Abstract
IMPORTANCE
Strabismus causes irreversible vision loss if not detected and treated early. It is unclear whether birth weight (BW) and gestational age (GA) are risk factors for strabismus.
OBJECTIVE
To estimate the impact of BW and GA on the likelihood of developing strabismus among premature infants.
DESIGN
In this longitudinal cohort analysis, we followed a group of premature children from birth to determine the proportion which developed strabismus and the timing of first strabismus diagnosis. Multivariable Cox regression analyses assessed the relationships of BW and GA and the development of strabismus. Regression models were adjusted for other known risk factors for strabismus, sociodemographic factors, and ocular comorbidities.
SETTING
Communities throughout the United States
PARTICIPANTS
38055 otherwise healthy children born prematurely who were enrolled for >6 months in a nationwide US managed care network between 2001–2011.
EXPOSURE
BW <2000g or GA <32 weeks
MAIN OUTCOME MEASURES
Hazard ratios (HRs) for strabismus with 95% confidence intervals (CIs)
RESULTS
Of 38055 otherwise healthy children who were born prematurely, 587 were diagnosed with strabismus later in life. Cumulative incidence of strabismus was 3.0% at 5 years. Controlling for GA and other factors, infants born with BW <2000g had a 61% increased hazard (HR=1.61; [CI 1.22–2.13]) of developing strabismus. Controlling for BW and other covariates, there was no significant association between strabismus and GA (HR=0.98, [CI, 0.69–1.38]). Among premature infants with BW <2000g, GA ≤32 weeks conveyed no additional increased risk for developing strabismus relative to those born after 32 weeks (HR=1.27, [CI, 0.86–1.88]). In contrast, among those with GA ≤32 weeks, BW <2000g conveyed a 14-fold increase in the risk of strabismus relative to BW >2000g (HR=14.4, [CI 1.99–104]).
CONCLUSIONS AND RELEVANCE
Independent of GA, very low BW conferred a large increase in strabismus risk among premature infants. In contrast, independent of BW, GA did not significantly impact the risk of strabismus. Updates to existing guidelines in the pediatric and ophthalmic literature should be considered, highlighting the importance of BW rather than GA and alerting clinicians about the need for careful monitoring of premature infants of low BW for strabismus.
Strabismus is a common childhood ocular condition estimated to affect 2–4% of children between the ages 6 months and 5 years.1–4 When left untreated, children with strabismus are at increased risk for amblyopia.5 Strabismus can have a dramatic effect on quality of well-being, affecting self-image and social interactions of preschool and early school age children.6–8 Studies have found that intervening early to correct strabismus results in improved best corrected visual acuity, a reduced need for later surgical interventions, and reduced societal costs.9,10
Well-recognized risk factors for strabismus include anisometropia and refractive error11,12, genetics13,14, older age of parents1,15, maternal cigarette smoking during pregnancy15,16, neurodevelopmental impairment17,18, low APGAR (Appearance, Pulse, Grimace, Activity, Respiration) scores19, craniofacial and chromosomal abnormalities20,21, in utero toxin exposure22, retinopathy of prematurity (ROP) 23,24, and caesarian delivery5,25. For children who are born premature, there is debate in the literature regarding two other potential risk factors, birth weight (BW) and gestational age (GA) 26,27,28,29 (eTable 1) 1,2,15,23–25,29–33. Six previous studies evaluated both full term and premature infants but came to disparate conclusions regarding the impact of BW and GA on strabismus risk. Of these, three found that both BW and GA were independent significant risk factors for strabismus, while one found that only BW was significant, one found only GA was significant, and one found that only infants with both low BW and GA were at increased risk. Two studies looked specifically at premature infants: Bremer and colleagues followed 2449 premature infants with BW <1251g enrolled in the Multicenter Trial of Cryotherapy for Retinopathy of Prematurity at 3 and 12 months of life, and VanderVeen and colleagues followed 702 infants with BW <1251g enrolled in the multicenter Early Treatment for Retinopathy of Prematurity Trial at 6 and 9 months of life.23,32 Both studies found that BW and GA were not significantly associated with the development of strabismus after multivariate analysis. These studies were limited because they only assessed severely premature infants who were at a substantially high risk for ROP, and did not also consider mildly premature infants.
To try to better understand the impact of BW and GA on risk of developing subsequent strabismus, we used health care claims data from a large, diverse sample of over 38000 premature but otherwise healthy children enrolled in a nationwide United States (US) managed care network.
