Abstract
Background & Aims:
In patients with cirrhosis, sleep disturbances are assumed to result from hepatic encephalopathy (HE). The effects of obstructive sleep apnea (OSA) on cognition, sleep parameters, or driving in patients with cirrhosis are unclear.
Methods:
We performed a cross-sectional, prospective study of 118 subjects. Subjects were assigned to 1 of 4 groups: those with OSA and cirrhosis (without HE or ascites, n=34), those with only cirrhosis (n=30), those with only OSA only (n=29), and those without OSA or cirrhosis (controls, n=25). None of OSA patients were receiving continuous positive airway pressure (CPAP) therapy. Subjects underwent cognitive testing (paper–pencil tests for psychomotor speed and attention, as well as executive function tests), sleep assessment (daytime sleepiness and night-time sleep quality) and a monotonous driving simulation (worsening lane deviations over time indicate poor performance). We also tested patients with OSA, with cirrhosis (n=10) and without cirrhosis (n=7), before and after CPAP therapy.
Results:
Daytime sleepiness and sleep quality were worse in subjects in the OSA groups (with or without cirrhosis) than subjects with cirrhosis alone or controls. Of subjects with only OSA, 36% had impaired psychomotor speed and attention, compared to >60% of subjects in both cirrhosis groups. In contrast, executive function was uniformly worse in subjects with OSA, with or without cirrhosis, than groups without OSA. Simulator performance (lane deviations) worsened over time in both OSA groups. CPAP therapy significantly increased executive function and sleep quality, and reduced simulator lane deviations and sleepiness, in subjects with and without cirrhosis. After CPAP therapy, performance on the paper–pencil test performance improved significantly only in subjects with OSA without cirrhosis.
Conclusion:
OSA should be considered in evaluating sleep impairment in patients with cirrhosis. In patients with cirrhosis and OSA, psychomotor speed and attention issues are likely related to the cirrhosis, whereas executive function and simulator performance are affected by the OSA. CPAP therapy improves executive function and simulator performance in patients with OSA, regardless of cirrhosis.
Keywords: covert hepatic encephalopathy, minimal hepatic encephalopathy, chronic liver disease, CPAP
Introduction
Cognitive dysfunction in cirrhosis can have an immense negative medical, psychological and socio-economic impact1. The majority of the cognitive dysfunction in cirrhosis is assumed to be related to the overt or covert hepatic encephalopathy (HE)2. Furthermore, changes in the sleep-wake cycle in cirrhosis are assumed to be related to overt HE3. With the rise in obesity, there is an increased prevalence of obstructive sleep apnea (OSA) that is also associated with cognitive dysfunction and driving impairment4, 5. A prior study showed that daytime sleepiness questionnaires could not differentiate between cirrhotic and non-cirrhotic patients with OSA6. Also OSA can worsen liver disease by causing ischemic liver injury7. Therefore a “missed” diagnosis of OSA because of the underlying assumption that all cognitive dysfunction and daytime sleepiness in cirrhosis is related to overt HE can potentially worsen the cardiorespiratory status and liver disease by delaying CPAP and can expose patients to the expense and adverse events of unnecessary HE treatments.
Therefore, the aims of this study were to evaluate (1) the interaction between OSA and cirrhosis cognition, sleep and driving simulation (2) evaluate the specific contribution of OSA to these changes by studying patients before and after CPAP therapy.
Methods
From the Liver clinics at VCU and McGuire VA Medical Centers, we prospectively recruited four age-matched groups, (i) OSA patients without cirrhosis, (ii) OSA patients with cirrhosis, (iii) cirrhotics patients without OSA and (iv) healthy controls, between November 2012 and June 2014.
Cirrhosis was diagnosed by histology, endoscopic or radiologic evidence of cirrhosis/varices in patients with chronic liver disease. We excluded cirrhotic patients with prior overt HE, on current overt HE therapy, ascites/hepatic hydrothorax, TIPS or an unclear cirrhosis diagnosis. As part of our clinical practice, compensated cirrhotic patients with sleep complaints, a neck circumference >17 inches in men and >16 inches in women and BMI >30 in the absence of overt HE are routinely referred for polysomnography. Therefore the diagnosis of OSA was made in all patients using polysomnography using guidelines [Apnea-hypopnea index (AHI) > 15 events/hour or AHI> 5/hour in a patient who reports any of the following: unintentional sleep episodes during wakefulness; daytime sleepiness; unrefreshing sleep; fatigue; insomnia; waking up breath holding, gasping, or choking; or the bed partner describing loud snoring, breathing interruptions, or both during the patient's sleep]8. AHI is calculated by first adding the total number of Apnea events, plus Hypopnea events, then dividing this value by the total number of minutes of actual sleep time and finally multiplying this value by 60. Age-matched OSA patients without cirrhosis or liver disease excluded through clinical/radiologic/laboratory review were recruited from the sleep clinics.
Age-matched healthy controls without chronic diseases including sleep issues were also enrolled. We excluded subjects on psychoactive medications, on sleep aids, abusing alcohol or illicit drugs for the last 6 months (confirmed by questioning, urine tests and corroboration from relatives), not current drivers, with concomitant diagnosed sleep disorders and those who were unable to give consent or understand English.
