Abstract
Purpose
We determined hormone concentrations (estradiol [E2], estrone [E1], estrone conjugates [E1-C], androstenedione [A], testosterone [T]) before and on anastrozole therapy where we also determined plasma concentrations of anastrozole and its metabolites.
Experimental
Postmenopausal women who were to receive adjuvant anastrozole for resected early breast cancer were studied. Pretreatment, blood samples were obtained for the acquisition of DNA and for plasma hormone measurements (E2, E1, E1-C, A, T). A second blood draw was obtained at least 4 weeks after starting anastrozole for hormone, anastrozole and metabolite measurements. For hormone assays, a validated bioanalytical method using gas chromatography negative ionization tandem mass spectrometry was used. Anastrozole and metabolite assays involved extraction of plasma followed by LC/MS/MS assays.
Results
649 patients were evaluable. Pretreatment and during anastrozole, there was large inter-individual variability in E2, E1, and E1-C as well as anastrozole and anastrozole metabolite concentrations. E2 and E1 concentrations were below the lower limits of quantitation in 79% and 70%, respectively, of patients on anastrozole therapy, but those with reliable concentrations had a broad range (0.627-234.0 pg/mL, 1.562-183.2 pg/mL, respectively). Considering E2, 8.9% had the same or higher concentration relative to baseline while on anastrozole, documented by the presence of drug.
Conclusions
We demonstrated large inter-individual variability in anastrozole and anastrozole metabolite concentrations as well as E1, E2, E1-C, A, and T concentrations before and while on anastrozole. These findings suggest that the standard 1 mg daily dose of anastrozole is not optimal for a substantial proportion of women with breast cancer.
Keywords: anastrozole, estrone, estradiol, estrone conjugates, androstenedione, testosterone, breast cancer
Introduction
The third-generation aromatase inhibitors (AIs) anastrozole, exemestane and letrozole play a major role in postmenopausal women with estrogen receptor (ER) positive breast cancer in the early (1,2) and metastatic (3) settings and have been shown to be of value as preventive agents in women at high risk of developing breast cancer (4,5). An American Society of Clinical Oncology (ASCO) Practice Guideline has recommended the use of AIs at some point during adjuvant endocrine therapy of early breast cancer, either as initial therapy or following some period of tamoxifen therapy (6).
Anastrozole is a nonsteroidal AI that was reported to maximally suppress plasma estradiol concentrations at doses of one and 10 mg per day (7) and Geisler et al. (8) reported equipotency of the one and 10 mg dose levels in terms of aromatase inhibition and suppression of plasma estrogen concentrations. These findings plus the results of clinical trials (9) led to the adoption of the one mg dose for clinical use, which was the dose approved by the U.S. Food and Drug Administration (FDA). However, based on the marked variability seen clinically in terms of outcomes and tolerability, the question can be asked whether the one mg daily dose is optimal for all patients.
Because this variability in clinical outcomes could be due to variability in suppression of estrogens, we developed a clinical study to examine anastrozole metabolism and its pharmacodynamic effect on estrogens, the ligand for the ER, as well as their precursors androstenedione and testosterone, in a large population of postmenopausal breast cancer patients. We have reported our findings with respect to anastrozole metabolism and pharmacodynamics in the first 191 patients entered on our clinical study (10) and demonstrated large inter-individual variation in anastrozole metabolism and its effect on circulating estrogens in these postmenopausal patients. In the current report, we expand this series to 649 women entered on our study who have hormone plasma concentrations (estradiol [E2}, estrone [E1], estrone conjugates [E1-C], androstenedione, testosterone), anastrozole and anastrozole metabolite plasma concentrations as well as genotyping data, as these patients will be utilized in a series of genome-wide association studies. To our knowledge, this population of patients is the largest series of women with hormone and anastrozole determinations plus genotyping data and provides clear insights into the large interindividual variability in the effect of anastrozole on circulating estrogens.