Methods
Data Source
The Clinformatics DataMart database (OptumInsight, Eden Prairie, MN) contains detailed de-identified records of beneficiaries in a nationwide managed care network including health care claims from January 1, 2001 through December 31, 2011, for 18.5 million enrollees aged 0 to 21 years. For each enrollee, we had access to all medical claims and sociodemographic information including age, sex, race, and family household net worth. We have used a similar database for older individuals.34–36 The University of Michigan Institutional Review Board determined use of this data was exempt from requiring its approval.
Sample Selection
We identified 38055 children born prematurely but were otherwise healthy and were enrolled in the managed care plan continuously since birth for at least six months. Prematurity status was identified using the International Classification of Diseases, 9th Revision, Clinical Modifications (ICD-9-CM) billing codes for “prematurity” (765.1x) or “extreme immaturity” (765.0x) (eTable 2)37. The overall health of each child was quantified using Clinical Risk Group (CRG) software (3M Health Information Systems, Wallingford, CT).
The CRG classification system for risk adjustment assigns each individual to one of 1080 mutually exclusive risk groups based on his or her historical clinical and demographic characteristics available in claims data.38 Chronic conditions are defined as physical, mental, emotional, behavioral or developmental disorders, expected to last at least 12 months, or having sequelae that last at least 12 months, and that require ongoing treatment and/or monitoring. All CRGs can be folded into 9 CRG statuses. We classified infants based on the information from their first year of life and included those whose CRG status was characterized as “Healthy” (status 1) or “History of Significant Acute Disease” (e.g, upper respiratory tract infection; status 2). Children who were chronically ill (CRG status 3 through 9) were excluded because some of these children may have been too sick for ophthalmologic evaluations or their medical comorbidities may have limited the ability for an eye provider to adequately assess the child for strabismus. Time in plan was measured starting at the date of the birth identified by Current Procedural Terminology (CPT-4) billing codes V30.xx-V39.xx.39
Key Predictors
The two key predictors of interest were GA and BW. ICD-9-CM code 765.2x identified the GA of children born prematurely and ranged from<24 weeks to 37 weeks. For selected analyses, GA was treated as a continuous variable. For others, it was treated as a binary variable: infants whose GA was ≤32 weeks were defined as “very premature” and others with GA >32 weeks (but less than 37 weeks) were defined as “mildly premature”. ICD-9-CM 765.0x or 765.1x identified BW of children who were born prematurely and ranged from <500 grams (g) to ≥2500g. For selected analyses, BW was treated as a continuous variable. For others, it was treated as a binary variable: infants whose BW was <2000g were defined as “very low BW” and others with BW ≥2000g at birth were considered “mildly low BW”. Those with missing or “unspecified” values were excluded from analyses involving either of these predictor variables.
Primary Outcome
The outcome of interest, diagnosis of strabismus, was captured using ICD-9-CM code 378.xx. To reduce errors in characterizing children with strabismus as a result of miscoding, we required a confirmatory strabismus diagnosis submitted on a separate date. The distribution of the time to initial strabismus diagnosis was estimated by the method of Kaplan and Meier.40 Enrollees were censored at the end of plan coverage or end of study. Cumulative incidence is one minus the survival probability. To determine whether our conclusions were sensitive to inclusion of heterophoria in the definition of strabismus, the analysis was rerun excluding 378.4x. No substantive differences were noted.
Analyses
Statistical analyses were conducted using SAS software, version 9.3 (SAS Institute, Cary, NC). Enrollee characteristics were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. For all analyses, p<0.05 were considered statistically significant.
Hazard ratios were estimated by the Cox proportional hazards model.41 Time-to-event regression models were fitted for BW alone, GA alone, an additive (or no-interaction) model of BW and GA, and a joint exposure model. We chose to use both continuous and discrete models since past studies have used both as well. A continuous model makes the most sense intuitively given the continuous nature of BW and GA, but a discrete model allows for more actionable recommendations and valuable comparison to past studies. Additional models were fitted adjusting for ROP, other ocular comorbidities (eTable 2), delivery method, sex, race, urban/rural residence, and household net worth. Children with missing covariates were excluded from multivariable analyses. The proportional hazards assumption was not violated for key predictors (checked graphically and by Kolmogorov-Smirnov Test).42
Results
38055 premature infants met the inclusion criteria (Figure 1). The mean time in the plan for eligible children was 2.5 years, and ranged from 6 months (inclusion criterion) to 11 years (maximum available data 2001–2011). The sample included 18234 infant girls (47.9%); among those with known race, there were 25022 (74.3%) whites, 2831 (8.4%) blacks, 3377 (10.0%) Latinos, 1900 (5.6%) Asians, and 567 (1.7%) individuals of other races. The majority of children came from urban households (34651, or 91.9%), and 13996 (45.5%) had household net worth of over $150000 (Table 1). The cumulative incidence of diagnosis of amblyopia (ICD-9-CM 368.00–368.03) in the infants in our study was 4.2% at 10 years.