All subjects were enrolled prospectively and the following was analyzed:
Analysis of the effect of OSA on Cognition and Driving Simulation in Cirrhosis: A cross-sectional analysis of the subject groups defined using traditional polysomnography but not currently on CPAP, and
Analysis of CPAP: Longitudinal analysis of OSA patients with and without cirrhosis before and after CPAP initiation.
At each visit, all subjects underwent (a) physical examination and mini-mental status exam to exclude current overt HE, and body mass index evaluation (BMI), (b) Sleep questionnaires: (i) Epworth Sleepiness Scale (ESS)9 and (ii) Pittsburgh Sleep Quality Index (PSQI)10, (c) cognitive testing using paper-pencil tests of psychomotor speed, attention and visuo-motor coordination: number connection tests A/B (NCT-A/B), digit symbol (DST), block design (BDT), and a computerized test of executive function inhibitory control test (ICT; outcomes are lures and targets). Impairment on ≥ 2 of NCT-A/B, DST and BDT compared to our population norms were defined as impaired cognition (also covert HE in cirrhosis) while ICT lures were compared between groups as markers of executive function11, 12.
This was followed by a validated driving simulation program (STISIM Inc.) to assess sustained attention and vigilance5. The subjects were given a Likert Scale to assess their current sleepiness from 0 (completely alert) to 10 (sleepiest). The program started with a 10-minute long training session. This was followed by an hour-long simulated drive during which subjects were instructed to drive along a straight road at a constant speed limit. There were no built-in interruptions, stop signs, speed warnings, oncoming cars, and opportunities for crashes or changes in scenery. The simulator audio and room lights were switched off and no disturbances were allowed. There was no feedback throughout the procedure, even if the subject deviated from their lane or had a speeding citation. At the end of the simulation, mean speed, total lane deviations (including off the road and onto the opposite lane; supplementary figures 1 A-C) and speeding events were automatically recorded. In order to analyze changes over time, we studied lane deviations and speeding in the 2nd half of the simulation compared to the 1st half (delta, Δ being events in the 2nd half minus the 1st half). The Likert scale for sleepiness was readministered after the simulation and change in sleepiness post compared to pre-simulation was calculated as Δ sleepiness.
Based on prior studies, we hypothesized that with increasing sleepiness over time, the speeding instances will decrease with a corresponding increase in lane deviations5.
The study was approved by the Institutional Review Board at the McGuire VA Medical Center.
Statistical analysis
For the cross-sectional analyses, we used standard tests (ANOVA, Kruskal-Wallis and Chi-square) as appropriate to compare data between groups using patients at baseline with Holm-Bonferroni corrections as needed. Paired t-tests were used to compare patients before/after CPAP initiation. Sleepiness scales were correlated with cognition and driving simulation using Pearson correlations. AHI was also correlated with cognition and sleepiness scales.
Results
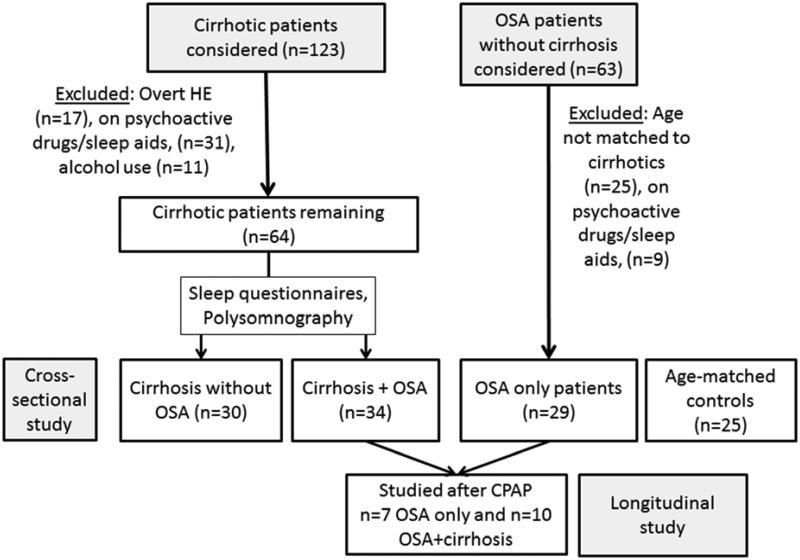
The detailed patient flow is shown in figure 1. The cirrhotic patients were enrolled first and subsequently OSA subjects without cirrhosis and healthy controls that were age-matched to the cirrhosis group were recruited. Ultimately we enrolled 118 subjects after informed consent which included 25 controls, 29 OSA patients without cirrhosis, 30 cirrhotic patients without OSA and 34 OSA patients with cirrhosis. All OSA patients (cirrhotic and non-cirrhotic) were diagnosed on polysomnography. Of the 30 cirrhotic patients without OSA, 11 patients had polysomnography non-diagnostic for OSA or other sleep disorders while 19 patients had a low pre-test probability for a positive OSA diagnosis (no sleep complaints, normal BMI and neck circumferences) and therefore were not referred for polysomnography. The cirrhotic patients had a MELD 8±2, 65% Hepatitis C, 24% NASH and 11% hepatitis C and alcohol as an etiology. None of the patients had ascites or hepatic hydrothorax, were on diuretics, had atrial fibrillation or other breathing disorders.
