Abstract
Background & Aims
Esophageal adenocarcinoma is believed to result from the progression of gastroesophageal reflux disease to erosive esophagitis and re-epithelialization of the esophagus with a columnar cell population termed Barrett's esophagus (BE). Men develop BE and esophageal adenocarcinoma more frequently than women, and the ratio is increasing; approximately 7 men are diagnosed with malignancy for every woman, yet little is known about the mechanisms of this difference. We assessed whether sex steroid hormones were associated with BE in a male population.
Methods
We analyzed data from the Barrett's Esophagus Early Detection Case Control Study, based at the Walter Reed National Military Medical Center. Blood samples were collected from 173 men with BE and 213 men without BE (controls, based on endoscopic analysis); 13 sex steroid hormones were measured by mass spectrometry and sex hormone binding globulin was measured by ELISA. We also calculated free estradiol, free testosterone and free dihydrotestosterone (DHT). We used multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) adjusted for age, race, smoking status, alcohol consumption, body mass index (BMI; kg/m2), heartburn, regurgitation, and gastroesophageal symptom score (excluding heartburn and regurgitation).
Results
Levels of free testosterone and free DHT were positively associated with BE risk; patients in the highest quartile for these hormones were most likely to have BE (for free testosterone, OR=5.36; 95% CI, 2.21–13.03; P=0.0002 and for free DHT, OR=4.25, 95% CI, 1.87–9.66; P=.001). Level of estrone sulfate was inversely associated with BE risk (P for trend=.02). No other hormone was associated with BE risk. Relationships were not modified by age or BMI.
Conclusions
In an analysis of men, levels of free testosterone and free DHT were significantly associated with risk of BE.
Keywords: BEEDS, SHBG, gonadal steroid hormones, esophageal neoplasms, cancer risk
Introduction
The extraordinary and progressively widening sex ratio observed during the natural history of erosive esophagitis to Barrett's esophagus (BE) to esophageal adenocarcinoma is dramatic, peaking at malignancy with more than seven males diagnosed for every female 1, 2. Analyses of Barrett's and Esophageal Adenocarcinoma CONsortium (BEACON) data 3-8 and other population-based studies 9-13 have enabled assessment of how risk factors may differ between the sexes, yet no analysis to-date has been able to explain the large sex disparities of this disease.
Gastroesophageal reflux is one of the primary risk factors for development of BE and esophageal adenocarcinoma, yet reflux symptoms are approximately equal between the sexes 2, 9 and associations of reflux with esophageal adenocarcinoma are equal or stronger for females compared with males 8. Body mass index (BMI) is another major risk factor for esophageal adenocarcinoma 5 and an analysis of BE has shown that abdominal obesity may be of greatest importance 6; these relationships do not appear to be altered or attenuated when adjusted for, or stratified by, symptomatic gastroesophageal reflux. Moreover, the strengths of these associations are similar in both sexes, and population attributable risks estimable from published associations between waist circumference and BE (females=55%, males=37%) 6 and BMI and esophageal adenocarcinoma (females=28%, males=33%) 5 are approximately equal between the sexes or stronger for females compared with males. This evidence, as well as postulated mechanisms of association between obesity and other inflammation-related cancers, have led to the proposition that systemic inflammation may partly account for the strong relationship between obesity and the pathogenesis of esophageal adenocarcinoma. The evidence base that sex steroid hormones are involved in inflammatory processes 14-17 and the fact that sex steroid hormone receptor proteins are expressed in esophageal tissues 18, 19 supports the hypothesis that sex steroid hormones may underlie sex disparities in the pathogenesis of BE and esophageal adenocarcinoma 20. We assessed this hypothesis by conducting the first analysis of circulating sex steroid hormones in relation to BE.
Methodology
Study Population
BE cases and endoscopy controls macroscopically-negative for BE were recruited between 2004 and 2012 as part of the Barrett's Esophagus Early Detection Case Control Study (BEEDS) which was based at the Walter Reed National Military Medical Center (WRNMMC) in Bethesda, MD. BE cases were required to have histologic confirmation of specialized intestinal metaplasia with goblet cells; prevalent and incidence cases were eligible. Endoscopy control patients were referred for endoscopy for a variety of reasons including dyspepsia, reflux symptoms, and anemia and were frequency-matched to BE patients on sex. Individuals had to be at least 18 years of age to be eligible for inclusion and were excluded if they had severe pulmonary or cardiac disease, were pregnant, had an inability to give consent, had an active malignancy or were diagnosed with such in the past 5 years (excluding non-melanoma skin cancer). Participants provided a 15 mL blood sample, were interviewed for information on demographics, BMI, and lifestyle factors—including a modified version of a 7-question “GERD Questionnaire” 21—and had clinical data abstracted from medical records. For this analysis, selection was restricted to males because there were too few females to provide adequate statistical power for a female-only analysis. We selected all males that had ≥0.6 ml of serum for analysis, which resulted in 212 controls and 173 BE cases providing 80% power to detect an odds ratio (OR) of 1.8 based on a median split at alpha 0.05. BEEDS was approved by the NCI Clinical Center IRB and the National Naval Medical Center IRB.
Laboratory analysis
In collaboration with the Pharmacogenomics Laboratory of Laval University, Quebec, Canada, we quantitatively assessed: dehydroepiandrosterone (DHEA), androstenediol, androstenedione, testosterone, dihydrotestosterone (DHT), androsterone (ADT), estrone (E1) and estradiol (E2) using gas chromatography–mass spectrometry (GC-MS); dehydroepiandrosterone-sulphate (DHEAS), 3-androstanediol-3 glucuronide (3α-diol-3G), 3-androstanediol-17 glucuronide (3α-diol-17G), androsterone glucuronide (ADT-G), and estrone sulfate (E1S) using liquid chromatography tandem mass spectrometry (LC-MS/MS); and sex hormone binding globulin (SHBG) using ELISA (Diagnostics Biochem Canada, Inc.). These selected sex steroid hormones cover a wide array and key positions of the sex steroid biosynthesis pathway (Figure). We included 5% quality control (QC) samples from three healthy male individuals, aged 24, 39, and 46 years at blood draw. All coefficients of variation (CVs) were <15% (mean 8%, standard deviation 3%) except DHEAS (17%), E1S (18%), and ADT (19%). The CV for SHBG quantified by ELISA was 23% which is within manufacturer's expected range of <25%.