Figure 1. Selection of premature infants.
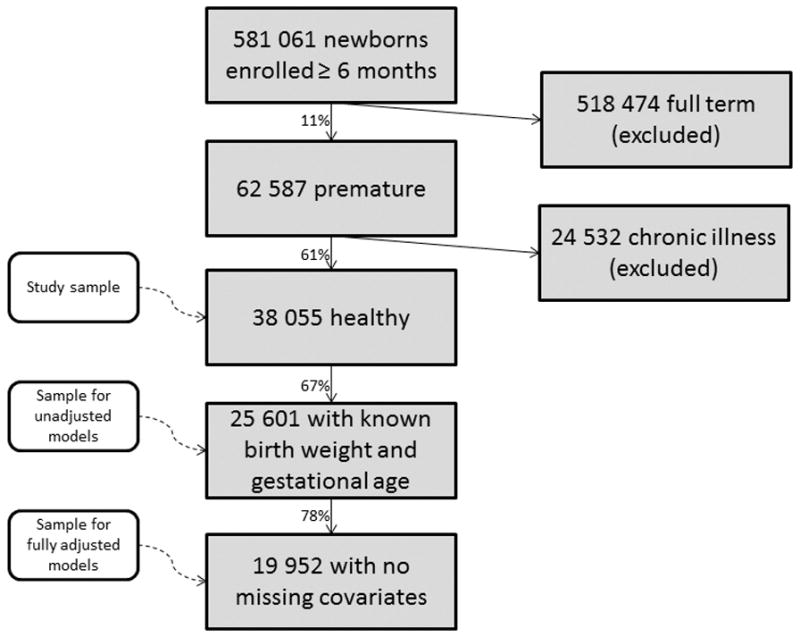
Plan participants who were enrolled for at least six-months, born prematurely, and were not chronically ill were eligible for this study. Those with missing covariates (birth weight, gestational age, race, sex, residency, household net worth, delivery method, retinopathy of prematurity, other ocular conditions) were excluded from some analyses.
Table 1.
Descriptive statistics of healthy, premature children included in study
| Total | Strabismus | No Strabismus | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| All Eligible | 38055 | 587 | 37468 | |||
|
| ||||||
| Race | ||||||
| White | 25022 | 74.3% | 443 | 82.6% | 24579 | 74.1% |
| Black | 2831 | 8.4% | 12 | 2.2% | 2819 | 8.5% |
| Latino | 3377 | 10.0% | 43 | 8.0% | 3334 | 10.1% |
| Asian | 1900 | 5.6% | 31 | 5.8% | 1869 | 5.6% |
| Other | 567 | 1.7% | 7 | 1.3% | 560 | 1.7% |
| Missing | 4358 | 47 | 4311 | |||
|
| ||||||
| Sex | ||||||
| Male | 19811 | 52.1% | 289 | 49.3% | 19522 | 52.1% |
| Female | 18234 | 47.9% | 297 | 50.7% | 17937 | 47.9% |
| Missing | 10 | 0 | 10 | |||
|
| ||||||
| Residency | ||||||
| Urban | 34651 | 91.9% | 539 | 93.6% | 34112 | 91.9% |
| Large Rural | 1597 | 4.2% | 22 | 3.8% | 1575 | 4.2% |
| Small Rural | 1441 | 3.8% | 15 | 2.6% | 1426 | 3.8% |
| Missing | 366 | 366 | ||||
|
| ||||||
| Household Net Worth | ||||||
| <25K | 6606 | 21.5% | 72 | 13.7% | 6534 | 21.6% |
| 25–75K | 4261 | 13.9% | 55 | 10.5% | 4206 | 13.9% |
| 75–150K | 5896 | 19.2% | 103 | 19.6% | 5793 | 19.2% |
| 150–500K | 10813 | 35.2% | 219 | 41.7% | 10594 | 35.0% |
| 500K+ | 3183 | 10.3% | 76 | 14.5% | 3107 | 10.3% |
| Missing | 7292 | 58 | 7234 | |||
|
| ||||||
| Delivery Method | ||||||
| Cesarean | 20723 | 54.7% | 325 | 56.3% | 20398 | 54.7% |
| Vaginal | 17151 | 45.3% | 252 | 43.7% | 16899 | 45.3% |
| Missing | 181 | 6 | 175 | |||
|
| ||||||
| Retinopathy of Prematurity | 860 | 2.3% | 31 | 5.3% | 829 | 2.2% |
|
| ||||||
| Other Ocular Conditions | 68 | 0.2% | 6 | 1.0% | 62 | 0.2% |
Birth weight and gestational age at birth
A total of 8549 premature infants (22.5%) had very low BW, 25265 (66.4%) had a mildly low BW, and 4241 (11.1%) had either an unspecified or missing value for BW. There were 3747 (9.8%) infants who were born very prematurely, 22763 (60.3%) were mildly premature, and 11545 (30.3%) had an unspecified or missing value for GA. (Table 2) As expected, among those who were born very prematurely, 82% also had very low BW; among infants with mildly low BW, 96% were mildly premature.