Figure 1.
Flow of patients through the study
Cross-sectional comparison
Cognition and sleep questionnaires
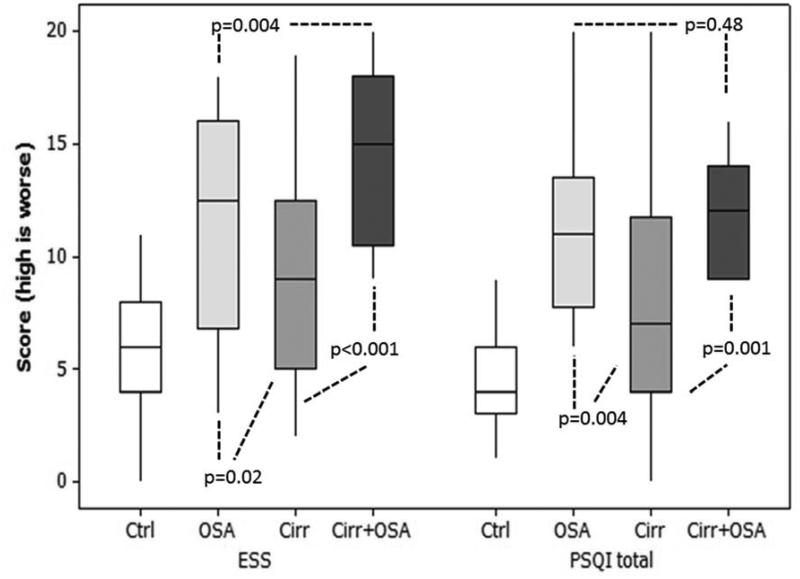
In OSA patients, the median AHI was 19.6 (IQR 16-87) suggested mild-moderate OSA. All OSA patients were awaiting CPAP therapy. There was a worsening of daytime sleepiness (ESS) from controls to non-OSA cirrhotics, OSA without cirrhosis and was the worst in OSA+cirrhosis (Figure 2B/Table 1). Night-time quality (PSQI) was best in controls, impaired further in non-OSA cirrhotics and equally worse in both OSA groups.
Figure 2. Overarching title: Sleep and cognitive differences between subject groups. Ctrl: control, OSA: obstructive sleep apnea alone, OSA+Cirrhosis: both cirrhosis and OSA.
Figure 2A: Cognitive impairment between groups: Cirrhotic patients had a higher proportion of cognitive impairment compared to OSA and controls.
Figure 2B: Sleep questionnaires between groups: There was a significant worsening of daytime sleepiness and sleep quality in OSA patients with cirrhosis compared to other groups. ESS: Epworth Sleepiness Scale, PSQI: Pittsburgh Sleep Quality Index. Boxplots demonstrate median and inter-quartile range of scores. Controls were significantly better compared to all disease groups (p<0.0001) on ESS and PSQI.
Table 1.
Comparison between groups for the cross-sectional analysis in subjects not on CPAP
| Controls (n=25) |
OSA without cirrhosis (n=29) |
Cirrhosis without OSA (n=30) |
Cirrhosis with OSA (n=34) |
P value between all groups |
|
|---|---|---|---|---|---|
| Age | 54.5±9.1 | 56.1±9.7 | 56.7±6.2 | 58.5±7.6 | 0.10 |
| Gender (Men/Women) | 17/8 | 20/9 | 20/10 | 21/13 | 0.35 |
| BMI* | 27.5±5.3 | 33.4±5.1 | 30.5±4.5 | 32.4±6.3 | 0.007 |
| Neck circumference (inches)* |
14±2 | 17±2 | 15±1 | 17±2 | <0.0001 |
| ESS†* | 5.7±3.1 | 11.8±4.7 | 9.1±4.9 | 14.5±3.9 | <0.0001 |
| PSQI*‡ | 4.2±2.3 | 11.5±3.9 | 8.0±5.1 | 12.1±2.6 | <0.0001 |
| Hours of sleep/night in the past month*‡ |
7.1±1.3 | 5.6±1.2 | 6.2±1.6 | 5.0±1.1 | 0.001 |
| Coanitive tests | |||||
| Impaired Cognition on paper-pencil tests†* |
0% | 36% | 60% | 62% | 0.002 |
| Number connection-A (sec)†* |
24.5±8.1 | 29.8±11.2 | 37.6±14.9 | 38.5±17.3 | 0.004 |
| Number connection-B (sec)†* |
63.6±21.5 | 76.4±29.1 | 108.7±77.2 | 103.9±45.5 | 0.021 |
| Digit Symbol†* | 78.5±17.5 | 65.3±19.2 | 58.0±15.9 | 52.7±13.0 | <0.0001 |
| Block design | 40.6±11.2 | 38.4±15.1 | 33.5±15.2 | 31.0±11.9 | 0.13 |
| ICT lures (number)*‡ | 6.0±3.0 | 13.1±7.7 | 11.0±6.4 | 13.9±10.9 | 0.009 |
| ICT targets (% correct) | 98.7±1.3 | 95.2±3.1 | 93.1±12.3 | 95.5±2.9 | 0.047 |
| Driving Simulation | |||||
| Total speeding instances†* | 32.7±18.6 | 31.2±22.6 | 27.3±20.3 | 20.0±17.1 | 0.05 |
| Total lane deviations* | 169.8±113.2 | 237.3±112.3 | 239.4±167.4 | 236.5±173.5 | 0.24 |
| Δ Speeding (median) |
1.0 | 1.5 | 2.0 | −1.0 | 0.34 |
| Δ lane deviations*‡ | 7.0 | 35.0 | 19.0 | 30.5 | 0.03 |
| Δ Sleepiness (median) |
2.5 | 3.0 | 3.0 | 3.0 | 0.32 |
:p<0.05 comparing cirrhosis to non-cirrhotics
: p<0.05 comparing controls and all patient groups,
: p<0.05 comparing OSA groups to cirrhosis without OSA,
Δ: 2nd half minus first half of the simulation. For continuous variables ANOVA was performed while for medians, we performed Kruskal-Wallis tests to determine p-values between all four groups. PSQI: Pittsburgh Sleep Quality Index, ESS: Epworth Sleepiness Scale. High score on block design, digit symbol and ICT targets is good while a high score on the remaining tests indicates poor performance.
