Abstract
Background
There are limited data on prognostic utility of interferon-gamma (IFN-γ) release assays (IGRAs) for active tuberculosis (TB) among HIV-1 infected individuals.
Methods
Samples from a perinatal cohort of HIV-1 infected women in Kenya, obtained during pregnancy were tested using T-SPOT.TB IGRAs to detect Mycobacterium tuberculosis (MTB)-specific IFN-γ responses. IFN-γ (cut-off values>0, ≥6 and ≥10 spot forming cells/well (SFCs/well)) and CD4 cell count (cut-off values<250 and <350 cells/μL) were evaluated for sensitivity and specificity using a time dependent receiver operating characteristic (ROC) curve and positive predictive value (PPV) using Kaplan Meier method for future TB within one year postpartum.
Results
Of 327 women, 9 developed TB within one year postpartum (Incidence rate (IR): 3.5/100 person-years of follow-up (pyfu); 95% confidence interval: 1.6–6.7/100 pyfu). IFN-γ≥6 SFCs/well was associated with an optimal trade-off between sensitivity (78%) and specificity (55%) and PPV of 5.9%. In women with CD4<250 cells/μL, sensitivity and specificity of IFN-γ≥6 SFCs/well were 89% and 63%, respectively and PPV was 19.2%.
Conclusion
Among HIV-1 infected women, IFN-γ response (≥6 SFCs/well) during pregnancy lacked high positive predictive value for postpartum TB but had higher sensitivity and positive predictive value among immunosuppressed women (CD4<250 cells/μL).
Keywords: TB IGRA, Predictive value, Sensitivity, Specificity, HIV-infected women
Background
Tuberculosis (TB) and HIV-1 are major health problems for women during reproductive years.1 Latent TB infection (LTBI) during pregnancy in HIV-1 infected women is associated with increased risk of postpartum tuberculosis, maternal and infant mortality and infant HIV-1 transmission.2–4 Although the World Health Organization (WHO) recommends isoniazid prophylaxis therapy (IPT) to all HIV-1 infected individuals, including pregnant women, global coverage of this intervention is <1%.5 Alternative targeted strategies may be useful.
Tuberculin skin tests (TSTs) are commonly used to detect LTBI and inform IPT.6,7 IGRAs are currently recommended for detection of LTBI, alone or with TST in TB-exposed individuals in the US, Canada and the UK. 8–12 However, WHO currently does not recommended IGRAs in low- and middle-income countries (LMICs) due to insufficient data on their utility in these settings.13
Several studies have shown associations between IGRA responses and development of active TB, with some evaluating magnitude of IFN-γ responses as a predictor.14–20 Among HIV-1 infected individuals, some studies have reported associations between positive IGRA and future active TB, however, predictive value of magnitude of IFN-γ responses has not been described.3,21,22
Conventional test cut-offs for IGRAs were developed based on concurrent active TB as a gold-standard. The same IGRA cut-offs have been used for detection of LTBI, because there is no gold-standard for latent infection. Many studies have evaluated IGRA for diagnosis of active TB, which is distinct from using IGRA to detect LTBI and predict future active TB.23 It is important to assess prognostic merit of IGRAs in HIV-1 infected individuals at high risk for TB.21,24 Further, because immunosuppression might influence IGRA results and performance, combining IGRAs with CD4 counts might enhance diagnostic utility.
We previously reported a significant association between T-SPOT.TB IGRA (Oxford Immunotec) during pregnancy with postpartum active TB in HIV-1 infected women (adjusted hazard ratio (HR), 4.5; 95% confidence interval (CI), 1.1–18.0; p=0.03).3 Using IGRA results from this cohort, we developed prognostic models to estimate sensitivity, specificity and positive predictive value (PPV) of IFN-γ response, and CD4 count measured during pregnancy for diagnosis of tuberculosis within 1 year postpartum.
Methods
Data from a historical perinatal cohort of 535 HIV-1 seropositive women in Nairobi, Kenya, accrued and followed between 1999 and 2005, were used. Details of the cohort have been described.3,25,26 Written informed consent for the parent study was obtained. The Human Subjects Division at University of Washington and Ethical Review Committee at Kenyatta National Hospital approved the parent and current studies.
Of 535 women in the cohort, 393 with at least one available cryopreserved peripheral blood mononuclear cell (PBMC) sample at enrollment were included for IGRA testing.3 Of the 393, valid IGRA results were obtained for 361; 7 had no viable cells and 25 had invalid assays. Women with indeterminate IGRAs due to spot count>10 SFCs/well in the negative control were included, however 8 with spot count<20 SFCs/well in the positive control were excluded. Of the remaining 353 women (361 minus 8), 327 were seen after delivery and included in this analysis (Online Appendix Figure 1). The denominator of 327 women in this analysis differs from the past published analysis (269 women) because the prior analysis excluded women with a prior history of TB and all women with indeterminate responses (in the previous analysis, of 361 women with valid IGRAs, we excluded 28 with a history of TB, 12 with no postpartum follow-up and 52 with indeterminate IGRA responses, resulting in 269 women).3
PBMCs were evaluated for MTB-specific IFN-γ responses using the T-SPOT.TB manufacturer-defined procedures, previously described.3 Measurement of CD4 counts was conducted using a FACScan flow cytometer (Becton Dickinson).27
None of the women included in this analysis had clinically diagnosed TB at enrollment; however women who reported prior history of TB were included.