Experimental
Details relating to the study design and assays for E2, E1, E1-C, androstenedione, and testosterone and for anastrozole and anastrozole metabolites have been presented in detail previously (10) and they will be briefly summarized here. The design of this clinical study involved postmenopausal women who were to receive anastrozole as adjuvant therapy for resected early stage breast cancer at Mayo Clinic, M.D. Anderson Cancer Center, or Memorial Sloan Kettering Cancer Center. Eligibility criteria included age of at least 18 years, postmenopausal status, breast cancer stage I, II, or III according to the American Joint Committee on Cancer (AJCC) Staging Manual (Sixth Edition), a tumor that was estrogen receptor positive and/or progesterone receptor positive, and a planned treatment with anastrozole at the clinically approved dose of one mg per day. Patients could have received prior tamoxifen, but other prior endocrine therapy was not permitted and none of the patients were receiving hormone replacement therapy. Within two weeks prior to starting anastrozole, a blood sample was obtained for the acquisition of DNA and for pre-treatment hormone measurements. A second blood draw for hormone measurements, anastrozole and anastrozole metabolite concentrations in plasma was scheduled for at least four weeks after initiation of anastrozole. Patients were instructed not to take their dose of anastrozole for that day until after the blood was drawn. This trial (ClinicalTrials.gov study number NCT00283608) was performed after approval by local Institutional Review Boards in accordance with assurances filed with and approved by the United States Department of Health and Human Services. Written informed consent was provided by each patient before entry on study.
For the hormone assays, a validated bioanalytical method using gas chromatography negative ionization tandem mass spectrometry was used to measure physiologically relevant concentrations of the following steroids from 1.0 mL of human plasma (Taylor Technology, Prinston, NJ), with lower limits of quantitation in this study of: E2, 0.625 pg/mL; E1, 1.56 pg/mL; E1-C: 6.25 pg/mL; testosterone, 25.0 pg/mL; and androstenedione, 25.0 pg/mL. Anastrozole and metabolite assays involved liquid-liquid extraction of plasma, followed by LC/MS/MS assay as previously reported (11). Although the subject of future publications, genotypes were determined by the RIKEN Center for Integrative Medical Science (Yokohama, Japan) with the Illumina Human610-Quad platform.
Results
Patients analyzed
There were 695 patients with hormone, anastrozole and anastrozole metabolite, and genotyping data. 46 (6.6%) patients were excluded from the analysis for the following reasons: 22 patients had no detectable anastrozole or anastrozole metabolite, one patient had no anastrozole or hydroxy-anastrozole assay performed, three patients had their “on-anastrozole” blood drawn less than four weeks after starting anastrozole, and 20 patients had a date for the hormone determinations that differed from that for the anastrozole. Thus, 649 patients were evaluable in these analyses and their characteristics are listed in Table 1.
Table 1. Patient characteristics.
