Abstract
Studies in hamsters, mice and rats have demonstrated that estradiol (E2), its interconvertible metabolite estrone (E1) and their catechol metabolites, in particular 4-hydroxy E2/E1, are carcinogenic in the kidney, uterus and mammary gland. Observational studies and clinical trials consistently show that sustained exposure to E2/E1 is associated with the development of sporadic breast cancer. The weight of evidence supports the contribution of two complementary pathways in the initiation, promotion and progression of breast cancer. One pathway involves activation of nuclear and cytoplasmic signaling pathways through the binding of estrogen to nuclear and membrane-bound estrogen receptors leading to increased cell proliferation. The other pathway involves the oxidative metabolism of E2/E1 to catechols and then reactive quinones that can contribute to oxidative DNA damage and form specific, mutagenic depurinating adducts with adenine and guanine which then in turn can serve as biomarkers for the occurrence of these processes. Both pathways can serve as portals to preventive intervention. Antiestrogens are used clinically to block receptor-mediated signaling to block tumor growth. Various chemopreventive agents such as sulforaphane (SFN) and resveratrol have been shown in cell culture to block oxidative metabolism of E2/E1 and thus prevent DNA damage. Pretreatment of MCF-7 and MCF-10F cells with and inhibitor of catechol-O-methyltransferase (COMT) followed by treatment with E2 or 4-OH E2 caused increased oxidative DNA damage (8-oxo-dG) and depurinating DNA adducts showing the importance of E2-catechol O-methylation by COMT as a protective pathway. E2 Treatment of MCF-10A cells with E2 or 4-OH E2 caused an increase in E2-adenine and guanine adducts. Treatment with sulforaphane increased NAD(P)H:quinone oxidoreductase 1 (NQO1) and glutathione-S-transferase A1 (GSTA1) expression without affecting expression of catechol-O-methyltransferase (COMT) or cytochrome P450 1B1. Pretreatment with SFN decreased depurinating DNA adducts while increasing levels of 4-OCH3E1/2 and 4-OHE1/2-glutathione conjugates. Treatment of MCF-10F cells with E2 or 4-OH-E2 also caused increased depurinating DNA adducts and neoplastic transformation while pretreatment with resveratrol caused a reduction in adduct levels and neoplastic transformation. Increased levels of estrogen-quinone conjugates and DNA adducts have also been detected in urine of women at increased risk for and with breast cancer. These observations support the notion that targeting the estrogen/estrone metabolism pathway may be another way to reduce breast cancer risk.
Keywords: breast cancer, estrogen, estrogens, estrogen oxidative metabolism, estrogen-quinone adducts, sulforaphane, resveratrol, chemoprevention, chemoprotection, DNA damage, DNA adducts
Introduction
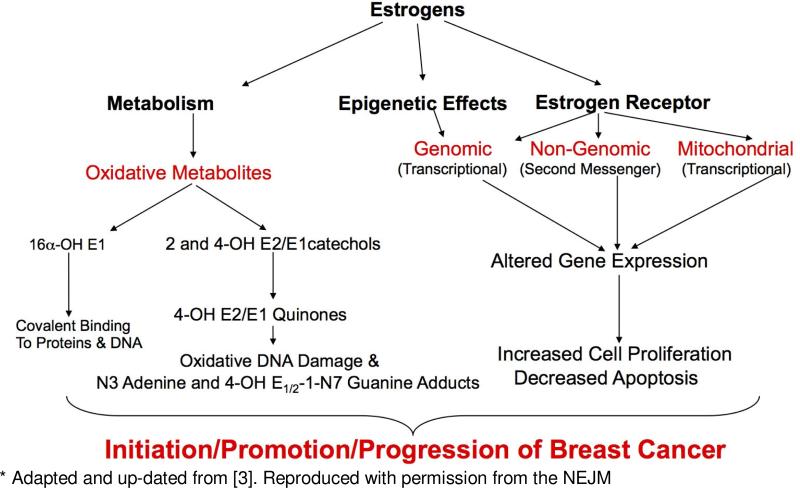
Observational studies and clinical trials in women have assessed the relationship of breast cancer risk and estrogen exposure. The results demonstrate that prolonged exposure to elevated levels of endogenous estrogen (estradiol, E2 and estrone, E1), hormone replacement treatment using conjugated equine estrogens plus medroxyprogesterone acetate and oral contraceptives containing ethinyl estradiol, are associated with a significantly increased relative risk for development of breast cancer of between approximately 1.2 to 2.5 [1-3]) (and Hankinson, Santen, Zeigler papers in this issue of the Journal. In addition, numerous studies in various animal and in vitro models have demonstrated a causal association between estrogen exposure and cancer. Figure 1 shows estrogen receptor (ER) and non-receptor pathways that likely contribute to estrogen carcinogenesis in the breast.