Mothers with suspected TB during follow-up were referred to the Kenyan Ministry of Health (MOH) TB Clinic. MOH diagnostic protocol involved a chest radiograph and 3 sputum samples. Adults with at least 1 positive smear received sputum-positive pulmonary TB diagnosis and those with 3 negative smears but chest radiograph and clinical presentation suggestive of tuberculosis received sputum-negative pulmonary tuberculosis diagnosis. Culture was not routinely used for tuberculosis diagnosis.28 Women reported the tuberculosis diagnosis and treatment they received from MOH during follow-up visits. Since the perinatal cohort was not established to address TB, data regarding specific TB diagnostic procedures and disease classification were not obtained from MOH. Use of IPT is not routine in Kenya and IGRA assays were conducted after the clinical cohort was completed. Thus, none of the women received IPT.
In our previous analysis, we determined the HR of postpartum tuberculosis and mortality in mothers with a positive IGRA, during pregnancy.3 For the current analyses, we used results from the same IGRA assays but derived cut-off values in the quantitative IFN-γ response (spot forming cells (SFCs)/well) and CD4 count to assess their sensitivity, specificity and PPV for tuberculosis postpartum. IFN-γ response was defined as SFCs/well in the antigen-containing minus negative control. Negative values of IFN-γ response were set to 0. We calculated SFCs/well for combined IFN-γ response (maximum of ESAT-6 and CFP-10 response above background) and antigen-specific response (ESAT-6- or CFP-10-specific response above background). We defined, a priori, three cut-points in the combined and antigen-specific IFN-γ response: >0, ≥6 and ≥10 SFCs/well. Above background (>0) represents the least conservative cut-off, ≥6 represents cut-off for a positive IGRA defined by T.SPOT.TB manufacturer and ≥10 represents a more conservative cut-off. CD4 count cut-off values were set at <250 and <350 cells/μL. Lastly, we determined the performance of the 3 IFN-γ cut-offs within the strata of the two CD4 cell count cut-offs.
Active TB diagnosed during the first year postpartum was the gold standard for test performance. We defined sensitivity as probability of being test positive (above IFN-γ or below CD4 cut-offs) during pregnancy in women who developed tuberculosis and specificity, the probability of being test negative (below IFN-γ or above CD4 cut-offs) during pregnancy in women who did not develop tuberculosis within 1 year postpartum. PPV was defined as cumulative incidence of tuberculosis in women who were above IFN-γ or below CD4 cut-offs.
For analysis of time to tuberculosis, person-time was initiated from delivery and women were censored at initiation of antiretroviral therapy, death, last study clinic visit, or 12 months postpartum. Some women reported tuberculosis but did not report a diagnosis of TB at subsequent study visit. These women were not counted as TB cases and were censored at last visit documented to be free of TB.
Sensitivity, specificity, and area under the Receiver Operating Characteristic (ROC) curve (AUC) were calculated, from a time-dependent ROC curve using the Kaplan Meier method, taking censoring into account.29 CI for sensitivity, specificity and AUC were based on quantiles of 1000 samples from the bootstrap procedure. AUC (95% CI) values were estimated for each cut-off value as well as using the markers as continuous measures. PPV for postpartum tuberculosis are Kaplan-Meier estimates and 95% CI, calculated for each marker cut-off (above IFN-γ or below CD4 cut-offs). Log-rank test was used to determine equality of survival curves at different levels of a marker. Cox proportional hazards (PH) regression analysis was conducted to estimated HR for incident active TB, associated with being above IFN-γ or below CD4 cut-offs.
Sensitivity and specificity were derived using the survivalROC package and CIs using the boot package in R version 2.9.2.30,31 Kaplan Meier survival analyses and Cox PH regression analyses were conducted using STATA 11.1.32
Results
Baseline characteristics of 327 women in this analysis are shown in Table 1. Nine of the 327 women developed tuberculosis within one year postpartum (incidence rate (IR): 3.5/100 person years of follow-up (pyfu); 95% CI: 1.6–6.7). Online Appendix Figure 2 displays the survival curve for tuberculosis.
Table 1.