| Total (N=649) | |
|---|---|
| Age, years | |
| Median | 67.4 |
| Range | (39.7-96.0) |
| Race | |
| American Indian or Alaska Native | 4 (0.6%) |
| Asian | 14 (2.2%) |
| Black or African American | 37 (5.7%) |
| White | 594 (91.5%) |
| Ethnicity | |
| Hispanic or Latino | 55 (8.5%) |
| Not Hispanic or Latino | 586 (90.3%) |
| Unknown | 8 (1.2%) |
| BMI | |
| Median | 27.2 |
| Range | (16.0-53.6) |
| AJCC tumor stage | |
| 1 | 367 (56.5%) |
| 2 | 215 (33.1%) |
| 3 | 67 (10.3%) |
| ER/PgR status | |
| Negative/Positive | 4 (0.6%) |
| Positive/Negative | 125 (19.3%) |
| Positive/Positive | 519 (80.0%) |
| Positive/Unknown | 1 (0.2%) |
| HER2 status | |
| Negative | 555 (85.5%) |
| Positive | 82 (12.6%) |
| Unknown | 12 (1.8%) |
| Smoking status | |
| No | 346 (53.3%) |
| Unknown | 71 (10.9%) |
| Yes | 232 (35.7%) |
| Prior Chemotherapy | |
| No | 384 (59.2%) |
| Yes | 265 (40.8%) |
| Prior tamoxifen | |
| No | 583 (89.8%) |
| Yes | 66 (10.2%) |
| Blood Draw Days | |
| N | 649 |
| Mean (SD) | 108.0(65.2) |
| Median | 91.0 |
| Range | (29.0-471.0) |
Anastrozole and Anastrozole Metabolites
Besides anastrozole, three metabolites were detected in plasma: anastrozole conjugates, hydroxy-anastrozole and hydroxy-anastrozole conjugates. Note that the triazole metabolite was not detected. The median plasma concentration of free anastrozole was 33.2 ng/mL (quartile [Q] 1: 23.5 ng/mL, Q3: 44.8 ng/mL), with a range from 0.0 to 132.1 ng/mL. The median plasma concentration of anastrozole conjugates was 4.70 ng/mL (Q1: 0.76 ng/mL, Q3: 9.07 ng/mL), with a range: 0.0 to 206.4 ng/mL. The majority of the hydroxy-anastrozole was recovered as conjugates. A lack of internal standards prevented absolute quantitation of these two metabolites, but there was a 22-fold range in the medians of the hydroxy-anastrozole conjugate concentrations vis-à-vis the hydroxy-anastrozole concentrations.
Estradiol
Figure 1A displays the pre-anastrozole and on-anastrozole E2 plasma concentrations with the quartile of free anastrozole concentrations indicated. Substantial interindividual variability in the pre-anastrozole E2 concentrations was seen with a median value of 4.0 pg/mL (Q1: 2.4 pg/mL, Q3: 6.5 pg/mL) and a range of 0.0 pg/mL to 62.2 pg/mL). Substantial interindividual variability was also seen in the on-anastrozole E2 concentrations with a median value of 0.0 pg/mL (Q1: 0.0 pg/mL, Q3: 0.0 pg/mL) and a range of 0.0 to 234.0 pg/mL.
Figure 1.
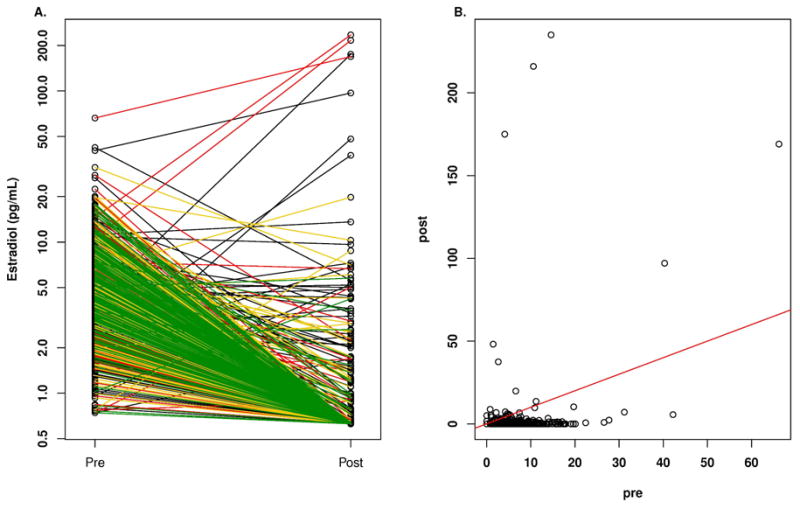
A. Plasma concentrations of estradiol according to quartile of anastrozole concentration in breast cancer patients before and after treatment with one mg/day oral dose of anastrozole. Key for line color in A: black, lowest quartile; red, second quartile; yellow, third quartile; green highest quartile. B. Plasma concentrations of estradiol in breast cancer patients before (pre) and while on treatment (post) with one mg/day oral dose of anastrozole.