Figure. Schematic of Sex Steroid Hormone Metabolism.
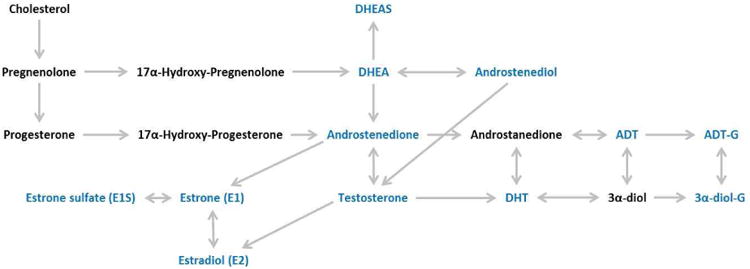
Sex steroid hormones that were quantitated are shown in blue font. Note that only 12 names are shown in blue yet 14 assays were conducted. This is because 3α-diol-G was quantitated as the separate metabolites of 3-androstanediol-3 glucuronide (3α-diol-3G) and 3-androstanediol-17 glucuronide (3α-diol-17G), and Sex Hormone Binding Globulin (SHBG) is not shown as it is not a part of the sex steroid biosynthesis pathway. Abbreviations: 3α-diol, 3-androstanediol glucuronide; ADT, androsterone; ADT-G, androsterone glucuronide; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone-sulphate; DHT, dihydrotestosterone.
Statistical Analysis
We used unconditional logistic regression models to estimate ORs and 95 percent confidence intervals (95%CI). Each exposure was assessed as quartiles using cut-points based on the control distribution, as well as a continuous metric with standardization to half the value of the interquartile range. In addition to assessing individual exposures, we also assessed a priori specified combinations and ratios of hormones that are near each other in the metabolic pathway: parent estrogens (the sum of E1 and E2), testosterone:parent estrogens ratio, testosterone:E2 ratio, and androstenedione:E1 ratio. We calculated free estradiol 22, free testosterone 23, and free DHT 24 using formulas that include the individual hormone, SHBG and a constant for albumin.
Minimally adjusted models included age (quartiles) as a covariate. We also assessed whether adjustment for race (white/non-white or unknown) smoking status (ever/never), pack-years of smoking (tertiles), alcohol consumption (never/monthly/weekly/daily), BMI (kg/m2), heartburn (never/monthly/weekly/daily), regurgitation (never/monthly/weekly/daily), gastroesophageal symptom score (excluding heartburn and regurgitation) and separate groupings of current medications (proton pump inhibitors, H2 receptor antagonists, antacids, non-steroidal anti-inflammatory drugs) consistently changed OR estimates by more than 10%. None of these covariates consistently affected ORs, but we included age, race, smoking status, alcohol consumption, BMI, heartburn, regurgitation, and gastroesophageal symptom score in the fully-adjusted model given previous evidence that these exposures are associated with BE. We also assessed whether relationships between exposures and BE were modified by age or BMI by conducting stratified analyses based on the median control values. All tests were two-sided and p-values <0.05 were considered to be statistically significant. Analyses were conducted using STATA version 11 (Stata-Corp LP, College Station, TX).
Results
There were 212 controls and 173 BE cases for analysis (Table 1). Cases were more likely to be older, to have ever-smoked, and to have consumed alcohol daily.
Table 1. Distributions of examined variables by case-control status.
| Variable | Controls (n=212) | Barrett's Esophagus (n=173) |
|---|---|---|
| Demographic Variables | ||
| Age (years; mean (SD)) | 47.25 (11.88) | 56.49 (11.35) |
| Body mass index (kg/m2; mean (SD)) | 28.37 (4.51) | 28.41 (4.08) |
| Race (%) | ||
| White | 70.9 | 81.6 |
| Non-white/unknown | 29.1 | 19.4 |
| Ever-Smoked (%) | 31.5 | 48.0 |
| Pack-years smoked | 9.8 (2.2, 21.0) | 22.0 (9.0, 47.0) |
| Frequency of Alcohol Use (%) | ||
| Never/Less than monthly | 15.7 | 16.1 |
| Monthly | 21.3 | 16.7 |
| Weekly | 47.7 | 44.4 |
| Daily | 15.2 | 22.8 |
| GERD Score (out of 13) | 6 (4, 8) | 6 (4, 8) |
| GERD Score without Heartburn and Regurgitation (out of 7) | 2 (2, 3) | 2 (1, 3) |
| Ever Experienced Daily Heartburn (%) | ||
| Never | 10.3 | 11.0 |
| Monthly | 22.1 | 20.8 |
| Weekly | 29.1 | 28.9 |
| Daily | 38.5 | 39.3 |
| Ever Experienced Daily Regurgitation (%) | ||
| Never | 10.3 | 13.3 |
| Monthly | 36.2 | 25.4 |
| Weekly | 32.4 | 36.4 |
| Daily | 21.1 | 24.9 |
| Hormone Variables | ||
| DHEA (nmol/L) | 9.19 (5.90, 11.84) | 6.33 (3.66, 9.25) |
| DHEAS (umol/L) | 3.32 (2.25, 4.72) | 2.38 (1.23, 3.93) |
| Androstenediol (pmol/L) | 2323.6 (1715.3, 3162.9) | 2004.2 (1508.8, 2877.4) |
| Androstenedione (nmol/L) | 2.83 (2.31, 3.87) | 2.69 (2.2, 3.4) |
| Testosterone (nmol/L) | 14.1 (10.7, 18.3) | 14.2 (11.2, 18.3) |
| DHT (pmol/L) | 1107.0 (767.4, 1559.0) | 1130.3 (768.7, 1442.6) |
| 3adiol3g (nmol/L) | 3.33 (2.41, 4.95) | 3.36 (2.28, 4.82) |
| 3adiol17g (nmol/L) | 8.66 (6.00, 12.36) | 8.87 (5.38, 11.87) |
| ADT (pmol/L) | 803.6 (617.1, 1031.6) | 656.1 (490.3, 885.3) |
| ADT-G (nmol/L) | 78.0 (56.4, 119.0) | 69.8 (45.4, 108.2) |
| E1 (pmol/L) | 128.0 (97.5, 161.6) | 123.4 (97.0, 146.0) |
| E1S (nmol/L) | 1.58 (1.04, 2.33) | 1.39 (0.82, 2.04) |
| E2 (pmol/L) | 90.4 (73.3, 112.7) | 90.7 (73.4, 107.5) |
| SHBG (nmol/L) | 31.5 (19.2, 44.5) | 30.8 (22.3, 43.6) |
| Free Testosterone (nmol/L) | 0.29 (0.24, 0.38) | 0.29 (0.24, 0.37) |
| Free DHT (pmol/L) | 27.13 (21.21, 35.44) | 27.41 (20.91, 37.09) |
| Free Estradiol (pmol/L) | 2.42 (1.93, 3.08) | 2.32 (1.87, 2.94) |
Unless otherwise stated, statistics shown are the median and interquartile range.