Baseline sociodemographic and clinical characteristics and distribution of baseline markers in pregnant HIV-1 infected women
| Indicator | Women,a no.(n=327) | Valueb |
|---|---|---|
| Sociodemographic characteristic | ||
| Age, years | 326 | 25.0 (22.0–28.0) |
| Ever married | 327 | 302 (92.4) |
| More than primary education | 323 | 139 (43.0) |
| Employed | 327 | 111 (33.9) |
| Living in >1 room household | 325 | 67 (20.6) |
| Number of rooms | 325 | 1.0 (1.0–1.0) |
| Use of flush toilet | 327 | 164 (50.2) |
| Use of shared toilet | 327 | 297 (90.8) |
| Number of persons per room | 326 | 3.0 (2.0–4.0) |
| Medical history | ||
| History of tuberculosis | 327 | 28 (8.6) |
| History of HIV-1 related illness | 327 | 40 (12.2) |
| T-SPOT.TB IGRA response | 327 | |
| Positive | 125 (38.2) | |
| Negative | 157 (48.0) | |
| Indeterminate | 45 (13.8) | |
| ESAT-6 spot countc | 327 | |
| >0 | 193 (59.0) | |
| ≥ 6 | 130 (39.8) | |
| ≥ 10 | 113 (34.6) | |
| CFP-10 spot countc | 327 | |
| >0 | 186 (56.9) | |
| ≥ 6 | 123 (37.6) | |
| ≥ 10 | 101 (30.9) | |
| Maximum (ESAT-6, CFP-10) c | 327 | |
| > 0 | 212 (64.8) | |
| ≥ 6 | 152 (46.5) | |
| ≥ 10 | 132 (40.4) | |
| HIV-1 markers | ||
| CD4 cell count, cells/μL | 317 | 413.0 (300.0–618.0) |
| <250 | 58 (18.3) | |
| <350 | 115 (36.3) | |
| HIV-1 viral load, copies/mL | 326 | 4.8 (4.2–5.3) |
| >50,000 | 170 (52.0) | |
For whom data was available
Median (interquartile range) or number (percent) of women
Spot count above background
Sensitivity of IFN-γ>0 and ≥6 SFCs/well was approximately 78%; IFN-γ≥10 SFCs/well was associated with lower sensitivity (65%). There was gain in specificity with increasing magnitude of IFN-γ with highest specificity (60%) observed at ≥10 SFCs/well. T-SPOT.TB manufacturer-defined cut-point (≥6 SFCs/well above background) was associated with an optimal trade-off between sensitivity and specificity (Table 2). AUC values for IFN-γ as baseline marker for postpartum tuberculosis was 0.64 (95% CI: 0.48–0.80) (Figure 1). AUC values (95% CIs) for each cut-off are shown in Table 2.
Table 2.
Sensitivities, specificities and positive predictive values (PPVs) for tuberculosis within 1 year postpartum associated with cut-off values in IFN-γ response measured at 32 weeks’ gestation in HIV-1 infected women.
| Predictor | Cut-off value | Events | PYFU | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive value (95% CI) | AUC (95% CI) | Hazard Ratio* (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| IFN-γ | >0 | 7 | 164.3 | 78.9 (47.8–100.0) | 35.7 (30.3–41.4) | 4.3 (1.7–8.8) | 0.57 (0.41–0.69) | 1.9 (0.4–9.3) | 0.41 |
| ≥ 6 | 7 | 118.7 | 77.7 (45.7–100.0) | 54.6 (49.3–60.1) | 5.9 (2.4–12.2) | 0.66 (0.50–0.78) | 4.0 (0.8–19.3) | 0.08 | |
| ≥ 10 | 6 | 102.1 | 65.0 (29.0–98.7) | 60.5 (55.2–65.7) | 5.9 (2.2–12.8) | 0.63 (0.45–0.80) | 3.0 (0.7–12.0) | 0.12 | |
| ESAT-6 | >0 | 6 | 148.6 | 69.1 (36.3–100.0) | 41.3 (35.9–46.8) | 4.0 (1.5–8.8) | 0.55 (0.37–0.70) | 1.4 (0.4–5.7) | 0.61 |
| ≥ 6 | 6 | 101.8 | 67.4 (30.7–98.0) | 61.2 (56.1–66.8) | 5.9 (2.2–12.8) | 0.64 (0.45–0.80) | 3.0 (0.8–12.1) | 0.12 | |
| ≥ 10 | 5 | 86.7 | 55.5 (21.3–90.2) | 66.2 (60.8–71.5) | 5.8 (1.9–13.5) | 0.61 (0.43–0.79) | 2.4 (0.7–9.1) | 0.18 | |
| CFP-10 | >0 | 7 | 143.9 | 78.9 (44.1–100.0) | 43.9 (38.3–49.1) | 4.9 (2.0–10.0) | 0.61 (0.46–0.74) | 2.7 (0.6–13.1) | 0.21 |
| ≥ 6 | 6 | 93.6 | 66.0 (28.5–99.2) | 63.4 (57.7–68.6) | 6.4 (2.4–14.0) | 0.65 (0.46–0.82) | 3.4 (0.9–13.8) | 0.08 | |
| ≥ 10 | 5 | 76.7 | 50.5 (13.9–85.2) | 69.8 (64.7–74.8) | 6.5 (2.1–15.2) | 0.60 (0.43–0.78) | 2.9 (0.8–10.8) | 0.11 |
Note: PYFU Person years of follow-up; CI Confidence Interval
Crude Hazard Ratio for active TB in women above IFN-γ cut-off value compared with women below IFN-γ cut-off value
Figure 1.
