 1977. [Google Scholar]
- Donlin WD, Knealing TW, Needham M, Wong CJ, Silverman K. Attendance rates in a workplace predict subsequent outcome of employment-based reinforcement of cocaine abstinence in methadone patients. Journal of Applied Behavior Analysis. 2008;41(4):499–516. doi: 10.1901/jaba.2008.41-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Defulio A, Everly JJ, Donlin WD, Aklin WM, Nuzzo PA, et al. Employment-based reinforcement of adherence to oral naltrexone treatment in unemployed injection drug users. Experimental and Clinical Psychopharmacology. 2013;21(1):74–83. doi: 10.1037/a0030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everly JJ, Defulio A, Koffarnus MN, Leoutsakos JM, Donlin WD, Aklin WM, et al. Employment-based reinforcement of adherence to depot naltrexone in unemployed opioid-dependent adults: A randomized controlled trial. Addiction (Abingdon, England) 2011;106(7):1309–1318. doi: 10.1111/j.1360-0443.2011.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: Protective effect of coping responses. Addiction (Abingdon, England) 2002;97(10):1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, O'Brien CP, Greenstein R, Ternes J, Long M, Steinberg-Donato S. Effects of contingent payment on compliance with a naltrexone regimen. The American Journal of Drug and Alcohol Abuse. 1979;6(3):355–365. doi: 10.3109/00952997909001724. [DOI] [PubMed] [Google Scholar]
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Management and Healthcare Policy. 2014;7:35–44. doi: 10.2147/RMHP.S19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri A, Perucci CA. A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction (Abingdon, England) 2002;97(10):1241–1249. doi: 10.1046/j.1360-0443.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Knealing TW, Roebuck MC, Wong CJ, Silverman K. Economic cost of the therapeutic workplace intervention added to methadone maintenance. Journal of Substance Abuse Treatment. 2008;34(3):326–332. doi: 10.1016/j.jsat.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunoe N, Lobmaier P, Vederhus JK, Hjerkinn B, Gossop M, Hegstad S, et al. Challenges to antagonist blockade during sustained-release naltrexone treatment. Addiction (Abingdon, England) 2010;105(9):1633–1639. doi: 10.1111/j.1360-0443.2010.03031.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA : The Journal of the American Medical Association. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the addiction severity index. reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews (Online) 2011;4 doi: 10.1002/14651858.CD001333.pub4. CD001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, et al. Preparations for AIDS vaccine trials. an automated version of the risk assessment battery (RAB): Enhancing the assessment of risk behaviors. AIDS Research and Human Retroviruses. 1994;10(Suppl 2):S281–3. [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: A ceiling on effectiveness? The American Journal of Drug and Alcohol Abuse. 2006;32(4):503–517. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. The New England Journal of Medicine. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Pal Singh Balhara Y, Jain R. A urinalysis-based comparative study of treatment adherence on buprenorphine and buprenorphine/naloxone combination used as opioid substitution therapy. Innovations in Clinical Neuroscience. 2012;9(7-8):24–29. [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug and Alcohol Dependence. 1999;54(2):127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Shoptaw SJ, Miotto KA, Frosch DL, Obert JL, et al. Naltrexone for opioid dependence: Evaluation of a manualized psychosocial protocol to enhance treatment response. Drug and Alcohol Review. 2001;20:67–78. [Google Scholar]
- Roozen HG, Kerkhof AJ, van den Brink W. Experiences with an outpatient relapse program (community reinforcement approach) combined with naltrexone in the treatment of opioid-dependence: Effect on addictive behaviors and the predictive value of psychiatric comorbidity. European Addiction Research. 2003;9(2):53–58. doi: 10.1159/000068808. [DOI] [PubMed] [Google Scholar]
- San L, Pomarol G, Peri JM, Olle JM, Cami J. Follow-up after a six-month maintenance period on naltrexone versus placebo in heroin addicts. British Journal of Addiction. 1991;86(8):983–990. doi: 10.1111/j.1360-0443.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, DeFulio A, Sigurdsson SO. Maintenance of reinforcement to address the chronic nature of drug addiction. Preventive Medicine. 2012;55:S46–53. doi: 10.1016/j.ypmed.2012.03.013. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, et al. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. Journal of Applied Behavior Analysis. 2007;40(3):387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, et al. Management of relapse in naltrexone maintenance for heroin dependence. Drug and Alcohol Dependence. 2007;91(2-3):289–292. doi: 10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A, Stone S. A description of opiate detoxification using buprenorphine in a community setting. Journal of Substance Use. 2002;7:96–99. [Google Scholar]
- Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: A systematic review of the literature. Journal of the American Medical Informatics Association : JAMIA. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Controlled Clinical Trials. 1988;9(4):345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]