E2 concentrations were below the LLQ in 79% of patients on anastrozole therapy, but those with reliable detectable concentrations had a broad range (0.627 pg/mL to 234.0 pg/mL). Importantly, 57 of 643 patients (8.9%) patients had a stable or increased E2 level, compared with pre-anastrozole concentrations, which are displayed in Figure 1B where the patients at or above the red line of unity can be seen. These 57 patients had stable or increased E2 concentrations despite the majority (51/57 [89%]) having detectable free anastrozole with a median concentration of 26.7 ng/mL (Q1: 17.5 ng/mL, Q3: 32.7 ng/mL) and a range of 0.0 to 59.6 ng/mL, which was significantly lower than those patients who had a decrease in E2 concentrations where the median concentration of free anastrozole was 34.2 ng/mL (Q1: 23.8 ng/mL, Q3: 45.6 ng/mL) with a range of 0.0 ng/mL to 132.1 ng/mL (p= 1.6 E-05, rank sum test). The 6 patients with undetectable free anastrozole levels had detectable anastrozole metabolites indicating that they were taking the drug, raising the possibility that they were rapid metabolizers of the anastrozole. The median baseline E2 for these 57 patients was 0.0 pg/ml (Q1: 0.0 pg/mL, Q3: 2.6 pg/mL; range: 0.0 to 66.2 pg/mL), which was significantly lower than for those patients who had a decrease in E2 concentrations while receiving anastrozole where the median concentration of E2 was 4.1 pg/mL (Q1: 2.7 pg/mL, Q3: 6.7 pg/mL; range: 0.7 pg/mL to 42.2 pg/mL) (p=1.8 E-15, rank sum test). These 57 patients had a median age of 66.3 years with a range of 49.4 to 88.4 years and a median BMI of 26.3 with a range of 16.0 to 40.6.
Seventy-five of 643 (11.7%) patients had pre-anastrozole E2 concentrations >10 pg/mL, the conventional point of separating premenopausal and postmenopausal women. The median baseline E2 for these 75 patients was 12.8 pg/ml (Q1: 11.2 pg/mL, Q3: 16.3 pg/mL; range: 10.2 pg/mL to 66.2 pg/mL), which was significantly higher than those patients who had pre-anastrozole E2 concentrations ≦ 10 pg/mL (p=2.2 E-16, rank sum test). These 75 patients had a median age of 68.2 years with a range of 51.0 to 87.6 years and a median BMI of 35.0 with a range of 18.3 to 53.7.
Estrone
Figure 2A displays the pre-anastrozole and on-anastrozole E1 plasma concentrations with the quartile of free anastrozole concentrations. Substantial interindividual variability in the pre-anastrozole E1 concentrations was seen with a median value of 18.6 pg/mL (Q1: 13.2 pg/mL, Q3: 26.6 pg/mL) and a range of 0.0 to 111.0 pg/mL. Substantial interindividual variability was also seen in the on-anastrozole E1 concentrations with a median value of 0.0 pg/mL (Q1: 0.0 pg/mL, Q3: 1.7 pg/mL) and a range of 0.0 to 183.0 pg/mL. E1 concentrations were below the LLQ in 70% of patients on anastrozole therapy, but those with reliable detectable concentrations had a broad range (1.6 pg/mL to 183.2 pg/mL).
Figure 2.