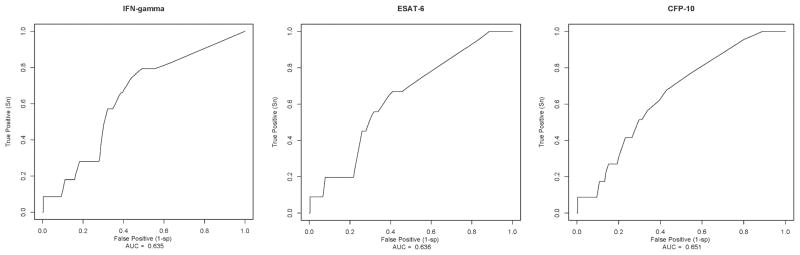
Receiver Operating Characteristic Curves for interferon-γ, ESAT-6 and CFP-10 as baseline markers for postpartum tuberculosis and the corresponding area under the ROC curve (AUC) value in HIV-1 infected women
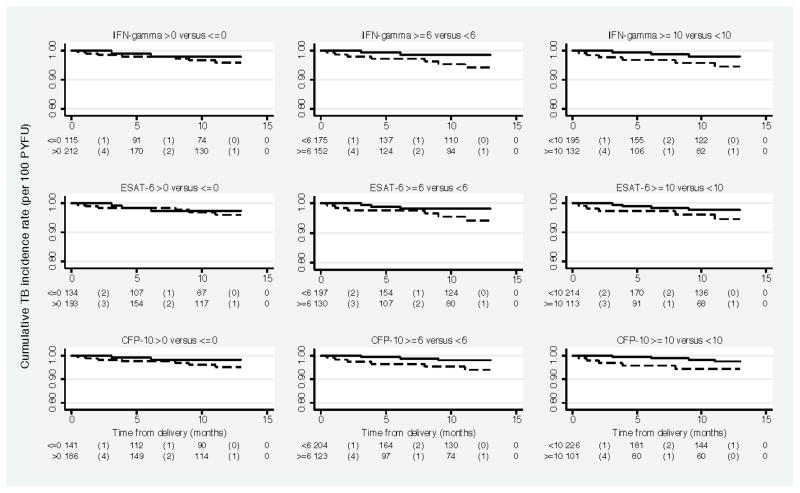
Of the women with IFN-γ >0, ≥6 and ≥10 SFCs/well, 4.3%, 5.9% and 5.9% developed tuberculosis within one year postpartum, respectively (Table 2). The PPVs associated with the cut-off values in ESAT-6 were similar to those for the combined response, whereas the PPVs associated with CFP-10 were slightly higher. Survival curves for postpartum TB for women above and below each of these cut-offs are shown in Figure 2 and the unadjusted HRs for TB associated with each cut-off are shown in Table 2. IFN-γ≥6 SFCs/well was associated with a 4-fold increased risk of postpartum active TB (HR: 4.0; 95% CI: 0.8–19.3; P=0.08).
Figure 2.
Kaplan Meier estimates of cumulative tuberculosis incidence rate in HIV-1 infected women between delivery and 1 year postpartum by various cut-points in combined interferon-γ response and response to individual antigens, measured during pregnancy.
Note: In each plot, dashed line represents the survival curve for women below the cut-off value and bold line represents the survival curve for women above the cut-off value. The risk table shown below each plot shows the number at risk (number of events) within each time interval represented on the x-axis.
p-values for Log-rank test for equality of survival functions at each of the cut-off values displayed were >0.05.
The estimates if sensitivity, specificity, PPV, AUC and HR of IFN-γ for a 2 year follow-up are presented in Online Appendix Table 1.