Figure 1.
Receptor and Non-Receptor Pathways to Estrogen Carcinogenesis*
A selection of the animal models where estrogen treatment was associated with tumor formation in various tissues is shown in Table 1.Two animal models pertain to the development of mammary tumors, the ERKO/Wnt mouse and the ACI rat. Santen and colleagues [1] (and Santen in this issue of the Journal) have demonstrated that oophorectomy of ERKO/Wnt mice significantly delayed the time of mammary tumor development, demonstrating a significant role for estrogen in the absence of the estrogen receptor. Treatment of the oophrectomized mice with E2 significantly shortened the time to tumor appearance to that of intact controls. These results provide evidence in support of a non-receptor mediated process as contributing to tumor development.
Table 1.
Tumors caused by estrogen exposure on selected animal models
| Tumor Type | Animal Model | Treatment | References |
|---|---|---|---|
| Renal | Syrian Golden Hamster | E2, E1, 4-OH E2/E1, DES | [28-30] |
| Uterine | CD-1 Mice | 2-OH E2, 4-OH E2, DES, EE | [31, 32] |
| Liver | Female Rats | Ethinyl estradiol following initiation with DEN | [33] |
| Mammary | ERKO/Wnt Mice | E2 | [1] AND Santen from this Workshop |
| Mammary | ACI Female Rat | E2 | [4-7] |
The female ACI rat develops mammary tumors in a dose-dependent response when treated with E2, but not spontaneously ([4-7] and references therein). In response to E2 treatment, the mammary gland undergoes progression from ductal hyperplasia to atypical hyperplasia leading to the appearance of adenocarcinomas beginning after 16-18 weeks of treatment, with tumor incidence reaching 30-100 percent depending on E2 dose [5]. Tumor development was only partially inhibited by tamoxifen [8] suggesting a role for a non-receptor dependent process in the mechanism of E2 induced mammary tumors in this model, similar to what has been observed in the ERKO/Wnt oophrctomized mouse model. However, ACI rat E2-induced mammary tumor development was significantly reduced from an incidence of 82% to 24% by treatment with the antioxidant butylated hydroxyanisole [9]. This decrease was associated with a significant reduction in E2-induced levels of 8-isoprostane, a marker of oxidative stress, supporting a role for oxidative damage and stress in ACI mammary tumor development.