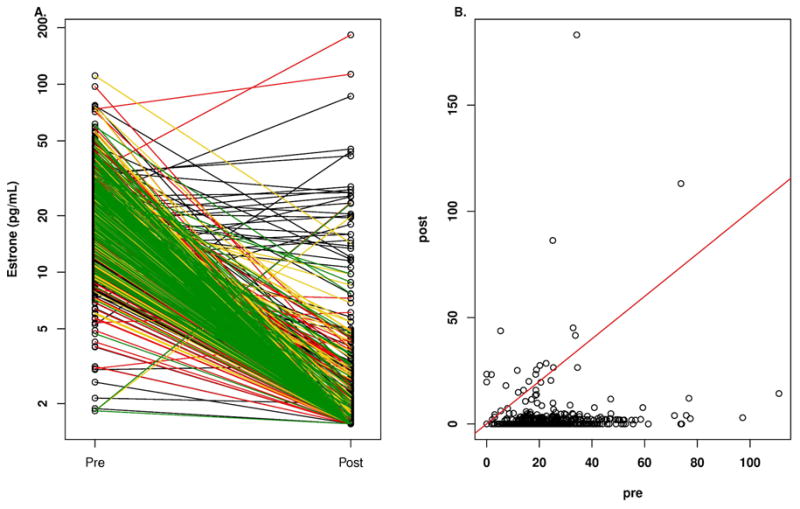
A. Plasma concentrations of estrone according to quartile of anastrozole concentration in breast cancer patients before and after treatment with one mg/day oral dose of anastrozole. Key for line color in A: black, lowest quartile; red, second quartile; yellow, third quartile; green highest quartile. B. Plasma concentrations of estrone in breast cancer patients before (pre) and while on treatment (post) with one mg/day oral dose of anastrozole.
Thirty-seven of 639 patients (5.8%) had a stable or increased E1 level, compared with pre-anastrozole concentrations, which are displayed in Figure 2B where the patients at or above the red line of unity can be seen. These 37 patients had a stable or increased E1 level despite the majority (31/37 [84%]) having detectable free anastrozole with a median concentration of 26.5 ng/mL (Q1: 0.4 pg/mL, Q3: 32.2 pg/mL; range: 0.0 to 69.6 ng/mL), which was significantly lower than for those patients who had a decrease in E1 concentrations where the median concentration of anastrozole was 34.1 ng/mL (Q1: 24.0 ng/mL, Q3: 45.7 ng/mL; range: 0.0 ng/mL to 132.1 ng/mL) (p= 6.7 E-05, rank sum test). The 6 patients with undetectable free anastrozole levels had detectable anastrozole metabolites indicating that they were taking the drug. The median baseline E1 for these 37 patients was 3.0 pg/ml (Q1: 0.0 pg/mL, Q3: 18.6 pg/mL; range: 0.0 to 73.8 pg/mL), which was significantly lower than for those patients who had a decrease in E1 concentrations while receiving anastrozole where the median concentration of E1 was 18.9 pg/mL (Q1: 13.7 pg/mL, Q3: 26.9 pg/mL; range: 1.9 pg/mL to 111.0 pg/mL) (p=1.2 E-08, rank sum test). These 37 patients had a median age of 65.3 years with a range of 51.4 to 85.5 years and a median BMI of 27.2 with a range of 18.3 to 40.6. Twenty-seven of 36 (75%) (one patient with missing E2 value) patients with a stable or rising E1 on anastrozole also had a stable or rising E2 concentration while receiving anastrozole.