Table 2.
Descriptive statistics of birth covariates
| Total | Strabismus | No Strabismus | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| All Enrollees | 38055 | 587 | 37468 | |||
|
| ||||||
| Birth Weight (grams) | ||||||
| <500 | 206 | 0.6% | 7 | 1.4% | 199 | 0.6% |
| 500–<750 | 82 | 0.2% | 0 | 0.0% | 82 | 0.2% |
| 750–<1000 | 104 | 0.3% | 1 | 0.2% | 103 | 0.3% |
| 1000–<1250 | 647 | 1.9% | 18 | 3.5% | 629 | 1.9% |
| 1250–<1500 | 1368 | 4.0% | 22 | 4.3% | 1346 | 4.0% |
| 1500–<1750 | 2336 | 6.9% | 51 | 9.9% | 2285 | 6.9% |
| 1750–<2000 | 3806 | 11.3% | 72 | 14.0% | 3734 | 11.2% |
| 2000–<2500 | 11274 | 33.3% | 168 | 32.6% | 11106 | 33.4% |
| 2500+ | 13991 | 41.4% | 177 | 34.3% | 13814 | 41.5% |
|
| ||||||
| Unspecified | 3765 | 9 | 3756 | |||
| Missing | 476 | 58 | 418 | |||
|
| ||||||
| Gestation Age (weeks) | ||||||
| <24 | 39 | 0.1% | 0 | 0.0% | 39 | 0.1% |
| 24 | 14 | 0.1% | 0 | 0.0% | 14 | 0.1% |
| 25–26 | 42 | 0.2% | 0 | 0.0% | 42 | 0.2% |
| 27–28 | 96 | 0.4% | 2 | 0.5% | 94 | 0.4% |
| 29–30 | 839 | 3.2% | 16 | 4.2% | 823 | 3.1% |
| 31–32 | 2717 | 10.2% | 56 | 14.9% | 2661 | 10.2% |
| 33–34 | 7138 | 26.9% | 100 | 26.5% | 7038 | 26.9% |
| 35–36 | 13314 | 50.2% | 172 | 45.6% | 13142 | 50.3% |
| 37+ | 2311 | 8.7% | 31 | 8.2% | 2280 | 8.7% |
|
| ||||||
| Unspecified | 187 | 4 | 183 | |||
| Missing | 11358 | 202 | 11156 | |||
|
| ||||||
| Birth Weight and Gestational Age | ||||||
| GA≤32 BW<2000 |
2980 | 11.6% | 68 | 18.7% | 2912 | 11.5% |
| GA>32 BW<2000 |
3591 | 14.0% | 60 | 16.5% | 3531 | 14.0% |
| GA>32 BW≥2000 |
18365 | 71.7% | 233 | 64.0% | 18132 | 71.8% |
| GA≤32 BW≥2000 |
665 | 2.6% | 3 | 0.8% | 662 | 2.6% |
|
| ||||||
| Missing at least one | 12454 | 219 | 60.2% | 12235 | ||
BW = Birth Weight (grams); GA = Gestational Age (weeks)
Development of strabismus
A total of 587 premature infants were diagnosed with strabismus. The cumulative incidence of strabismus estimated by Kaplan-Meier was 1.4% by age 2 and 3.0% by age 5. Cumulative incidence curves for the entire sample and for each of the four primary exposure groups display the age of onset of strabismus from 0 to 5 years (Figure 2).