Partial correlation coefficients of hormones and SHBG adjusted for age (continuous) and BMI (continuous) amongst control subjects (Supplemental Table 1) provided strongest coefficients for the pairings of free estradiol and E2 (r=0.92), free E2 and E1 (r=0.75), free testosterone and testosterone (r=0.75), estradiol and estrone (r=0.74), and free DHT and DHY (r=0.74).
Table 2 shows the results of the multivariable analyses of all quantitated exposures. Testosterone did share a positive association with BE, with the fourth quartile compared with the first providing an OR of 2.26 (95% CI: 1.03, 4.95, p=0.04), although the test for trend was not statistically significant (p=0.29). High levels of E1S were significantly inversely associated with BE, as shown by the OR for the continuous analysis (0.59, 95%CI: 0.38, 0.92, p=0.02).
Table 2. Multivariable analysis of the associations between individual sex steroid hormones, SHBG and Barrett's esophagus.
| Variable | Controls (n=213) | Cases (n=174) | OR | 95% CI | pvalue |
|---|---|---|---|---|---|
| DHEA (nmol/L) | |||||
| <5.90 | 52 | 70 | |||
| 5.90-<9.20 | 48 | 48 | 1.06 | (0.57, 2.00) | 0.85 |
| 9.20-<11.85 | 47 | 18 | 0.57 | (0.26, 1.23) | 0.15 |
| >11.85 | 50 | 23 | 0.93 | (0.41, 2.10) | 0.86 |
| continuous | 197 | 159 | 0.74 | (0.46, 1.17) | 0.19 |
| DHEAS (umol/L) | |||||
| <2.25 | 47 | 67 | |||
| 2.25-<3.32 | 48 | 32 | 0.69 | (0.33, 1.41) | 0.31 |
| 3.32-<4.72 | 44 | 23 | 0.74 | (0.34, 1.63) | 0.46 |
| >4.72 | 43 | 21 | 1.13 | (0.48, 2.64) | 0.79 |
| continuous | 182 | 143 | 1.11 | (0.66, 1.86) | 0.70 |
| Androstenediol (pmol/L) | |||||
| <1715.26 | 51 | 53 | |||
| 1715.26-<2323.60 | 48 | 45 | 1.41 | (0.72, 2.78) | 0.32 |
| 2323.60-<3162.90 | 51 | 30 | 0.98 | (0.48, 2.00) | 0.95 |
| >3192.9 | 47 | 29 | 1.62 | (0.75, 3.52) | 0.22 |
| continuous | 197 | 157 | 1.21 | (0.72, 2.03) | 0.47 |
| Androstenedione (nmol/L) | |||||
| <2.32 | 48 | 52 | |||
| 2.32-<2.83 | 51 | 36 | 0.64 | (0.32, 1.29) | 0.21 |
| 2.83-<3.87 | 49 | 44 | 0.93 | (0.46, 1.88) | 0.83 |
| >3.87 | 49 | 27 | 0.71 | (0.34, 1.48) | 0.36 |
| continuous | 197 | 159 | 1.01 | (0.63, 1.62) | 0.96 |
| Testosterone (nmol/L) | |||||
| <10.72 | 48 | 34 | |||
| 10.72-<14.14 | 51 | 44 | 1.49 | (0.72, 3.07) | 0.28 |
| 14.14-<18.31 | 49 | 42 | 1.96 | (0.92, 4.16) | 0.08 |
| >18.31 | 49 | 38 | 2.26 | (1.03, 4.95) | 0.04 |
| continuous | 197 | 158 | 1.39 | (0.76, 2.55) | 0.29 |
| DHT (pmol/L) | |||||
| <767.38 | 49 | 38 | |||
| 767.38-<1107.03 | 49 | 38 | 1.08 | (0.52, 2.22) | 0.84 |
| 1107.03-<1559.02 | 51 | 51 | 1.53 | (0.74, 3.13) | 0.25 |
| >1559.02 | 48 | 32 | 1.14 | (0.52, 2.47) | 0.75 |
| continuous | 197 | 159 | 1.19 | (0.72, 1.96) | 0.49 |
| 3α-diol-3G (nmol/L) | |||||
| <2.42 | 50 | 47 | |||
| 2.42-<3.31 | 49 | 27 | 0.89 | (0.43, 1.86) | 0.76 |
| 3.31-<4.96 | 45 | 43 | 1.42 | (0.70, 2.89) | 0.33 |
| >4.96 | 50 | 38 | 1.36 | (0.66, 2.80) | 0.40 |
| continuous | 194 | 155 | 0.99 | (0.76, 1.28) | 0.93 |
| 3α-diol-17G (nmol/L) | |||||
| <6.00 | 50 | 44 | |||
| 6.00-<8.65 | 47 | 30 | 1.17 | (0.56, 2.44) | 0.67 |