Estrone Conjugates
Figure 3 displays the pre-anastrozole and on-anastrozole E1-C plasma concentrations with the quartile of free anastrozole concentrations indicated in panel A. Substantial interindividual variability in the pre-anastrozole E1-C concentrations was seen with a median value of 226.0 pg/mL and a range of 0.0 to 3320.0 pg/mL. Substantial interindividual variability was also seen in the on-anastrozole E1-C concentrations with a median value of 14.2 pg/mL and a range of 0.0 to 2990.0 pg/mL. Twenty-five of 585 patients (4.3%) had a stable or increased E1-C level, compared with pre-anastrozole concentrations, and are displayed in Figure 3B where the patients at or above the red line of unity can be seen. These 25 patients had stable or increasing E1-C levels despite the majority (18/25 [72%] having detectable free anastrozole with a median concentration of 13.3 ng/m (Q1: 0.0, Q3: 27.3 pg/mL; range of 0.0 to 98.8 ng/mL), which was significantly lower than for those patients who had a decrease in E1 concentrations where the median concentration of free anastrozole was 33.9 ng/mL (Q1: 23.9 ng/mL, Q3: 44.8 ng/mL; range: 0.0 ng/mL to 132.1 ng/mL) (p= 1.3 E-05, rank sum test). The 7 patients with undetectable free anastrozole levels had detectable anastrozole metabolites indicating that they were taking the drug. The median baseline E1-C for these 25 patients was 127.0 pg/mL (Q1: 53.8, Q3: 249.0 pg/mL; range: 7.7 pg/mL to 544.0 pg/mL), which was significantly lower than for those patients who had a decrease in E1-C concentrations while receiving anastrozole where the median concentration of E1-C was 33.9 pg/mL (Q1: 23.9 pg/mL, Q3: 44.8 pg/mL; range: 0.0 pg/mL to 132.1 pg/mL) (p= 2.5 E-03, rank sum test). These 25 patients had a median age of 65.8 years with a range of 51.4 to 86.2 years and a median BMI of 27.9 with a range of 19.9 to 41.7.
Figure 3.
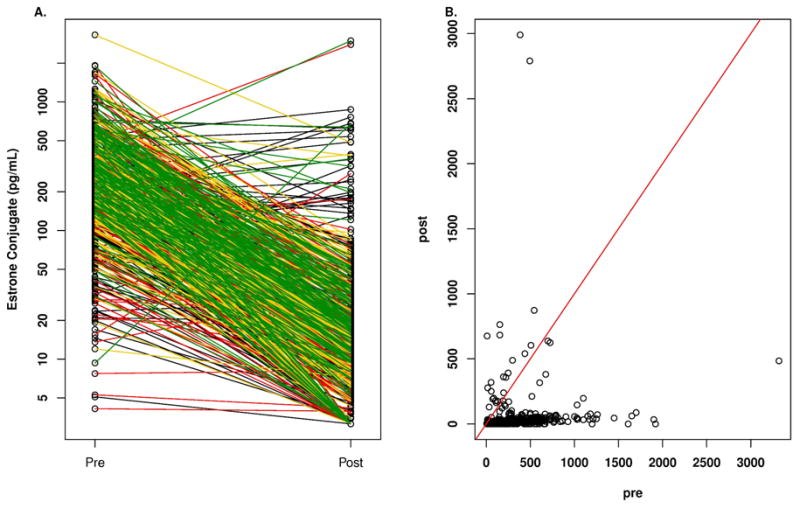
A. Plasma concentrations of estrone conjugates according to quartile of anastrozole concentration in breast cancer patients before and after treatment with one mg/day oral dose of anastrozole. Key for line color in A: black, lowest quartile; red, second quartile; yellow, third quartile; green highest quartile. B. Plasma concentrations of estrone conjugates in breast cancer patients before (pre) and while on treatment (post) with one mg/day oral dose of anastrozole.
Androstenedione
Figure 4A displays the pre-anastrozole and on-anastrozole androstenedione plasma concentrations and shows substantial variability in the pre-anastrozole concentrations but no consistent change while on anastrozole. The median androstenedione concentrations were 423.0 pg/mL (Q1: 293.0 pg/mL, Q3: 565.5 pg/mL) and 443.0 pg/mL (Q1: 324.0, Q3: 640.0 pg/mL), respectively, with ranges of 0.0 to 2470.0 pg/mL and 0.0 to 1600.0 pg/mL, respectively. In 639 patients with both pre-anastrozole and on-anastrozole concentrations, 60% had no change or an increase in androstenedione concentrations and 40% had a decrease.
Figure 4.