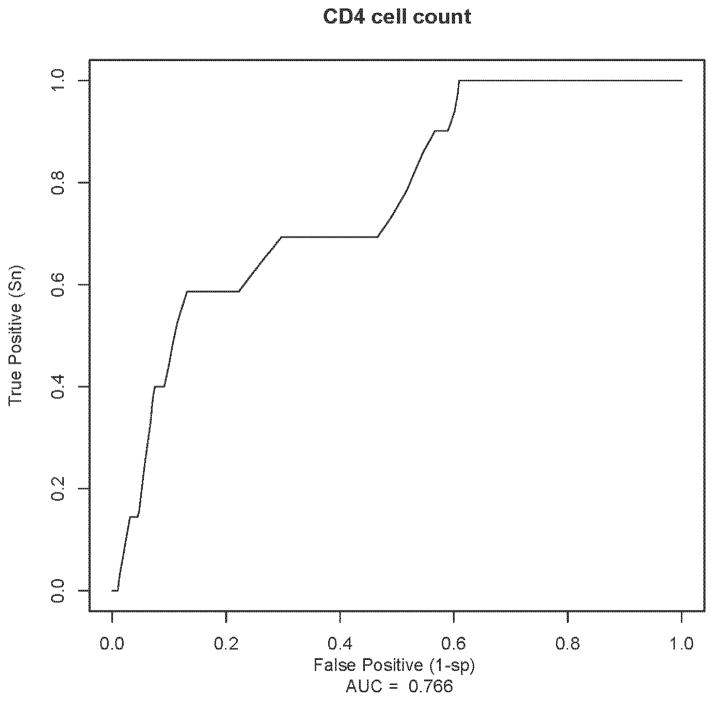
Sensitivity of CD4<350 and <250 cells/μL was 65% and 52%, respectively. Specificity was higher for CD4<250 cells/μL (82%) yielding 18% false positive results (1 minus specificity) compared to 35% false positive results at CD4<350 cells/μL (Table 3). ROC curve for CD4 as a baseline marker for postpartum tuberculosis had an AUC value of 0.76 (95% CI: 0.56–0.90) (Figure 3). AUC values for CD4<250 and <350 cells/μL was 0.67 (95% CI: 0.48–0.87) and 0.65 (95% CI: 0.44–0.86), respectively.
Table 3.
Sensitivities, specificities and positive predictive values for tuberculosis within one year postpartum associated with cut-off values in CD4 cell count alone and in combination with IFN-γ responses measured at 32 weeks’ gestation in HIV-1 infected women
| Predictor cut-off value | Events | PYFU | Sensitivity* | Specificity | Positive Predictive value | Hazard Ratio** (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|
| CD4 cell count | ||||||||
| <250 | 4 | 44.4 | 51.5 (14.1–91.7) | 82.8 (78.4–87.0) | 9.0 (2.5–23.1) | 4.7 (1.2–18.6) | 0.03 | |
| <350 | 5 | 84.4 | 64.6 (24.6–100.0) | 64.7 (59.3–69.8) | 5.9 (1.9–13.8) | 3.3 (0.8–13.7) | 0.10 | |
| CD4 cell count | IFN-γ | |||||||
| ≥250 | >0 | 3 | 137.5 | 78.0 | 33.4 (27.3–39.5) | 2.2 (0.4–6.4) | 1.5 (0.2–14.4) | 0.73 |
| ≥ 6 | 3 | 98.9 | 76.3 | 53.5(47.1–59.8) | 3.0 (0.6–8.9) | 3.3 (0.3–31.6) | 0.30 | |
| ≥ 10 | 2 | 85.4 | 47.5 | 59.2(53.3–65.2) | 2.3 (0.3–8.5) | 1.4 (0.2–10.1) | 0.72 | |
| <250 | >0 | 3 | 21.7 | 84.6 | 47.7(33.8–61.4) | 13.8 (2.9–40.4) | 3.2 (0.3–30.7) | 0.32 |
| ≥ 6 | 3 | 15.6 | 89.4 | 63.3(50.4–78.4) | 19.2 (4.0–56.2) | 5.8 (0.6–55.9) | 0.13 | |
| ≥ 10 | 3 | 13.6 | 87.5 | 68.8(56.0–82.2) | 22.1 (4.5–64.5) | 7.0 (0.7–67.8) | 0.09 | |
| ≥350 | >0 | 2 | 113.7 | 74.5 | 30.3(24.1–36.2) | 1.8 (0.2–6.4) | 1.0 (0.1–10.6) | 0.97 |
| ≥ 6 | 2 | 81.1 | 73.8 | 51.0(44.0–58.2) | 2.5 (0.3–8.9) | 2.2 (0.2–23.8) | 0.53 | |
| ≥ 10 | 1 | 69.5 | 35.6 | 57.3(50.5–64.1) | 1.4 (0.04–8.0) | 0.7 (0.1–7.8) | 0.78 | |
| <350 | >0 | 4 | 45.5 | 80.8 | 45.9(36.8–55.8) | 8.8 (2.4–22.5) | 3.4 (0.4–30.5) | 0.27 |
| ≥ 6 | 4 | 33.5 | 78.9 | 62.3(54.0–71.7) | 11.9 (3.3–30.6) | 6.0 (0.7–54.1) | 0.11 | |
| ≥ 10 | 4 | 29.4 | 79.4 | 67.0(57.6–75.7) | 13.6 (3.7–34.8) | 7.4 (0.8–66.1) | 0.07 | |
Note: PYFU Person years of follow-up;
Confidence Intervals for sensitivity could not be calculated due to the very small number of events that the sensitivity estimate was based on.