Cognitive performance and proportion impaired on paper-pencil tests was significantly worse in the cirrhotic groups (with/without OSA) compared to the non-cirrhotic groups (Figure 2A). Based on standard tests, 36% of OSA patients without cirrhosis would also have been classified as having covert HE if they were cirrhotic. Interestingly, while cognitive performance on paper-pencil tests was worse in cirrhotic patients (with/without OSA), executive function through the study of ICT lures were worse in those with OSA (with/without cirrhosis) compared to the rest (table 1).
ESS and PSQI were correlated (0.5, p<0.0001) with each other. There were only modest correlations between sleep questionnaires and cognitive performance. PSQI was positively linked to lures (0.3, p=0.02), and negatively with targets (−0.3, p=0.006) and digit symbol (−0.3, p=0.03). Hours of sleep per night was modestly linked with digit symbol (0.3, p=0.01) and targets (0.3, p=0.02) and negatively with lures (0.2, p=0.05). Similarly, ESS negatively correlated with digit symbol (−0.3, p=0.001). There was a significantly higher ESS (11.4±4.6 vs. 8.7±4.1, p=0.02) and PSQI (9.9±5.2 vs. 7.1±3.5, p=0.04) in those with impaired cognition on the paper-pencil tests.
Similar to earlier experience, AHI and ESS (r=0.02, p=0.9) and PSQI (r=−0.1, p=0.6, supplementary figures 2A/B) were not significantly correlated13. However, a high AHI was linked with poor cognition (NCT-A, r=0.6,p=0.005, NCT-B, r=0.5,p=0.03, block design, r=−0.5,p=0.03 and targets, r=−0.7,p<0.0001).
Driving simulation cross-sectional
There were significantly lower total speeding events in the cirrhosis+OSA group compared to the rest. We found a significantly higher total lane deviation number in both OSA groups compared to controls (Table 1). All subjects felt subjectively sleepier to a similar extent in the second half of the simulation. The lane deviations were significantly higher in the second half in both OSA groups compared to non-OSA cirrhotics and controls. We found that daytime sleepiness (ESS) was correlated positively with total and delta lane deviations (0.3, p=0.03 and 0.5, p<0.0001 respectively) and negatively with total speeding instances (−0.2, p=0.05). Similarly, PSQI was linked with total and delta lane deviations (0.3, p=0.04 and 0.4, p=0.02) respectively but not with speed.
Longitudinal analysis before and after CPAP initiation
We followed ten patients with OSA and cirrhosis (age 53±5 years, MELD 8±2, 7 HCV and 3 NASH) and seven OSA patients without cirrhosis (age 52±4 years) who were initiated on CPAP. Patients were re-tested a median of 2.5 months (2-6 months) post-CPAP. There was a significant reduction in the AHI post-CPAP in both cirrhotics (median 37 to 3, p=0.001) and non-cirrhotic groups (median 42 to 4, p=0.001). In both groups there was an improvement in PSQI, post-simulator sleepiness change and executive function (reduction in ICT lures). In the non-cirrhotic group, there was an additional improvement in number connection A and B performance (Table 2). In the cirrhotic group, however, no other cognitive change was seen. With respect to driving simulation, there was a significant reduction in lane deviations over time after CPAP compared to pre-CPAP in both cirrhotic and non-cirrhotic patients (table 3, Figure 3). No change in speeding was seen.
Table 2.