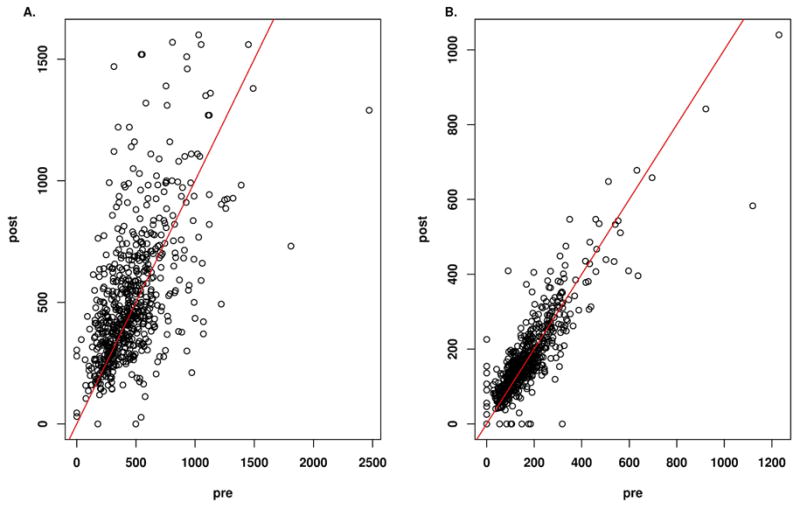
Plasma concentrations of androstenedione (A) and testosterone (B) in breast cancer patients before (pre) and while on treatment (post) with one mg/day oral dose of anastrozole.
Testosterone
Figure 4B displays the pre-anastrozole and on-anastrozole testosterone plasma concentrations and again shows substantial variability in the pre-anastrozole concentrations but no consistent change while on anastrozole. The median testosterone concentrations were 144.0 pg/mL (Q1: 9.5, Q3: 208.2 pg/mL) and 153.0 pg/mL (Q1: 104.0, Q3: 209.0 pg/mL), respectively, with ranges of 0.0 to 1230.0 pg/mL and 0.0 to 1040.0 pg/mL, respectively. In 646 patients with both pre-anastrozole and on-anastrozole concentrations, 57% had no change or an increase in testosterone concentrations and 43% had a decrease.
Discussion
The most remarkable findings in our study were the substantial interindividual variability in baseline hormones E2, E1, and E1-C, the variability seen in the metabolism of anastrozole, and the pharmacodynamic effects of anastrozole on E2, E1, and E1-C. The patients enrolled in this study were entered from the oncology practice of three large institutions and represented standard practice management in that conventional therapy was employed using the FDA-approved dose of anastrozole (one mg daily). The importance of having blood anastrozole concentrations when examining these pharmacodynamic effects was clearly seen from the fact that 22 patients (3.2%) had no detectable anastrozole or anastrozole metabolite blood levels and had to be excluded from the analysis.
Substantial interindividual variability was identified in the metabolism of anastrozole with a median plasma concentration of 33.2 ng/mL but a broad range, i.e., 0.0 ng/mL to 98.8 ng/mL. This variability is currently being explored in a GWAS utilizing anastrozole and anastrozole metabolites as phenotypes.
A particularly remarkable finding was that a substantial proportion of patients had an increase in E2, E1, and E1-C while receiving anastrozole despite the majority having measurable levels of the active free anastrozole and the remaining having measurable anastrozole metabolites, indicating they had been taking the drug. This included 8.9% of patients for E2, 5.8% of patients for E1, and 4.3% of patients for E1-C. These patients who had stable or increasing E2, E1, and E1-C had significantly lower baseline concentrations of their respective hormones and also significantly lower concentrations of free anastrozole compared with the patients who had a decrease in their respective hormones. It is understood that patient compliance for taking drugs may be a possible confounding factor, but the availability of anastrozole and anastrozole metabolite levels provides assurance that patients were taking the anastrozole. Regarding the E2 findings in our study, the 8.9% of patients who had a stable or rising concentration is similar to that seen in a study of 66 patients by Nagao et al. (12) in which six of 66 (9%) patients treated with anastrozole had an increase in E2 at six or nine months after initiation of therapy. These authors concluded that in some breast cancer patients with ordinary menopause, E2 rebounds with AI therapy.