There are also several in vitro models employing the use of MCF-7, MCF-10A and MCF-10F cells, the former being a widely used mammary tumor-derived epithelial line and the latter two being non-transformed, immortalized human mammary epithelial cell lines that do not express ERα [10]. These cell lines have been used to investigate the mechanisms of non-receptor-mediated processes associated with E2 treatment. E2 treatment of MCF-7 cells, enhanced in their capacity for oxidative metabolism of E2 to catechols and reactive quinones, caused an E2 concentration-dependent increase oxidative DNA damage, represented by increased 8-oxo-dG levels, when catechol-O-methylase (COMT) was inhibited [11]. Methylation of catechol estrogens by COMT blocks their oxidative metabolism to reactive quinones and the associated formation of reactive oxygen species (Figure 2). Cavalieri and colleagues have demonstrated that the oxidative E2/E1-quinone metabolites cause DNA damage represented by mutagenic depurinating adducts with adenine and guanine, 4-OH E1/2-1-N3 adenine and 4-OH E1/2- 1-N7 guanine [12]. Similar to what was observed in MCF-7 cells regarding oxidative DNA damage, inhibition of COMT in E2-treated MCF-10F cells resulted in increased formation of the depurinating adenine and guanine adducts [13]. These results demonstrate the importance of O-methylation by COMT for blocking further oxidative metabolism of estrogen catechols to reactive quinone metabolites [14] and provide additional support for the ability of oxidative quinone metabolites to cause DNA damage that could contribute to the carcinogenic process. Thus, a weight of evidence supports both receptor-mediated and non-receptor-mediated oxidative metabolism (Figure 1), with the latter resulting in the formation of DNA adducts and oxidative DNA damage, as contributors to the initiation, promotion and progression of breast cancer [1].
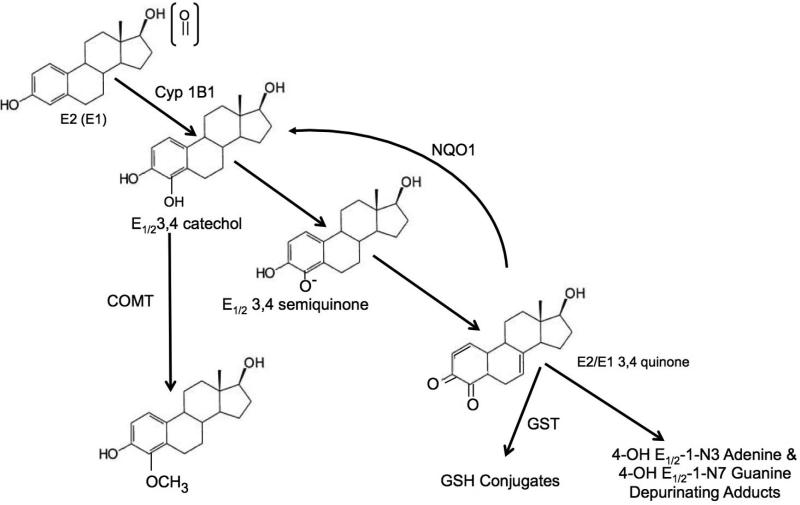
Figure 2.
The 4-oxidation of E2/E1 and the Protective Phase II Pathways
The detailed pathways of E2/E1 oxidative metabolism to 2-OH and 4-OH catechols, semi-quinones and quinones and the associated formation of reactive oxygen species (ROS) and 4-OH E1/2-1-N3 adenine and 4-OH E1/2-1-N7 guanine adducts are shown in Figure 17 of Cavalieri and Rogan [12]. The 2-hydroxylation pathway results in formation of ROS and formation of a low level of depurinating adducts. In contrast, the 4-hydroxylation pathway (Figure 2) and the resulting 4-E2/E1 quinones cause the formation of much greater levels of depurinating adenine and guanine adducts.
Cytochrome P450 1B1 has a high catalytic efficiency for formation of the 4-OH catechol and thus has been referred to as the estrogen 4-hydroxylase, catalyzing the oxidation of E2/E1 to 4-OH E2/E1 [15]. CYP1B1 has been shown to be expressed in normal human breast ductal tissue [16] and in a high percentage of invasive ductal carcinomas [17] demonstrating the potential for the oxidative metabolism of E2/E1 in normal and breast tumor tissue and the likely possibility that the resulting metabolites may contribute to the initiation and progression of breast cancer. This possibility receives additional support from the observation by Rogan and colleagues [12, 18] of an imbalance in E2/E1 and their oxidative catechol metabolites and quinone conjugates in breast tissue obtained from controls and women with breast cancer. Furthermore, in three separate studies, it has been found that levels of estrogen-quinone depurinating adducts were greater in women at high risk for developing breast cancer and in women with breast cancer compared to healthy women without breast cancer [12, 19]. Two recent studies have also reported the detection in serum of E2-2,3-quinone-4-S- and E2-3,4-quinone-2-S-albumin [20] and hemoglobin [21] adducts in Taiwanese women, with greater levels of both present in women with breast cancer compared to healthy controls. These findings suggest that detection of albumin and/or hemoglobin adducts might represent a ”body burden” for the oxidative metabolism of estrogen to its reactive quinone metabolites. While the tissue of origin of the adducts has yet to be traced, the expression of CYP1B1 in several peripheral tissues including breast, but typically not liver, along with the association of their levels with breast cancer suggests the possibility that at least a percentage of the urinary E2/E1-quinone adenine and guanine adducts and the adducts in albumin and hemoglobin may originate from estrogen metabolites formed in the breast. It will be important to conduct prospective longitudinal studies to determine if adduct levels begin to rise prior to development of breast cancer.