Figure 2. Cumulative incidence of strabismus among premature infants.
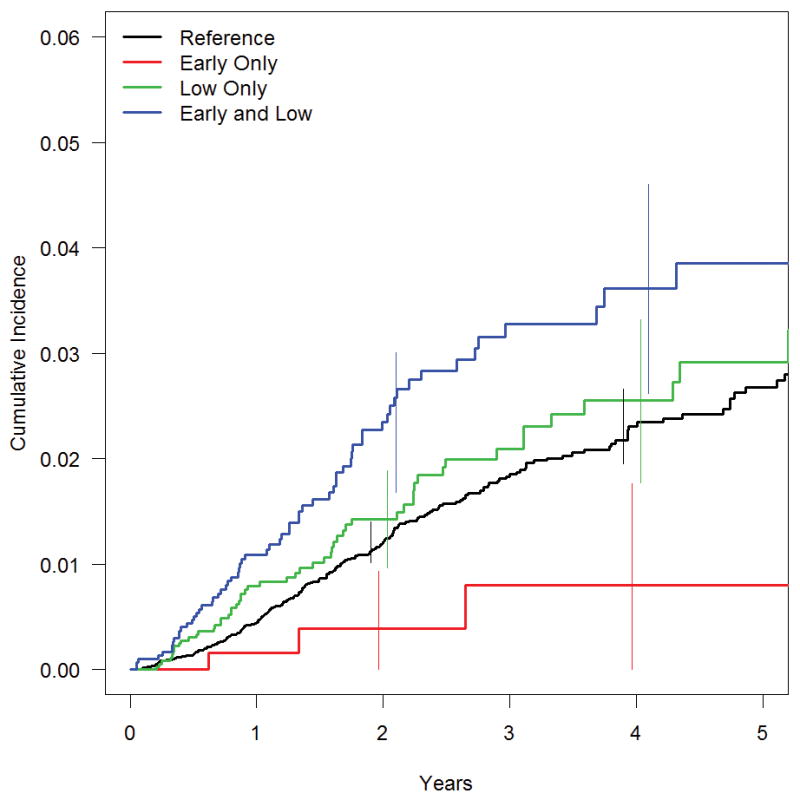
The cumulative incidence of strabismus estimated by Kaplan-Meier for all study participants and for infants in four primary exposure groups: Very low birth weight, very premature (LBW, VP; n=2980), Very low birth weight, mildly premature (LBW, MP; n=3591), Mildly low birth weight, mildly premature (MLBW, MP; n=18365), and Mildly low birth weight, very premature (MLBW, VP; n=665). 95% confidence intervals for each exposure group are shown at two and at four years. The effect of birth weight (low birth weight increases incidence of strabismus) is consistent across different levels of gestational age; the effect of gestational age is not.
Association between strabismus and birth weight
Premature infants born with very low BW had 47% increased hazard of strabismus compared to those with mildly low BW (unadjusted HR=1.47, [CI, 1.22–1.76]) (Table 3, Figure 3). When we considered both BW and GA together in an additive model to estimate their relative impacts, very low BW increased the risk of strabismus by 49% (HR=1.49, [CI, 1.16–1.92]). After adjusting for sex, race, urban/rural residence, household net worth, delivery method, ROP, and other ocular conditions, premature infants with very low BW had 61% increased hazard of developing strabismus (adjusted HR=1.61, [CI, 1.22–2.13]). Adjusting for the same covariates in a separate model, every 250g below 2500g at birth was associated with a 13% increased hazard of developing strabismus (adjusted HR=1.13, [CI, 1.04–1.23]). For example, an infant born at 1500g had 63% (1.134=1.63) increased risk relative to a 2500g, premature infant. Thus the models using continuous and binary forms of the primary predictor exhibit substantively equal conclusions.
Table 3.