Also of note in our study is that, whereas E2 >10 pg/ml is generally associated with a premenopausal status, these levels were found in 11.7% of the patients who were judged to be postmenopausal by their clinicians. A review of these patients revealed a median age of 68.2 years (range: 51.0 to 87.6 years) and a tendency to be overweight with a median BMI of 35.0 (range: 18.3 to 53.7). These findings indicate that there is not a definite cut-point for identifying a postmenopausal woman particularly since the age of patients was as high as 87 years.
In summary, these results show marked inter-individual variability in anastrozole metabolism and its pharmacodynamic effects, and suggest the standard 1 mg daily dose of anastrozole is not optimal for a substantial proportion of women with breast cancer. This cohort of 649 patients is the subject of ongoing analyses of multiple GWAS with different phenotypes including E2, E1, E1-C, substrate/product relationships, anastrozole, anastrozole metabolites, and mammographic breast density. It is expected that these studies will provide insights into the genetic contributions to the remarkable variability identified and will provide focus for future studies that will help make it possible to individualize treatment of patients with anastrozole, a step towards precision medicine.
Table 2. Pre-treatment hormone levels (pg/mL): pre-treatment and during treatment with anastrozole.
| Pre-treatment | During Anastrozole | |
|---|---|---|
| Estradiol | ||
| N. patients | 643 | 643 |
| Median | 4.0 | 0.0 |
| Range | 0.0-62.2 | 0.0-234.0 |
| Estrone | ||
| No. patients | 643 | 643 |
| Median | 18.6 | 0.0 |
| Range | 0.0-111.0 | 0.0-183.2 |
| Estrone conjugates | ||
| No. patients | 585 | 585 |
| Median | 226.0 | 14.2 |
| Range | 0.0-3320.0 | 0.0-2990.0 |
| Androstenedione | ||
| No. patients | 639 | 639 |
| Median | 423.0 | 443.0 |
| Range | 0.0-2470.0 | 0.0-1600.0 |
| Testosterone | ||
| No. patients | 646 | 646 |
| Median | 144.0 | 153.0 |
| Range | 0.0-1230.0 | 0.0-1040.0 |
Acknowledgments
The authors acknowledge the participation of the patients who participated the prospective clinical study from which the data presented were derived.
Financial support: Funded in part by National Institutes of Health grants U19 GM61388 and U01 GM061373 (Pharmacogenomics Research Network), K24RR020815, P50 CA166201 (Mayo Clinic Breast Cancer Specialized Program of Research Excellence), R01 CA138461, and a PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ingle JN. Overview of adjuvant trials of aromatase inhibitors in early breast cancer. Steroids. 2011;76:765–7. doi: 10.1016/j.steroids.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–18. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 3.Ingle JN, Suman VJ. Aromatase inhibitors for therapy of advanced breast cancer. J Steroid Biochem Molec Biol. 2005;95:113–9. doi: 10.1016/j.jsbmb.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Ales-Martinez JE, et al. NCIC CTG. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 6.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology Clinical Practice Guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plourde PV, Dyroff M, Dukes M. Arimidex®: a potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–11. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- 8.Geisler J, King N, Dowsett M, et al. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74:1286–91. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzdar A, Jonat W, Howell A, et al. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma. Cancer. 1998;83:1142–52. [PubMed] [Google Scholar]
- 10.Kamden LK, Liu Y, Stearns V, et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol. 2010;70:854–69. doi: 10.1111/j.1365-2125.2010.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingle JN, Buzdar AU, Schaid DJ, et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010;70:3278–86. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagao T, Kira M, Takahashi M, et al. Serm estradiol should be monitored not only during the peri-menopausal period but also the post-menopausal period at the time of aromatase inhibitor administration. World J Surg Oncol. 2009;7:88–92. doi: 10.1186/1477-7819-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]