| 8.65-<12.36 | 48 | 45 | 1.75 | (0.88, 3.49) | 0.11 |
| >12.36 | 49 | 32 | 1.59 | (0.74, 3.41) | 0.23 |
| continuous | 194 | 151 | 1.02 | (0.74, 1.41) | 0.90 |
| ADT (pmol/L) | |||||
| <617.13 | 50 | 77 | |||
| 617.13-<803.65 | 52 | 33 | 0.58 | (0.30, 1.15) | 0.12 |
| 803.65-<1031.64 | 48 | 28 | 0.62 | (0.31, 1.26) | 0.19 |
| >1031.64 | 47 | 21 | 0.79 | (0.35, 1.78) | 0.57 |
| continuous | 197 | 159 | 1.05 | (0.63, 1.74) | 0.86 |
| ADT-G (nmol/L) | |||||
| <56.45 | 47 | 54 | |||
| 56.45-<78.02 | 50 | 36 | 0.90 | (0.45, 1.79) | 0.76 |
| 78.02-<118.98 | 50 | 35 | 1.41 | (0.68, 2.94) | 0.35 |
| >118.98 | 46 | 30 | 0.95 | (0.46, 1.97) | 0.89 |
| continuous | 193 | 155 | 0.95 | (0.67, 1.33) | 0.76 |
| E1 (pmol/L) | |||||
| <97.50 | 50 | 36 | |||
| 97.50-<127.97 | 48 | 48 | 1.29 | (0.64, 2.60) | 0.47 |
| 127.97-<161.59 | 48 | 47 | 1.59 | (0.79, 3.19) | 0.20 |
| >161.59 | 51 | 28 | 0.71 | (0.33, 1.50) | 0.37 |
| continuous | 197 | 159 | 0.64 | (0.36, 1.14) | 0.13 |
| E1S (nmol/L) | |||||
| <0.92 | 45 | 54 | |||
| 0.92-<1.49 | 44 | 33 | 0.54 | (0.26, 1.10) | 0.09 |
| 1.49-<2.17 | 46 | 29 | 0.70 | (0.33, 1.46) | 0.34 |
| >2.17 | 44 | 29 | 0.48 | (0.23, 1.03) | 0.06 |
| continuous | 179 | 145 | 0.59 | (0.38, 0.92) | 0.02 |
| E2 (pmol/L) | |||||
| <73.33 | 50 | 38 | |||
| 73.33-<90.44 | 48 | 36 | 1.02 | (0.49, 2.11) | 0.95 |
| 90.44-<112.73 | 50 | 58 | 2.03 | (1.02, 4.05) | 0.04 |
| >112.73 | 49 | 27 | 0.87 | (0.41, 1.85) | 0.73 |
| continuous | 197 | 159 | 0.99 | (0.55, 1.80) | 0.99 |
| SHBG (nmol/L) | |||||
| <19.22 | 50 | 31 | |||
| 19.22-<31.53 | 49 | 56 | 1.10 | (0.54, 2.25) | 0.80 |
| 31.53-<44.53 | 48 | 35 | 0.67 | (0.30, 1.50) | 0.33 |
| >44.53 | 50 | 36 | 0.53 | (0.24, 1.16) | 0.11 |
| continuous | 197 | 158 | 0.71 | (0.44, 1.14) | 0.16 |
Adjusted for age, smoking status, alcohol consumption, heartburn, regurgitation, gastroesophageal symptom score (excluding heartburn and regurgitation), BMI, and race.
Continuous sex steroid hormone values were standardized to half of the difference between the 75th and 25th centiles of the distribution.
Table 3 shows the results of combinations and ratios as well as the calculated free hormones. Consistent with the testosterone results shown in Table 2, increasing free testosterone was also associated with BE. This association was particularly strong and progressively increased with each subsequent quartile (p for trend=0.003), peaking in the highest with an OR of 5.36 (95%CI: 2.21, 13.03, p=0.0002). Although the testosterone:parent estrogens ratio also provided results supportive of an effect for testosterone, there was no obvious trend in the quartile estimates and additional adjustment for free testosterone attenuated the estimates (data not shown) indicating that the effect was mediated by free testosterone. Free DHT was similarly positively associated with BE with an OR of 4.25 (95% CI: 1.87, 9.66) for the fourth quartile compared with the first. None of the other hormone metrics shown in Tables 2 and 3 appeared to share a relationship with BE.