Longitudinal assessment of OSA patients with/without cirrhosis before and after CPAP initiation
| Cirrhosis+OSA (n=10) | OSA only (n=7) | |||
|---|---|---|---|---|
| Pre-CPAP | Post-CPAP | Pre-CPAP | Post-CPAP | |
| ESS | 14.0±3.7 | 13.5±4.2 | 8.0±5.1 | 6.9±3.1 |
| PSQI | 11.4±2.2 | 8.6±1.2* | 11.0±1.4 | 7.5±1.8* |
| Cognition | ||||
| Number connection -A (sec) |
33.2±13.2 | 32.7±9.4 | 37±16 | 34±5* |
| Number connection -B (sec) |
79.9±21.3 | 76.3±10.9 | 86.1±14.9 | 74.5±14.8* |
| Digit Symbol | 53.5±14.3 | 59.2±11.0 | 52.0±11.7 | 54.2±12.4 |
| Block design | 32.2±11.9 | 34.6±12.1 | 32.0±14.3 | 36.4±9.5 |
| ICT lures (number) | 13.1±7.9 | 7.5±3.3* | 16.5±5.9 | 6.3±4.9* |
| ICT targets (% correct) | 95.7±2.9 | 95.8±7.4 | 95.8±3.5 | 96.1±4.9 |
| Driving Simulation | ||||
| Total speeding (mean) | 32.7±34.2 | 25.2±14.5 | 29.4±22.7 | 23.5±25.1 |
| Total lane deviations (mean) |
306.8±135.1 | 289.4±137.3 | 300.3±105.1 | 275.1±151.2 |
| Δ Sleepiness (median) | 4.0 | 1.5* | 3.5 | 2.0* |
p<0.05 pre-CPAP compared to post-CPAP. Sleep quality, executive function (ICT lures) and sleepiness change in the simulation improved in both groups while number connection tests improved only in the group without cirrhosis.
PSQI: Pittsburgh Sleep Quality Index, ESS: Epworth Sleepiness Scale. High score on block design, digit symbol and ICT targets is good while a high score on the remaining tests indicates poor performance.
Table 3.
Change in driving simulation over the first and second half before/after CPAP in cirrhotics and non-cirrhotic OSA patients
| Cirrhosis+OSA (n=10) | OSA only (n=7) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-CPAP | Post-CPAP | Pre-CPAP | Post-CPAP | |||||
| 1st half | 2nd half | 1st half | 2nd half | 1st half | 2nd half | 1st half | 2nd half | |
| Speeding | 15.9±15.6 | 16.8±19.5 | 11.6±8.6 | 13.6±7.6 | 15.3±12.5 | 13.3±12.2 | 14.6±12.4 | 15.2±13.5 |
| Total lane deviations |
132±70 | 169±70† | 151±69 | 155±72 | 136±98 | 176±68† | 139±89 | 145±79 |
There was a significantly higher mean number of lane deviations in the 2nd half compared to the 1st half before CPAP which became statistically similar after CPAP in both cirrhotics and non-cirrhotics.
p<0.05 on paired t-test
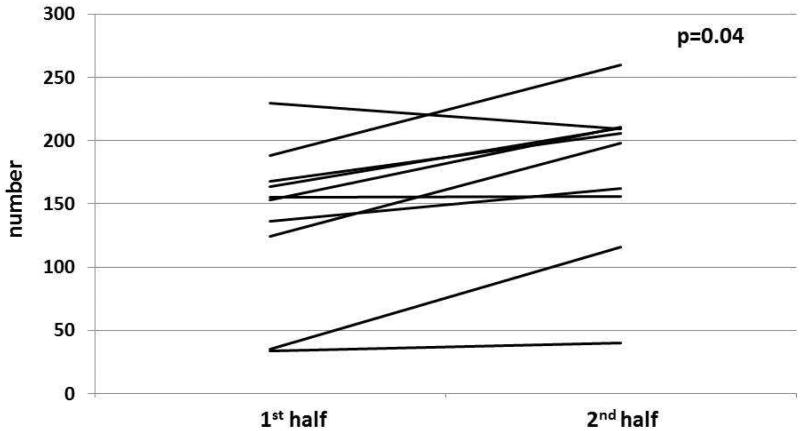
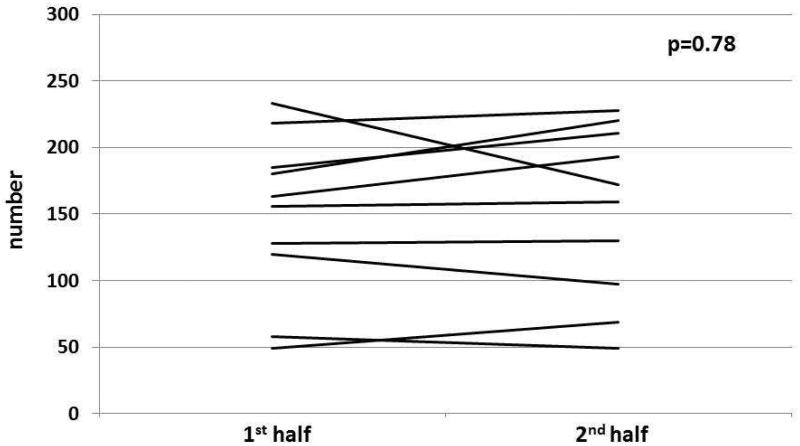
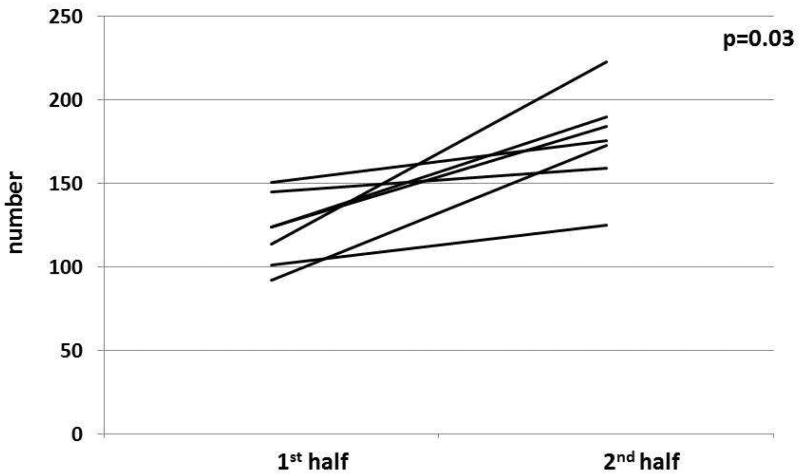
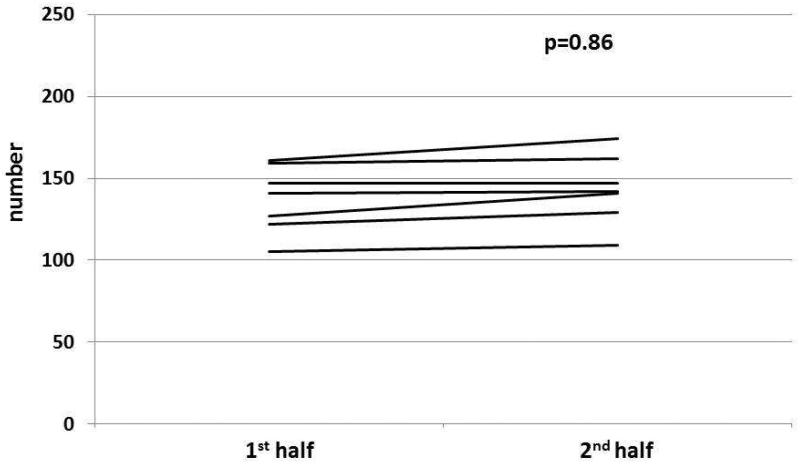
Figure 3. Overarching title: Change in lane deviations between 1st and 2nd half of simulation before and after CPAP in patients with OSA.
Figure 3A: In cirrhosis+OSA, there was a significant increase in lane deviations in the 2nd half compared to the 1st half before CPAP initiation.