Estrogen Metabolism as a Potential Target for Preventive Intervention
The phase II metabolism pathways that prevent formation of the adenine and guanine adducts are shown in Figure 2. As mentioned previously, O-methylation by COMT has a major role in blocking the further oxidation of the 4-OH catechols. A low activity polymorphism in COMT has been shown in some studies to cause a small, significant increased risk for developing breast cancer. However, other protective pathways may be modulated by polymorphisms that affect the activity of component enzymes, or environmental factors may modulate protective enzyme levels. These influences may reduce or increase levels of formation of reactive E2/E1 metabolites. Thus, future investigations of the role of polymorphisms in COMT or other protective enzymes should measure biomarkers that directly reflect their activities [14].
Other key phase II enzymes include gutathione-S-transferase (GST) and NAD(P)H quinone oxidoreductase 1 (NQO1) (Figure 2). Several studies have investigated the effects of induction of selected phase II enzymes on the levels of 4-quinone adenine and guanine adducts and on neoplastic transformation. Resveratrol, found in various foods including grapes, has both antioxidant and phase II enzyme inducing activity, although its bioavailability has been reported to be low. In one study in which E2 metabolism had been enhanced by pretreatment of MCF-10F cells with 2,3,7,8-tetrachlorodibenzo-pdioxin (TCDD), a known inducer of CYP1B1, treatment with resveratrol was observed to reduce expression of CYP1B1 and increase expression and activity of NQO1 up to two fold in concentration and time dependent responses [22, 23]. In E2 metabolism-enhanced MCF-10F cells treated with E2 and in MCF-10F cells treated with 4-OHE2, 72hr pretreatment with resveratrol dramatically reduced the levels of both the 4-OH E1/2- 1-N3 adenine and 4-OH E1/2-1-N7 guanine adducts to undetectable levels [22, 23]. MCF-10F cells can be caused to undergo “neoplastic transformation” characterized by acquisition of anchorage-independent growth as indicated by their ability to form colonies when grown in soft agar. Untreated MCF-10F fail to form colonies but do so after treatment with benzpyrene, 7,12-dimethylbenz[a]pyrene and other carcinogens [24] and estradiol in a concentration-dependent manner [25]. Tumorigenic breast tumor cell lines such as MCF-7 and T47D readily form colonies in soft agar. Treatment of normal and TCDD treated (metabolism-enhanced) MCF-10F cells with E2 at 0.1, 1 and 10μM increased colony efficiency from 0% in untreated controls to 3 and 6-7% in cells not TCDD pretreated and TCDD pretreated, respectively [23]. Pretreatment with resveratrol reduced E2 caused colony efficiency to approximately 1% and 2% in TCDD metabolism non-enhanced and enhanced cells, respectively. The reduction in neoplastic transformation was associated with reduced DNA depurinating adduct levels providing support for a causal role for the depurinating DNA adducts in cell transformation.