Table 3. Multivariable analysis of associations between combinations, ratios, and free (unbound) sex steroid hormones and Barrett's esophagus.
| Variable | Controls (n=212) | Cases (n=173) | OR | 95% CI | p value |
|---|---|---|---|---|---|
| Parent Estrogens (pmol/L) | |||||
| <175.89 | 49 | 35 | |||
| 175.89-<220.52 | 51 | 52 | 1.16 | (0.58, 2.31) | 0.67 |
| 220.52-<280.81 | 47 | 49 | 1.74 | (0.85, 3.55) | 0.13 |
| >280.81 | 50 | 23 | 0.62 | (0.29, 1.36) | 0.23 |
| continuous | 197 | 159 | 0.73 | (0.39, 1.35) | 0.31 |
| Testosterone: Parent Estrogens Ratio (pmol/L) | |||||
| <46.71 | 49 | 28 | |||
| 46.71-<69.53 | 51 | 61 | 2.27 | (1.11, 4.64) | 0.02 |
| 69.53-<89.55 | 50 | 43 | 1.79 | (0.83, 3.87) | 0.14 |
| >89.55 | 47 | 26 | 2.02 | (0.86, 4.77) | 0.11 |
| continuous | 197 | 158 | 1.56 | (0.86, 2.83) | 0.14 |
| Androstenedione: E1 Ratio (pmol/L) | |||||
| <17.81 | 51 | 51 | |||
| 17.81-<22.84 | 48 | 41 | 0.81 | (0.41, 1.60) | 0.55 |
| 22.84-<30.43 | 51 | 33 | 0.77 | (0.38, 1.55) | 0.46 |
| >30.43 | 47 | 34 | 1.32 | (0.62, 2.82) | 0.47 |
| continuous | 197 | 159 | 1.13 | (0.65, 1.94) | 0.67 |
| Testosterone: E2 Ratio (pmol/L) | |||||
| <119.31 | 50 | 30 | |||
| 119.31-<160.81 | 49 | 59 | 1.53 | (0.76, 3.09) | 0.23 |
| 160.81-<205.73 | 50 | 36 | 1.22 | (0.56, 2.64) | 0.61 |
| >205.73 | 48 | 33 | 1.59 | (0.71, 3.54) | 0.26 |
| continuous | 197 | 158 | 1.27 | (0.67, 2.43) | 0.47 |
| Free Testosterone (nmol/L) | |||||
| <0.24 | 48 | 39 | |||
| 0.24-<0.29 | 50 | 35 | 1.50 | (0.73, 3.11) | 0.27 |
| 0.29-<0.38 | 51 | 48 | 2.77 | (1.30, 5.87) | 0.01 |
| >0.38 | 48 | 35 | 5.36 | (2.21, 13.03) | 0.0002 |
| continuous | 197 | 157 | 2.67 | (1.41, 5.08) | 0.003 |
| Free DHT (pmol/L) | |||||
| <21.21 | 49 | 39 | |||
| 21.21-<27.13 | 49 | 37 | 1.28 | (0.63, 2.60) | 0.50 |
| 27.13-<35.44 | 49 | 37 | 1.98 | (0.93, 4.23) | 0.08 |
| >35.44 | 50 | 44 | 4.25 | (1.87, 9.66) | 0.001 |
| continuous | 197 | 157 | 1.98 | (1.16, 3.38) | 0.01 |
| Free Estradiol (pmol/L) | |||||
| <1.96 | 50 | 40 | |||
| 1.96-<2.42 | 49 | 46 | 1.35 | (0.68, 2.68) | 0.40 |
| 2.42-<3.09 | 50 | 45 | 1.38 | (0.69, 2.74) | 0.36 |
| >3.09 | 48 | 27 | 1.25 | (0.58, 2.70) | 0.56 |
| continuous | 197 | 158 | 1.28 | (0.68, 2.41) | 0.44 |
Adjusted for age, smoking status, alcohol consumption, heartburn, regurgitation, gastroesophageal symptom score (excluding heartburn and regurgitation), BMI, and race. Continuous sex steroid hormone values were standardized to half of the difference between the 75th and 25th centiles of the distribution.
There was little evidence for any effect modification by age (Supplemental Table 2) or by BMI (Supplemental Table 3) in the stratified analyses.
Discussion
In this analysis of serum sex steroid hormones in relation to BE in men, we found evidence for strong positive associations with free testosterone and with free DHT. In addition, we also observed an inverse association with high levels of E1S. There was no evidence that these relationships were modified by age or BMI.
The large sex disparities of BE 2 and esophageal adenocarcinoma 1, coupled with strong relationships with obesity 5, 6, have led to hypotheses that sex steroid hormones may underlie these observations 13, 20, 25-27. Hypotheses include a protective role for estrogens; a carcinogenic effect of androgens; and/or effects caused by alterations in the ratio of androgens to estrogens. While no previous study has assessed circulating sex steroid hormones in relation to BE, one previous small case-control study did relate hormone levels to esophageal adenocarcinoma 28. Pre-operative serum testosterone levels were significantly higher in 25 male esophageal adenocarcinoma patients (median=18.2 nM/L) compared with eight age-matched patients undergoing surgery for benign conditions (median=12.5 nM/L, p=0.01). Although this may offer support for our observations, the endpoint was different. Further, post-operative (≥ 3 months) levels in these esophageal adenocarcinoma cases were reduced to levels similar to controls (median=12.2 nM/L), which led the authors to conclude that the high pre-operative levels may have been partly attributable to production by the tumor.
There is other evidence that may support our observations, particularly for the effect of free testosterone and free DHT. Individuals diagnosed with prostate cancer, who often receive some form of androgen deprivation therapy which severely reduces testosterone and DHT levels, have shown reduced risks of esophageal adenocarcinoma with standardized incidence ratios (SIR) of 0.83 (p<0.05) in a US population 29 and 0.70 (p<0.05) in a UK population 30.
Studies of reproductive factors—as proxies of hormonal exposure—in relation to esophageal adenocarcinoma have been conducted, but these had limited statistical power and all but one 31 were restricted to women 10, 26, 32-35. Thus these prior results are unlikely to be of value in interpreting our findings here of quantitated sex steroid hormones in relation to the precursor metaplasia BE in a male population, especially given that altered levels of sex steroid hormones may have distinct effects within each sex.