Figure 3B: In cirrhosis+OSA, there was no significant change in lane deviations in the 2nd half compared to the 1st half after CPAP initiation.
Figure 3C: In OSA only patients, there was a significant increase in lane deviations in the 2nd half compared to the 1st half before CPAP initiation.
Figure 3D: In OSA only patients, there was no significant change in lane deviations in the 2nd half compared to the 1st half after CPAP initiation.
Discussion
Our data demonstrates the complex relationship between OSA and cirrhosis with respect to cognitive function, sleep quality and driving simulator performance. We found that OSA and cirrhosis affect cognition, sleep and driving differently and correction of underlying OSA using CPAP in cirrhotic patients can modulate the sleep quality, driving simulation (i.e., lane deviation errors) and executive function (i.e., number of lures). Impaired sleep quality and daytime sleepiness were higher in OSA groups, with no additive impact of cirrhosis. Interestingly, while performance on executive function tasks improved following CPAP in subjects with OSA alone, the combination of OSA and cirrhosis attenuated this beneficial treatment response.
The significantly worse daytime sleepiness in cirrhosis reflects prior studies and extends it on to OSA patients. This focus on OSA as a potential reason for daytime sleepiness in cirrhosis is particularly important because changes in sleep-wake cycle are assumed to be a sign of overt HE. As a result, these patients are then prescribed overt HE treatments that would be ineffective in case these sleep issues were caused by OSA. As expected, our OSA patients (cirrhotic/non-cirrhotic) had a significantly higher BMI and neck circumference compared to controls and cirrhotics without OSA without ascites. Therefore sleep issues in an obese subject with cirrhosis should direct clinicians to investigate about OSA before assuming it is due to overt HE.
The linkage between sleep pattern changes and cirrhosis has been controversial with studies yielding mixed results related to the interaction between sleep quality, daytime sleepiness and cognition 3, 14-17. Our findings of a modest correlation between cognition and sleep quality/daytime sleepiness with significantly worsened PSQI and ESS in cognitively impaired patients overall compared to those without confirms recent reports in cirrhotics and extends them on to OSA3, 14-16. This again shows that there may not be a direct link between sleep-wake cycle changes and cognitive dysfunction in cirrhosis and that OSA may be an important determinant of these changes.