Crude Hazard Ratio for active TB in women below CD4 cut-off value compared with women above CD4 cut-off value
Figure 3.
Receiver Operating Characteristic Curves for CD4 cell count as baseline marker for postpartum tuberculosis and the corresponding area under the ROC curve (AUC) value in HIV-1 infected women
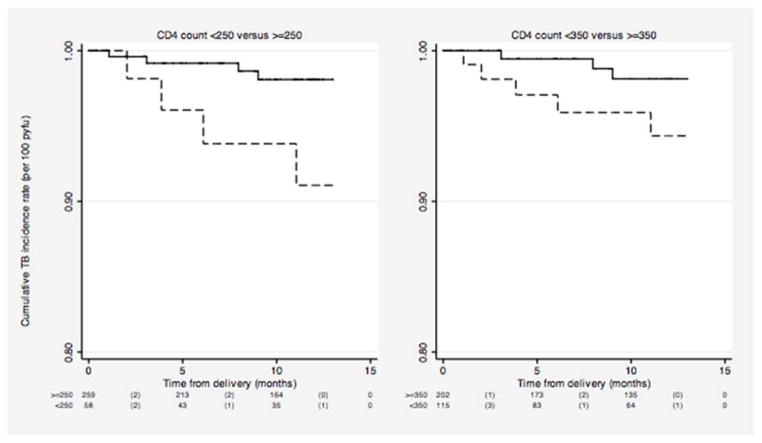
At CD4<250 and <350 cells/μL, 9.0% and 6% of women, respectively, would be expected to develop tuberculosis within 1 year postpartum (Table 3). The survival curves for incident TB associated with the two CD4 cut-offs are shown in Figure 4 and the unadjusted HR for future TB associated with CD4 cut-offs is shown in Table 3. CD4 cell count<250 cells/μL was associated with 4.7-fold increased risk of TB (HR: 4.7; 95% CI: 1.2–18.6; p=0.03).
Figure 4.
Kaplan Meier estimates of cumulative tuberculosis incidence rate in HIV-1 infected women between delivery and 1 year postpartum by various cut-points in CD4 cell count measured during pregnancy.
Note: In each plot, dashed lines represent the survival curve for women with CD4 <250 and <350 cells/μL and the bold lines represent survival curves for women with CD4>250 and >350 cells/μL. The risk table below each plot shows the number at risk (number of events) within each time interval represented on the x-axis.
p-values for the Log-rank test for equality of survival functions is 0.02 for CD4<250 compared to >=250 cells/μL and 0.08 for CD4<350 compared to >=350 cells/μL.
Sensitivity associated with each IFN-γ cut-off among women with CD4<250 (≥85%) and <350 cells/μL (>79%) was greater than sensitivity associated with each CD4 or IFN-γ cut-off alone. Specificity estimates increased with increasing IFN-γ cut-offs in women with CD4<250 and <350 cells/μL and were greater than the corresponding estimates for IFN-γ alone (Table 3). We did not estimate AUCs for this stratified analysis because of limited number of events per stratum.
PPV of the three IFN-γ cut-offs for postpartum TB was greatest in women with CD4<250 and <350 cells/μL. Moreover, PPVs increased with increasing IFN-γ cut-offs in women with CD4<250 and <350 cells/μL (Table 3).
Discussion
We estimated sensitivity, specificity and PPV of varied IFN-γ cut-offs associated with postpartum active TB among HIV-1 infected women with baseline IGRAs performed during pregnancy. We used a time-dependent ROC method to evaluate test characteristics of varied IFN-γ cut-offs for future active TB, which has not been previously reported. Manufacturer cut-offs for positive IGRAs are based on concurrent active TB as a gold standard rather than future TB. Previous studies suggest that binary positive/negative assays have some predictive ability.19–21 It is also possible that different cut-offs may be more sensitive, specific, or predictive of future TB. Despite the difference in purpose for the cut-off threshold, we found that sensitivity and specificity of IGRA cut-offs for future active TB was best at the manufacturer cut-off (≥6 SFCs/well).
The AUC values we obtained suggest that IFN-γ and CD4 cell count, measured at baseline, are weak in their ability to discriminate or accurately classify those who would and would not develop future postpartum TB.
In a scenario of IGRA-targeted IPT provision, PPVs for this analysis suggest that IGRA-targeted IPT would be delivered to 152 of 327 IGRA-positive (IFN-γ response≥6 SFCs/well) women to prevent 7 cases of TB but miss 2 TB cases in 175 IPT-untreated IGRA-negative women. In this population, CD4 cut-offs had higher sensitivity, specificity and PPV than IGRAs for the outcome of future TB. In addition, at all IGRA cut-offs the assay had higher sensitivity, specificity and PPV in immunosuppressed women (CD4 cell count<250 cells/μL) compared to women with higher CD4 counts. Together, our data suggest that IGRAs among immunosuppressed women during pregnancy would identify women most at-risk for TB.