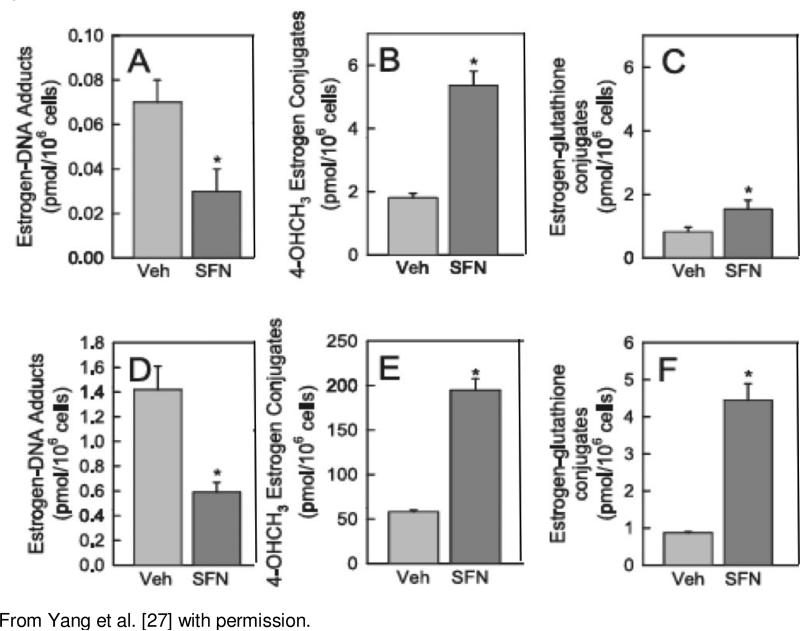
Sulforaphane, a isothiocyanate found in cruciferous vegetables, particularly 3-day old broccoli sprouts, has been demonstrated to induce a variety of genes including the phase II enzymes GST and NQO1 through the Keap1-NRF2 pathway [26]. In a recent study we investigated the effects of sulforaphane (SFN) and the NRF2 pathway on the formation of the combined levels of the 4-OH E1/2-1-N3 adenine and 4-OH E1/2-1-N7 guanine DNA adducts in MCF-10A cells [27]. MCF-10A cells were treated with SFN (3-10μM) for 24hr followed by treatment with 10μM E2 or 4-OHE2 for 24hr and then harvested. NQO1 and GSTA1 mRNA expression levels, determined by quantitative RTPCR, were significantly increased approximately 2 fold and 7 fold, respectively. No change in expression was detected for COMT or CYP1B1. Western blot analysis revealed a 3-fold increase in NQO1 protein that was accompanied by a similar increase in enzymatic activity. CYP1B1 protein expression was significantly inhibited, approximately 50%. Unexpectedly, western blot showed an approximately 2.5 fold significant increase in COMT protein levels. The mechanism by which SFN may alter COMT protein levels in the absence of increased mRNA expression requires further investigation. Analysis of the effects of SFN pretreatment on estrogen-DNA adducts, 4-OHCH3 methoxy conjugate and estrogen-glutathione conjugate levels was also conducted, Figure 3. The cells were treated with E2 (A-C) or 4-OHE2 (D-F) for 48 hrs. Treatment with the 4-OH catechol resulted in considerably greater levels of the 4- methoxy and glutathione conjugates as well as DNA adducts when compared with cells treated with E2, reflecting the low capacity of MCF-10A cells to metabolize E2 to the catechol metabolites. As expected, with E2 and 4-OHE2 treatment, SFN significantly increased the level of the 4-OHCH3 methyoxy metabolite (Figure 3 B and E), likely due to the increase in NQO1 activity resulting in reduction of the estrogen quinones back to the catechols and their subsequent 0-methylation by COMT (see Figure 2). Significant increases were also observed for the estrogen-glutathione conjugates (Figure 3 C and D). Associated with the increases in estrogen conjugates, the levels of the 4-OH E1/2-1- N3 adenine and 4-OH E1/2-1-N7 guanine DNA adducts were significantly reduced, Figures A and D. These results with SFN together with those with resveratrol demonstrate that the levels of E2/E1 reactive quinones formed by the oxidative metabolism of E2/E1 can be reduced by induction of protective phase II enzymes and that this leads to reduced levels of the mutagenic depurinating 4-OH E1/2-1-N3 adenine and 4-OH E1/2-1-N7 guanine DNA adducts.