There are at least three non-mutually exclusive mechanisms for our observed association with testosterone, assuming causality. The first is that testosterone and DHT are inversely associated with wound healing, possibly by inhibition of re-epithelialization 36-38, and in the esophagus this could potentially expand the interval for opportunistic metaplastic re-population. The second potential mechanism is related to inflammation. Although testosterone and DHT are generally considered anti-inflammatory 14, 39, it has also been proposed that these androgens may increase inflammation via immunosuppression 39-41. In theory, testosterone could also undergo intra-esophageal conversion to estradiol via aromatase with subsequent pro-inflammatory effects 14, although there is scant evidence that CYP19A1 is expressed in normal esophagus (GDS132142, GDS383843). The third possible mechanism is that testosterone could influence lower esophageal sphincter (LES) tone or frequency of transient LES relaxations, thus increasing the propensity for gastroesophageal reflux 44, 45. In this study, adjustment for reflux symptoms had negligible effect on the association between free testosterone and BE. However, comparison with an endoscopy control group hindered our ability to assess the effect of reflux symptoms on hormone-BE associations because only 10% reported never having had daily symptoms of reflux.
With regard to the receptors for these hormones, androgen receptor (AR) protein is mostly absent from normal esophageal squamous tissue 46, 47, although one study did report positive staining in seven of 23 specimens 28. In addition, AR gene transcription has been observed in both normal squamous epithelium 28(GEO accession: GDS3838 43) and—to a weaker extent—in BE (GDS3472 48, GDS1321 42). More importantly, perhaps, is evidence from mice of AR up-regulation in epithelial cells, fibroblasts and macrophages following wounding 37, which may support our re-epithelialization theory. To-date, assessment of AR transcription or translation has not been assessed in erosive esophagitis patients, which may be the disease-point of interest for further assessment of this idea.
There is more consistent evidence for the presence of estrogen receptor (ER) β protein in normal squamous tissue 49-52 and in BE 53-55, and weaker evidence for ER α protein 49-52. Gene expression data support the presence of both ER β and ER α receptors in stratified squamous epithelium and in BE (GDS4350 56, GDS1321 42, GDS3838 43, GDS3472 48).
We did observe an inverse association between E1S concentration and BE, although no single quartile was itself statistically significant which may warrant a cautious interpretation. Prior animal and in vitro studies of esophageal adenocarcinoma have shown that E2 57, 58 and 2-methoxyestradiol 59 exhibit anti-carcinogenic properties, and the normal esophagus is known to express certain 17-beta-hydroxysteroid dehydrogenases—such as HSD17B-1, 4, 5, 7, 8, 10 and 11 (GDS435056 GDS132142 GDS347248 GDS383843)—which indicates that local conversion between estrone and estradiol is possible. Animal and in vitro model systems have not tested the effects of E1. The inverse association between E1S and BE and the lack of associations with E1, E2, or androgen:estrogen ratios do not support the laboratory evidence of anti-carcinogenic effects in esophageal adenocarcinoma. However, estrogen metabolism is complex and we quantitated only three metabolites of this pathway.
Strengths of this analysis include: the use of mass spectrometry to accurately quantitate steroid hormones; histologic confirmation of specialized intestinal metaplasia with goblet cells for identification of a homogeneous case group; uniform assessment at a single institute; adjustment for separate groupings of current anti-reflux medications had no effect on our estimates, despite inconsistent evidence that specific formulations of these medications may affect circulating testosterone levels 60; and the fact that controls were recruited from the same endoscopy clinics, did not have macroscopically identifiable BE, and form the base population from which BE patients were identified for this study. Limitations include: phlebotomy was conducted after development of BE, thus we may have missed the relevant time window for disease pathogenesis; there was a small age difference between our cases and controls, although we adjusted for age in all of our analyses; endoscopy controls may not be optimal; we only quantitated our exposures once at a single age and point in time; BMI was self-reported and may not be the optimal anthropometric variable; and the study only included men seeking care thus the results may not be generalizable to non-healthcare seeking males with similar ailments.
In conclusion, we provide evidence for strong positive associations of free testosterone and free DHT with BE, which may partly explain the sex disparities of this metaplastic condition as well as esophageal adenocarcinoma. Future studies are needed to replicate this analysis, expand to population controls and esophageal adenocarcinoma cases with prediagnostic phlebotomy, and include quantitation of sex steroid hormone receptors in esophageal tissue.
Supplementary Material
Supplemental Table 1. Partial correlation coefficients among sex steroid hormone measures and SHBG in BEEDS control subjects, adjusted for age and BMI (n=212)
Supplemental Table 2. Multivariable analysis of the associations between sex steroid hormones, SHBG and Barrett's esophagus stratified by age
Supplemental Table 3. Multivariable analysis of the associations between sex steroid hormones, SHBG and Barrett's esophagus stratified by body mass index
Acknowledgments
We gratefully acknowledge the staff of the Gastroenterology Department at WRNMMC, particularly Molly Burman and Lilibeth Bardon without whom this study would not have been possible.
Funding: Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Financial support: This research was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Guarantor of the article: Michael B. Cook
Footnotes
Specific author contributions: Conception or design: MBC, BDC, PY, RDA, RTF, RMP, NH, SMD, CCA, PRT
Data acquisition: MBC, BDC, PY, RDA, NH, HA, LW, CW, BG, CD, VT, PC, CG, PRT
Data analysis: MBC, SNW, RTF, RMP, CG, PLH, PRT
Data interpretation: MBC, SNW, RTF, RMP, PC, CG, SMD, CCA, PLH, PRT
Drafting the work or revising it critically for important intellectual content: All authors
All authors have approved the final draft submitted.