Studies on sensitivity of IGRAs have typically used a cross-sectional study design in individuals with active TB where disease status and marker are measured concurrently and specificity is estimated in a separate healthy population at very low risk of tuberculosis exposure. A meta-analysis of IGRAs among HIV-1 infected individuals reported a pooled sensitivity of 72% for IGRAs for detection of active TB from studies in LMICs and 94% in high income countries.23 However, IGRAs are not recommended for active TB diagnosis but to detect LTBI to identify those at risk for future active TB. Hence, we evaluated test cut-offs for risk of future rather than concurrent TB and estimated sensitivity and specificity. We would expect lower sensitivity in our prognostic model than in a concurrent model because we used future active TB as the gold-standard.
Persistent TB exposure may decrease sensitivity of a single time-point IGRA. Because our cohort was based in a high TB prevalence setting, prognostic sensitivity of IGRA would be expected to be lower than in a setting with lower TB prevalence. Thus, in contrast to our prior analysis with 2-year follow-up, we restricted this analysis to one year because we anticipated that external TB exposures would accumulate during extended follow-up and attenuate sensitivity of the one-time diagnostic test to detect future active TB. IPT was not provided in the cohort; with IPT, associations with IGRA positivity or specific cut-offs could decrease.
This study has important strengths and limitation. We assessed usefulness of two clinically and programmatically relevant biomarkers for postpartum tuberculosis. In our previous study, we observed significant association between IGRA positivity and risk of postpartum tuberculosis.3 However, none of the IGRA cut-offs examined in the current study accurately discriminated individuals who would develop active TB, demonstrating that a marker with significant association is not sufficient to provide high prognostic utility. Our analysis takes into account the time lag between IGRA and TB detection. IGRAs are used to detect LTBI to reduce future TB, making this approach more relevant compared to the cross-sectional studies of IGRAs in individuals with active TB. We were limited by few cases of active TB, absence of TST for comparison and absence of TB diagnostic confirmation. Limitations in TB diagnosis could contribute to non-differential under-ascertainment of TB and may not influence sensitivity but could lead to underestimated positive predictive value.
In conclusion, our study estimates prognostic performance of IFN-γ and CD4 cell count, during pregnancy in identifying HIV-1 infected women at risk for postpartum tuberculosis. No IGRA cut-off provided a high predictive value or ability to discriminate those who would develop future TB. IFN-γ sensitivity and predictive value were highest in immunosuppressed women. Our study was limited in sample size and to increase precision of estimates the findings should be replicated in a cohort with more TB events. Cost-effectiveness or decision-tree analyses with these data or with larger cohort studies could inform alternative IGRA-targeted IPT strategies.
Supplementary Material
Acknowledgments
Sources of financial support: Supported by the US National Institutes Health (NIH) through grant #R21 HD058477-01 and Firland Foundation Grant # 200910. Barbara Lohman-Payne and Dalton Wamalwa were scholars in the International AIDS Training and Research Program, NIH Research Grant D43 TW000007, funded by the Fogarty International Center and the Office of Research on Women’s Health. Grace John-Stewart is supported by NIH Research Grant K24 HD054314-04. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank everyone at the Paediatrics Research Laboratory, Kenyatta National Hospital for laboratory support for this study and the study staff and participants of this perinatal cohort.
Footnotes
Conference presentation: 3rd Global Symposium on IGRAs, 2012 January 12–15, Hawaii, USA.
Author contributions
Study design – Sasi Jonnalagadda, Elizabeth Brown, Carey Farquhar, Grace John-Stewart
Data collection – Sasi Jonnalagadda, Barbara Lohman-Payne, Dalton Wamalwa
Analysis - Sasi Jonnalagadda, Elizabeth Brown, Grace John-Stewart
Preparation of the manuscript - Sasi Jonnalagadda, Grace John-Stewart, Barbara Lohman-Payne, Carey Farquhar, Dalton Wamalwa
Contributor Information
Sasi R. Jonnalagadda, Email: srj4@uw.edu.
Elizabeth Brown, Email: elizab@uw.edu.
Barbara Lohman-Payne, Email: bll@uw.edu.
Dalton Wamalwa, Email: dalton@africaonline.co.ke.
Carey Farquhar, Email: cfarq@uw.edu.
Grace C. John-Stewart, Email: gjohn@uw.edu.