Figure 3.
Effect of SFN on Estrogen-DNA Adducts, 4-OHCH3 and Estrogenglutathione Conjugate Levels in MCF-10A cells treated with E2 (A-C) or 4-OHE2 (D-F)*
Conclusions
Observational and clinical studies have demonstrated that persistent exposure to estrogen is associated with increased breast cancer risk. Studies in animal models have shown that E2, E1 and their oxidative catechol metabolites are carcinogenic. Results from in vivo and in vitro mechanistic studies indicate that the carcinogenicity of estrogen results from two complementary pathways, ER receptor-mediated effects on cell proliferation and oxidative metabolism of estrogen to reactive quinones that cause both oxidative DNA damage and formation depurinating 4-OH E1/2-1-N3 adenine and 4-OH E1/2-1-N7 guanine adducts. Phase II enzymes that catalyze the formation of estrogen catechol and quinone conjugates including COMT and GSTs are protective, as is NQO1 which catalyzes the reduction of the estrogen quinone to the catechol metabolites that are then O-methylated by COMT. Sulforaphane and Resveratrol induce these protective phase 2 enzymes resulting in the reduction of estrogen-induced DNA damage. Estrogen-quinone DNA adduct levels are detected in urine, and in serum, estrogenquinone adducts to albumin and hemoglobin have also been detected. The levels of these urinary and serum adducts were reported to be greater in women with breast cancer than in normal women. Prospective, longitudinal studies should be conducted to determine whether these urinary and serum adduct levels reflect the body burden of estrogen oxidative metabolism and represent biomarkers for breast cancer risk. The weight of evidence from human and experimental studies suggests that the E2/E1 oxidative metabolism pathway presents a chemoprotective target for reducing breast cancer risk.
Acknowledgements
Research done by the author and colleagues discussed in this review has been supported by grants: CA70655; CA77550; P50 CA88843; Army Grant DAMD17-03-1-0579; Maryland Cigarette Restitution Fund Research Grant at Johns Hopkins; T32 ES07141; P50 CA88843
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161–70. doi: 10.1016/j.steroids.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Yager JD, Santen RJ. Mechanisms relating estrogens to breast cancer. Translational Endocrinology & Metabolism. 2012;3:75–93. [Google Scholar]
- 3.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 4.Kovalchuk O, Tryndyak VP, Montgomery B, Boyko A, Kutanzi K, Zemp F, et al. Estrogen-induced rat breast carcinogenesis is characterized by alterations in DNA methylation, histone modifications and aberrant microRNA expression. Cell Cycle. 2007;6:2010–8. doi: 10.4161/cc.6.16.4549. [DOI] [PubMed] [Google Scholar]
- 5.Turan VK, Sanchez RI, Li JJ, Li SA, Reuhl KR, Thomas PE, et al. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol. 2004;183:91–9. doi: 10.1677/joe.1.05802. [DOI] [PubMed] [Google Scholar]
- 6.Harvell DM, Strecker TE, Tochacek M, Xie B, Pennington KL, McComb RD, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc Natl Acad Sci U S A. 2000;97:2779–84. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 8.Singh B, Bhat NK, Bhat HK. Partial inhibition of estrogen-induced mammary carcinogenesis in rats by tamoxifen: balance between oxidant stress and estrogen responsiveness. PloS one. 2011;6:e25125. doi: 10.1371/journal.pone.0025125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh B, Mense SM, Remotti F, Liu X, Bhat HK. Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J Biochem Mol Toxicol. 2009;23:202–11. doi: 10.1002/jbt.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr., Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–86. [PubMed] [Google Scholar]
- 11.Lavigne JA, Goodman JE, Fonong T, Odwin S, He P, Roberts DW, et al. The effects of catechol-O-methyltransferase inhibition on estrogen metabolite and oxidative DNA damage levels in estradiol-treated MCF-7 cells. Cancer Res. 2001;61:7488–94. [PubMed] [Google Scholar]