Our results are consistent with prior cognitive studies in non-cirrhotic OSA patients12. We found that non-cirrhotic OSA patients were significantly impaired on ICT lures, targets, digit symbol and number connection test-A/B compared to healthy controls. These tests evaluate several domains of executive function (working memory, response inhibition) while also employing attention and psychomotor speed resources18. The ICT has been shown to correlate with driving abnormalities in cirrhotics and mirrors findings seen in non-cirrhotic OSA driving studies19, 20. This cognitive dysfunction was compounded in cirrhotics who were further impaired on digit symbol and both number connection tests, highlighting the continuum of attention-centered cognitive impairments with increasing co-morbidities12. While we did not specifically evaluate these systemic covariates, poor performance on these tests have been linked to both sleep deprivation and hypoxemia of the pre-frontal cortex that leads to a reduction in shifting resources, as well as reduced cortical tone and arousal of subcortical structures with resultant cognitive impairment21.
Although cognition was the worst in cirrhotic groups on paper-pencil tests, it is also interesting that the use of a battery recommended for covert HE diagnosis in cirrhosis was impaired in 36% of non-cirrhotic OSA subjects and AHI was correlated with cognitive performance. This reiterates the non-specificity of these tests and the multiple mechanisms that could impact their performance. Specifically the improvement in number connection tests after CPAP in non-cirrhotic OSA but not in cirrhotics indicates that the mechanisms of abnormal performance in both groups varies and is related to hypoxemia in non-cirrhotic OSA patients and to cirrhosis-related pathogenesis in those with cirrhosis and OSA. Conversely improvement in ICT lures in both populations after CPAP shows that in patients with OSA, abnormal executive function is related to hypoxemia. Another implication for these non-specific cognitive results is to avoid including OSA subjects as “healthy” subjects to collect norms in populations1, 12, 18.
We further extended the cognitive testing into a realistic context using our monotonous driving simulation validated to evaluate inattention stemming from sleepiness5. In this program, proper speed maintenance and going over the limit requires both wakefulness and attention while lane deviations represent sleepiness, reduced vigilance and inattention5. Risser et al showed that, as time progressed, there was significantly higher lane and steering variability in OSA patients which was coupled with attention lapses on EEG5. Therefore with progressive sleepiness, we would expect a lowering of speeding and increase in lane deviations. We found that OSA patients (regardless of cirrhosis) had worse lane deviations over time compared to non-OSA cirrhotics who were in turn worse than the controls. This again points towards the differential effect of OSA compared to cirrhosis on cognitive functioning, and emphasizes the impact that OSA has on subcortical structures associated with cortical tone and arousal.
This differential impact was further demonstrated by only a partial reversibility of cognitive function after CPAP therapy in cirrhotic patients with OSA. Our findings extend this into the realm of cirrhosis and OSA. As in the cross-sectional component (Table 1), ICT lures performance was worst in OSA patients (with/without cirrhosis) compared to other groups which improved after CPAP. This indicates that lure performance was in part related to depressed oxygenation. Interestingly, number connection A/B also improved only in non-cirrhotic OSA patients, which is consistent with prior studies in non-cirrhotic OSA patients that showed only focused cognitive improvement in selected domains with an enhancement of driving simulator performance22-24. Interestingly, both cirrhosis+OSA and OSA only groups improved lane deviations after CPAP showing that vigilance is related to hypoxemia in OSA independent of cirrhosis. The treatment response dissociation in OSA between with and without cirrhosis suggests additional processes may account for illness phenomenology in cirrhosis+OSA patients. While CPAP may improve cognition by reducing hypoxemia in cirrhotic patients with OSA (resulting in improved cortical tone and executive function), the systemic inflammatory milieu associated with gut-bacteria remain unaffected3.
Our study is limited by the use of patients without overt HE, who have more pronounced sleep-wake cycle abnormalities14. The inclusion of those patients, who are advanced, however, could have skewed the results and their tendency to have ascites could have influenced their sleep quality and BMI. Our cognitive battery also did not use a delayed memory task since it was focused on cirrhosis-associated cognitive impairment25. We also used a simulator that is designed for testing sustained attention similar to that used in OSA studies but not reaction time which has been used in prior cirrhosis studies5, 26.
We conclude that there is a complex relationship between OSA and cirrhosis that affects sleep quality, daytime sleepiness, cognition and driving simulation. OSA is a potential cause of daytime sleepiness in cirrhosis and should be considered in obese cirrhotic patients before assuming it to be due to HE. The data show that in a cirrhotic patient with OSA, sleep issues and worsening driving over time are likely related to OSA. Our data also showed that executive dysfunction is similar in OSA patients with/without cirrhosis but cirrhosis augments the attention deficits present in OSA patients. CPAP results in improved executive function, sleep quality and driving simulation in OSA patients regardless of cirrhosis but only does not improve further cognition in those with OSA and cirrhosis. OSA should be considered as a modulator of cognitive dysfunction and sleep quality in cirrhosis.