Potential competing interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–82. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. American Journal of Epidemiology. 2005;162:1050–61. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- 3.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette Smoking and Adenocarcinomas of the Esophagus and Esophagogastric Junction: A Pooled Analysis From the International BEACON Consortium. Journal of the National Cancer Institute. 2010;102:1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON Consortium. Gut. 2011;60:1029–37. doi: 10.1136/gut.2010.233866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. International Journal of Epidemiology. 2012;41:1706–1718. doi: 10.1093/ije/dys176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubo A, Cook MB, Shaheen NJ, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett's oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013 doi: 10.1136/gutjnl-2012-303753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette Smoking Increases Risk of Barrett's Esophagus: An Analysis of the Barrett's and Esophageal Adenocarcinoma Consortium. Gastroenterology. 2012;142:744–753. doi: 10.1053/j.gastro.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook MB, Corley DA, Murray LJ, et al. Gastroesophageal Reflux in Relation to Adenocarcinomas of the Esophagus: A Pooled Analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON) PLoS ONE. 2014;9:e103508. doi: 10.1371/journal.pone.0103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutegard M, Nordenstedt H, Lu Y, et al. Sex-specific exposure prevalence of established risk factors for oesophageal adenocarcinoma. British Journal of Cancer. 2010;103:735–740. doi: 10.1038/sj.bjc.6605804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodelon C, Anderson GL, Rossing MA, et al. Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer Prev Res (Phila) 2011;4:840–50. doi: 10.1158/1940-6207.CAPR-10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng KK, Sharp L, McKinney PA, et al. A case-control study of oesophageal adenocarcinoma in women: a preventable disease. British Journal of Cancer. 2000;83:127–32. doi: 10.1054/bjoc.2000.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–94. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 13.Lofdahl HE, Lu Y, Lagergren J. Sex-specific risk factor profile in oesophageal adenocarcinoma. British Journal of Cancer. 2008;99:1506–1510. doi: 10.1038/sj.bjc.6604701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M, Naumann H, Weidler C, et al. Inflammation and sex hormone metabolism. Ann N Y Acad Sci. 2006;1069:236–46. doi: 10.1196/annals.1351.021. [DOI] [PubMed] [Google Scholar]
- 15.Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28:116–9. [PubMed] [Google Scholar]
- 16.Liao CH, Li HY, Yu HJ, et al. Low serum sex hormone-binding globulin: marker of inflammation? Clin Chim Acta. 2012;413:803–7. doi: 10.1016/j.cca.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Kupelian V, Chiu GR, Araujo AB, et al. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf) 2010;72:527–33. doi: 10.1111/j.1365-2265.2009.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashid F, Khan RN, Iftikhar SY. Probing the link between oestrogen receptors and oesophageal cancer. World J Surg Oncol. 2010;8:9. doi: 10.1186/1477-7819-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Sukocheva OA, Hussey DJ, et al. Estrogen, male dominance and esophageal adenocarcinoma: is there a link? World J Gastroenterol. 2012;18:393–400. doi: 10.3748/wjg.v18.i5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagergren J, Nyren O. Do sex hormones play a role in the etiology of esophageal adenocarcinoma? A new hypothesis tested in a population-based cohort of prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:913–5. [PubMed] [Google Scholar]
- 21.Manterola C, Muñoz S, Grande L, et al. Initial validation of a questionnaire for detecting gastroesophageal reflux disease in epidemiological settings. Journal of Clinical Epidemiology. 2002;55:1041–1045. doi: 10.1016/s0895-4356(02)00454-7. [DOI] [PubMed] [Google Scholar]
- 22.Sodergard R, Backstrom T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 24.Starka L, Pospisilova H, Hill M. Free testosterone and free dihydrotestosterone throughout the life span of men. Journal of Steroid Biochemistry and Molecular Biology. 2009;116:118–120. doi: 10.1016/j.jsbmb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Chandanos E, Lagergren J. The mystery of male dominance in oesophageal cancer and the potential protective role of oestrogen. European Journal of Cancer. 2009;45:3149–3155. doi: 10.1016/j.ejca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Cronin-Fenton DP, Murray LJ, Whiteman DC, et al. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. European Journal of Cancer. 2010;46:2067–76. doi: 10.1016/j.ejca.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutegard M, Lagergren P, Nordenstedt H, et al. Oesophageal adenocarcinoma: the new epidemic in men? Maturitas. 2011;69:244–8. doi: 10.1016/j.maturitas.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Awan AK, Iftikhar SY, Morris TM, et al. Androgen receptors may act in a paracrine manner to regulate oesophageal adenocarcinoma growth. European Journal of Surgical Oncology. 2007;33:561–8. doi: 10.1016/j.ejso.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Cooper SC, Trudgill NJ. Subjects with prostate cancer are less likely to develop esophageal cancer: analysis of SEER 9 registries database. Cancer Causes Control. 2012;23:819–25. doi: 10.1007/s10552-012-9950-9. [DOI] [PubMed] [Google Scholar]