References
- 1.Mofenson LM, Laughon BE. Human immunodeficiency virus, mycobacterium tuberculosis, and pregnancy: a deadly combination. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;45:250–3. doi: 10.1086/518975. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2007;45:241–9. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 3.Jonnalagadda S, Lohman Payne B, Brown E, et al. Latent tuberculosis detection by interferon gamma release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. The Journal of infectious diseases. 2010;202:1826–35. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. The Journal of infectious diseases. 2011;203:358–63. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. The global plan to stop TB 2011 – 2015: Transforming the fight towards elimination of tuberculosis. Geneva: World Health Organization; 2010. [Google Scholar]
- 6.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane database of systematic reviews. 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane database of systematic reviews. 2000:CD001363. doi: 10.1002/14651858.CD001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Annals of internal medicine. 2007;146:340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Annals of internal medicine. 2008;149:177–84. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2005;54:49–55. [PubMed] [Google Scholar]
- 11.Canadian Tuberculosis Committee. Updated recommendations on interferon gamma release assays for latent tuberculosis infection. An Advisory Committee Statement (ACS) Canada communicable disease report = Releve des maladies transmissibles au Canada. 2008;34:1–13. [PubMed] [Google Scholar]
- 12.National Collaborating Centre for Chronic Conditions (UK) Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for Its Prevention and Control. London: Royal College of Physicians (UK); 2006. [PubMed] [Google Scholar]
- 13.World Health Organization. Use of Interferon-γ Release Assays (IGRAs) in Tuberculosis Control in Low- and Middle-Income Settings. Expert Group Meeting Report; 20–21 July 2010; 2010. [Google Scholar]
- 14.Bakir M, Millington KA, Soysal A, et al. Prognostic value of a T-cell-based, interferon-gamma biomarker in children with tuberculosis contact. Annals of internal medicine. 2008;149:777–87. doi: 10.7326/0003-4819-149-11-200812020-00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Corral H, Paris SC, Marin ND, et al. IFNgamma response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PloS one. 2009;4:e8257. doi: 10.1371/journal.pone.0008257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi R, Narang U, Zwerling A, et al. Predictive value of latent tuberculosis tests in Indian healthcare workers: a cohort study. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2011;38:1475–7. doi: 10.1183/09031936.00014611. [DOI] [PubMed] [Google Scholar]
- 17.Lange B, Vavra M, Kern WV, Wagner D. Development of tuberculosis in immunocompromised patients with a positive tuberculosis-specific IGRA. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16:492–5. doi: 10.5588/ijtld.11.0416. [DOI] [PubMed] [Google Scholar]
- 18.Lienhardt C, Fielding K, Hane AA, et al. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PloS one. 2010;5:e10508. doi: 10.1371/journal.pone.0010508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kik SV, Franken WP, Mensen M, et al. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2010;35:1346–53. doi: 10.1183/09031936.00098509. [DOI] [PubMed] [Google Scholar]
- 20.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. Negative and Positive Predictive Value of a Whole-Blood Interferon-{gamma} Release Assay for Developing Active Tuberculosis: An Update. American journal of respiratory and critical care medicine. 2011;183:88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 21.Aichelburg MC, Rieger A, Breitenecker F, et al. Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-infected individuals. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:954–62. doi: 10.1086/597351. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Kim SI, Kim YR, Wie SH, Park YJ, Kang MW. Predictive value of interferon-gamma ELISPOT assay in HIV 1-infected patients in an intermediate tuberculosis-endemic area. AIDS research and human retroviruses. 2012;28:1038–43. doi: 10.1089/AID.2011.0360. [DOI] [PubMed] [Google Scholar]
- 23.Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. Journal of acquired immune deficiency syndromes. 2011;56:230–8. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangaka MX, Wilkinson KA, Glynn JR, et al. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. The Lancet infectious diseases. 2012;12:45–55. doi: 10.1016/S1473-3099(11)70210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otieno PA, Brown ER, Mbori-Ngacha DA, et al. HIV-1 disease progression in breast-feeding and formula-feeding mothers: a prospective 2-year comparison of T cell subsets, HIV-1 RNA levels, and mortality. The Journal of infectious diseases. 2007;195:220–9. doi: 10.1086/510245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walson JL, Brown ER, Otieno PA, et al. Morbidity among HIV-1-infected mothers in Kenya: prevalence and correlates of illness during 2-year postpartum follow-up. Journal of acquired immune deficiency syndromes. 2007;46:208–15. doi: 10.1097/QAI.0b013e318141fcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiarie JN, Richardson BA, Mbori-Ngacha D, Nduati RW, John-Stewart GC. Infant feeding practices of women in a perinatal HIV-1 prevention study in Nairobi, Kenya. Journal of acquired immune deficiency syndromes. 2004;35:75–81. doi: 10.1097/00126334-200401010-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenya National NLTP Guideline. [Accessed June 17, 2013];What the healthcare worker needs to know. 2005 at http://www.nltp.co.ke/docs/National_NLTP_Guideline.pdf.
- 29.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 30.Patrick J Heagerty and packaging by Paramita Saha. R package version 1.0.0. 2006. survivalROC: Time-dependent ROC curve estimation from censored survival data. [Google Scholar]
- 31.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. http://www.R-project.org. [Google Scholar]
- 32.StataCorp. Stata Statistical Software: Release 11. College Station: TSL; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.