- 12.Cavalieri E, Rogan E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Molecular aspects of medicine. 2014;36:1–55. doi: 10.1016/j.mam.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahid M, Saeed M, Lu F, Gaikwad N, Rogan E, Cavalieri E. Inhibition of catechol-O-methyltransferase increases estrogen-DNA adduct formation. Free Radic Biol Med. 2007;43:1534–40. doi: 10.1016/j.freeradbiomed.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yager JD. Catechol--methyltransferase: characteristics, polymorphisms and role in breast cancer. Drug discovery today Disease mechanisms. 2012;9:e41–e6. doi: 10.1016/j.ddmec.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996;93:9776–81. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr. 2000:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 17.Rahman M, Lax SF, Sutter CH, Tran QT, Stevens GL, Emmert GL, et al. CYP1B1 is not a major determinant of the disposition of aromatase inhibitors in epithelial cells of invasive ductal carcinoma. Drug Metab Dispos. 2008;36:963–70. doi: 10.1124/dmd.107.018960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogan EG, Badawi AF, Devanesan PD, Meza JL, Edney JA, West WW, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 19.Pruthi S, Yang L, Sandhu NP, Ingle JN, Beseler CL, Suman VJ, et al. Evaluation of serum estrogen-DNA adducts as potential biomarkers for breast cancer risk. J Steroid Biochem Mol Biol. 2012;132:73–9. doi: 10.1016/j.jsbmb.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C, Chen DR, Hsieh WC, Yu WF, Lin CC, Ko MH, et al. Investigation of the cumulative body burden of estrogen-3,4-quinone in breast cancer patients and controls using albumin adducts as biomarkers. Toxicol Lett. 2013;218:194–9. doi: 10.1016/j.toxlet.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, Hsieh WC, Chen DR, Kuo SJ, Yu WF, Hu SW, et al. Hemoglobin adducts as biomarkers of estrogen homeostasis: Elevation of estrogenquinones as a risk factor for developing breast cancer in Taiwanese Women. Toxicol Lett. 2014;225:386–91. doi: 10.1016/j.toxlet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Zahid M, Gaikwad NW, Ali MF, Lu F, Saeed M, Yang L, et al. Prevention of estrogen-DNA adduct formation in MCF-10F cells by resveratrol. Free Radic Biol Med. 2008;45:136–45. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu F, Zahid M, Wang CC, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev Res. 2008;1:135–45. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calaf G, Russo J. Transformation of human breast epithelial cells by chemical carcinogens. Carcinogenesis. 1993;14:483–92. doi: 10.1093/carcin/14.3.483. [DOI] [PubMed] [Google Scholar]
- 25.Russo J, Lareef MH, Tahin Q, Hu YF, Slater C, Ao X, et al. 17Beta-estradiol is carcinogenic in human breast epithelial cells. J Steroid Biochem Mol Biol. 2002;80:149–62. doi: 10.1016/s0960-0760(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 26.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–9. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L, Zahid M, Liao Y, Rogan EG, Cavalieri EL, Davidson NE, et al. Reduced formation of depurinating estrogen-DNA adducts by sulforaphane or KEAP1 disruption in human mammary epithelial MCF-10A cells. Carcinogenesis. 2013;34:2587–92. doi: 10.1093/carcin/bgt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–32. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- 29.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986;24:353–6. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 30.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 31.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–7. [PubMed] [Google Scholar]
- 32.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50:7677–81. [PubMed] [Google Scholar]
- 33.Yager JD, Shi YE. Synthetic estrogens and tamoxifen as promoters of hepatocarcinogenesis. Prev Med. 1991;20:27–37. doi: 10.1016/0091-7435(91)90004-n. [DOI] [PubMed] [Google Scholar]