- 30.Cooper SC, Croft S, Day R, et al. Patients with prostate cancer are less likely to develop oesophageal adenocarcinoma: could androgens have a role in the aetiology of oesophageal adenocarcinoma? Cancer Causes Control. 2009;20:1363–8. doi: 10.1007/s10552-009-9359-2. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Lagergren J. Reproductive factors and risk of oesophageal cancer, a population-based nested case-control study in Sweden. Br J Cancer. 2012;107:564–9. doi: 10.1038/bjc.2012.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green J, Roddam A, Pirie K, et al. Reproductive factors and risk of oesophageal and gastric cancer in the Million Women Study cohort. Br J Cancer. 2012;106:210–6. doi: 10.1038/bjc.2011.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman ND, Lacey JV, Jr, Hollenbeck AR, et al. The association of menstrual and reproductive factors with upper gastrointestinal tract cancers in the NIH-AARP cohort. Cancer. 2010;116:1572–81. doi: 10.1002/cncr.24880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagergren J, Jansson C. Sex hormones and oesophageal adenocarcinoma: influence of childbearing? British Journal of Cancer. 2005;93:859–61. doi: 10.1038/sj.bjc.6602810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindblad M, Garcia Rodriguez LA, Chandanos E, et al. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. British Journal of Cancer. 2006;94:136–41. doi: 10.1038/sj.bjc.6602906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engeland CG, Sabzehei B, Marucha PT. Sex hormones and mucosal wound healing. Brain Behav Immun. 2009;23:629–35. doi: 10.1016/j.bbi.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–24. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilliver SC, Ruckshanthi JP, Hardman MJ, et al. 5alpha-dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. 2009;217:73–82. doi: 10.1002/path.2444. [DOI] [PubMed] [Google Scholar]
- 39.Pergola C, Dodt G, Rossi A, et al. ERK-mediated regulation of leukotriene biosynthesis by androgens: a molecular basis for gender differences in inflammation and asthma. Proc Natl Acad Sci U S A. 2008;105:19881–6. doi: 10.1073/pnas.0809120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schooling CM. Androgen activity and markers of inflammation among men in NHANES III. American Journal of Human Biology. 2013;25:622–628. doi: 10.1002/ajhb.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimchi ET, Posner MC, Park JO, et al. Progression of Barrett's metaplasia to adenocarcinoma is associated with the suppression of the transcriptional programs of epidermal differentiation. Cancer Research. 2005;65:3146–54. doi: 10.1158/0008-5472.CAN-04-2490. [DOI] [PubMed] [Google Scholar]
- 43.Hu N, Clifford RJ, Yang HH, et al. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics. 2010;11:576. doi: 10.1186/1471-2164-11-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Close H, Mason JM, Wilson D, et al. Hormone replacement therapy is associated with gastro-oesophageal reflux disease: a retrospective cohort study. BMC Gastroenterol. 2012;12:56. doi: 10.1186/1471-230X-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon S, Prew S, Parkes G, et al. Do differences in female sex hormone levels contribute to gastro-oesophageal reflux disease? Eur J Gastroenterol Hepatol. 2013;25:772–7. doi: 10.1097/MEG.0b013e32835fbaab. [DOI] [PubMed] [Google Scholar]
- 46.Nordenstedt H, Younes M, El-Serag HB. Expression of androgen receptors in Barrett esophagus. J Clin Gastroenterol. 2012;46:251–2. doi: 10.1097/MCG.0b013e318238353e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tihan T, Harmon JW, Wan X, et al. Evidence of androgen receptor expression in squamous and adenocarcinoma of the esophagus. Anticancer Res. 2001;21:3107–14. [PubMed] [Google Scholar]
- 48.Stairs DB, Nakagawa H, Klein-Szanto A, et al. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett's esophagus. PLoS One. 2008;3:e3534. doi: 10.1371/journal.pone.0003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuguchi M, Miki Y, Onodera Y, et al. Estrogen receptor alpha and beta in esophageal squamous cell carcinoma. Cancer Sci. 2012;103:1348–55. doi: 10.1111/j.1349-7006.2012.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nozoe T, Oyama T, Takenoyama M, et al. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2007;13:4046–50. doi: 10.1158/1078-0432.CCR-07-0449. [DOI] [PubMed] [Google Scholar]
- 51.Kalayarasan R, Ananthakrishnan N, Kate V, et al. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus. 2008;21:298–303. doi: 10.1111/j.1442-2050.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 52.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–55. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 53.Liu L, Chirala M, Younes M. Expression of estrogen receptor-beta isoforms in Barrett's metaplasia, dysplasia and esophageal adenocarcinoma. Anticancer Research. 2004;24:2919–24. [PubMed] [Google Scholar]
- 54.Akgun H, Lechago J, Younes M. Estrogen receptor-beta is expressed in Barrett's metaplasia and associated adenocarcinoma of the esophagus. Anticancer Research. 2002;22:1459–61. [PubMed] [Google Scholar]
- 55.Tiffin N, Suvarna SK, Trudgill NJ, et al. Sex hormone receptor immunohistochemistry staining in Barrett's oesophagus and adenocarcinoma. Histopathology. 2003;42:95–6. doi: 10.1046/j.1365-2559.2003.01513_3.x. [DOI] [PubMed] [Google Scholar]
- 56.di Pietro M, Lao-Sirieix P, Boyle S, et al. Evidence for a functional role of epigenetically regulated midcluster HOXB genes in the development of Barrett esophagus. Proc Natl Acad Sci U S A. 2012;109:9077–82. doi: 10.1073/pnas.1116933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masaka T, Iijima K, Endo H, et al. Gender differences in oesophageal mucosal injury in a reflux oesophagitis model of rats. Gut. 2012 doi: 10.1136/gutjnl-2011-301389. [DOI] [PubMed] [Google Scholar]
- 58.Sukocheva OA, Wee C, Ansar A, et al. Effect of estrogen on growth and apoptosis in esophageal adenocarcinoma cells. Dis Esophagus. 2013;26:628–35. doi: 10.1111/dote.12000. [DOI] [PubMed] [Google Scholar]
- 59.Kambhampati S, Rajewski RA, Tanol M, et al. A second-generation 2-Methoxyestradiol prodrug is effective against Barrett's adenocarcinoma in a mouse xenograft model. Mol Cancer Ther. 2013;12:255–63. doi: 10.1158/1535-7163.MCT-12-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knigge U, Dejgaard A, Wollesen F, et al. The acute and long term effect of the H2-receptor antagonists cimetidine and ranitidine on the pituitary-gonadal axis in men. Clin Endocrinol (Oxf) 1983;18:307–13. doi: 10.1111/j.1365-2265.1983.tb03216.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Partial correlation coefficients among sex steroid hormone measures and SHBG in BEEDS control subjects, adjusted for age and BMI (n=212)
Supplemental Table 2. Multivariable analysis of the associations between sex steroid hormones, SHBG and Barrett's esophagus stratified by age
Supplemental Table 3. Multivariable analysis of the associations between sex steroid hormones, SHBG and Barrett's esophagus stratified by body mass index