Supplementary Material
Acknowledgements
We would like to acknowledge Theodore J Rosenthal from Systems Technology Incorporated for assistance with the simulator program.
Grant support: This work was partly supported by grant RO1AA020203 from the National Institute on Alcohol Abuse and Alcoholism, grant RO1DK087913 from the National Institute of Diabetes and Digestive and Kidney Diseases, grant UL1TR00058 from the National Center for Advancing Translational Sciences and the McGuire Research Institute. The sponsors did not have any role in design, collection or interpretation of the data.
Abbreviations
- OSA
obstructive sleep apnea
- HE
hepatic encephalopathy
- NCT-A/B
number connection test A/B
- DST
digit symbol test
- BDT
block design test
- ICT
inhibitory control test
- ESS
Epworth Sleepiness Scale
- PSQI
Pittsburgh Sleep Quality Index
- AHI
Apnea-hypopnea index
- CPAP
continuous positive airway pressure
- MELD
model for end-stage liver disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
Writing Assistance: none
Presentation: Portions of this manuscript were presented in abstract form at the Liver Meeting 2013 in Washington DC.
REFERENCES
- 1.Kappus MR, Bajaj JS. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10:1208–19. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Luria AR. The functional organization of the brain. Sci Am. 1970;222:66–72. doi: 10.1038/scientificamerican0370-66. passim. [DOI] [PubMed] [Google Scholar]
- 3.Montagnese S, De Pitta C, De Rui M, et al. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2013 doi: 10.1002/hep.26555. [DOI] [PubMed] [Google Scholar]
- 4.Crespo J, Cifrian J, Pinto JA, et al. Sleep apnea obstructive syndrome: a new complication previously undescribed in cirrhotic patients with ascites. Am J Gastroenterol. 2003;98:2815–6. doi: 10.1111/j.1572-0241.2003.08754.x. [DOI] [PubMed] [Google Scholar]
- 5.Risser MR, Ware JC, Freeman FG. Driving simulation with EEG monitoring in normal and obstructive sleep apnea patients. Sleep. 2000;23:393–8. [PubMed] [Google Scholar]
- 6.Kappus MR, Leszczyszyn DJ, Moses L, et al. Effect of obstructive sleep apnea on the sleep architecture in cirrhosis. J Clin Sleep Med. 2012;9:247–51. doi: 10.5664/jcsm.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musso G, Olivetti C, Cassader M, et al. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin Liver Dis. 2012;32:49–64. doi: 10.1055/s-0032-1306426. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 12.Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–73. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 13.Macey PM, Woo MA, Kumar R, et al. Relationship between obstructive sleep apnea severity and sleep, depression and anxiety symptoms in newly-diagnosed patients. PLoS One. 2010;5:e10211. doi: 10.1371/journal.pone.0010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samanta J, Dhiman RK, Khatri A, et al. Correlation between degree and quality of sleep disturbance and the level of neuropsychiatric impairment in patients with liver cirrhosis. Metab Brain Dis. 2013;28:249–59. doi: 10.1007/s11011-013-9393-3. [DOI] [PubMed] [Google Scholar]
- 15.Cordoba J, Cabrera J, Lataif L, et al. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27:339–45. doi: 10.1002/hep.510270204. [DOI] [PubMed] [Google Scholar]
- 16.Bersagliere A, Raduazzo ID, Nardi M, et al. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology. 2012;55:869–78. doi: 10.1002/hep.24741. [DOI] [PubMed] [Google Scholar]
- 17.Stewart CA, Auger R, Enders FT, et al. The effects of poor sleep quality on cognitive function of patients with cirrhosis. J Clin Sleep Med. 2014;10:21–6. doi: 10.5664/jcsm.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weissenborn K, Giewekemeyer K, Heidenreich S, et al. Attention, memory, and cognitive function in hepatic encephalopathy. Metab Brain Dis. 2005;20:359–67. doi: 10.1007/s11011-005-7919-z. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj JS, Hafeezullah M, Franco J, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600. doi: 10.1053/j.gastro.2008.07.021. e1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Varvarigou V, Parks PD, et al. Psychomotor vigilance testing of professional drivers in the occupational health clinic: a potential objective screen for daytime sleepiness. J Occup Environ Med. 2012;54:296–302. doi: 10.1097/JOM.0b013e318223d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sforza E, Roche F. Sleep apnea syndrome and cognition. Front Neurol. 2012;3:87. doi: 10.3389/fneur.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP) Brain Res Bull. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 23.Kylstra WA, Aaronson JA, Hofman WF, et al. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev. 2013;17:341–7. doi: 10.1016/j.smrv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Vakulin A, Baulk SD, Catcheside PG, et al. Driving simulator performance remains impaired in patients with severe OSA after CPAP treatment. J Clin Sleep Med. 2011;7:246–53. doi: 10.5664/JCSM.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 26.Bajaj JS, Hafeezullah M, Hoffmann RG, et al. Navigation skill impairment: Another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47:596–604. doi: 10.1002/hep.22032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.