Univariable and multivariable Cox regression analyses of birth weight and gestational age on risk of strabismus
| Birth Weight | Binary | Continuous | |||||
|---|---|---|---|---|---|---|---|
| Very Low (<2000g) vs. Mildly Low (>=2000g) | per 250g | ||||||
| Model | N | Hazard Ratio(a) | 95% Confidence Interval | p-value | Hazard Ratio(b) | 95% Confidence Interval | p-value |
| BW only | 33814 | 1.47 | (1.22,1.76) | <.0001 | 1.11 | (1.06, 1.16) | <.0001 |
| BW and GA | 25601 | 1.49 | (1.16,1.92) | 0.002 | 1.12 | (1.04, 1.21) | 0.002 |
| BW and other covariates | 25386 | 1.44 | (1.16,1.78) | 0.0008 | 1.09 | (1.03, 1.16) | 0.003 |
| BW, GA, and other covariates | 19952 | 1.61 | (1.22,2.13) | 0.0008 | 1.13 | (1.04, 1.23) | 0.006 |
| Gestational Age | Binary | Continuous | |||||
|---|---|---|---|---|---|---|---|
| Very premature (≤32 weeks) vs Mildly premature (>32weeks) | per week | ||||||
| Model | N | Hazard Ratio(c) | 95% Confidence Interval | p-value | Hazard Ratio(d) | 95% Confidence Interval | p-value |
| GA only | 26510 | 1.48 | (1.15,1.90) | 0.003 | 1.06 | (1.01, 1.11) | 0.02 |
| BW and GA | 25601 | 1.12 | (0.83,1.52) | 0.5 | 0.99 | (0.93, 1.06) | 0.9 |
| GA and other covariates | 20663 | 1.31 | (0.96,1.78) | 0.09 | 1.04 | (0.98, 1.10) | 0.2 |
| BW, GA, and other covariates | 19952 | 0.98 | (0.69, 1.38) | 0.9 | 0.98 | (0.91, 1.05) | 0.6 |
BW = Birth Weight (grams); GA = Gestational Age (weeks); p-values less than 0.05 are bold
Hazard ratios are of very low birth weight (<2000g) to mildly low birth weight infants (≥2000g)
Hazard ratios are for each 250g difference in birth weight
Hazard ratios are of very premature (≤32 weeks) to mildly premature infants (>32 weeks)
Hazard ratios are for each one week difference in gestational age
Figure 3. The hazard of developing strabismus according to two key predictors: birth weight and gestational age.
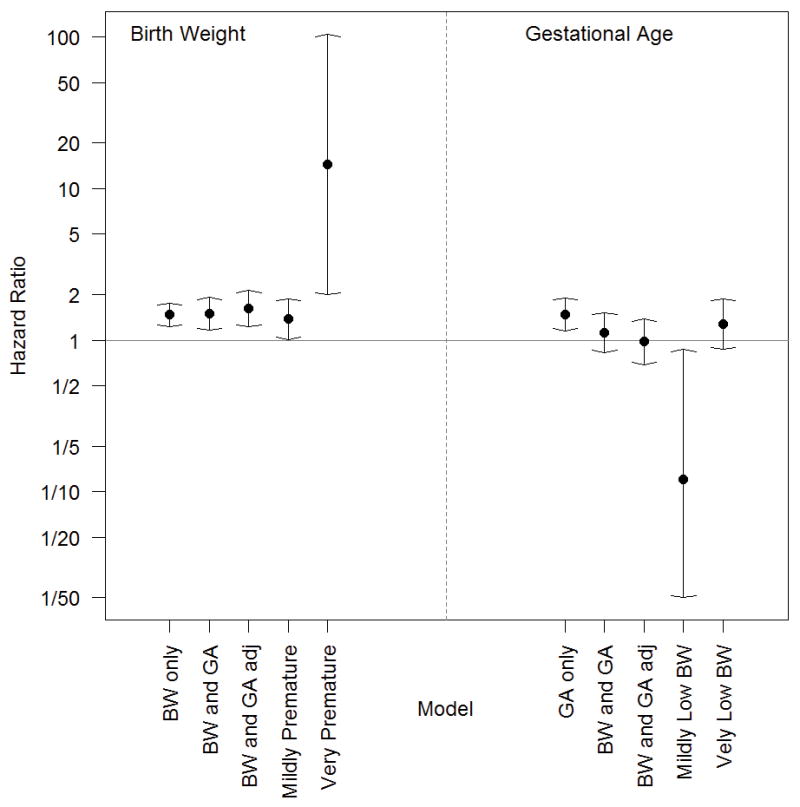
The effect of each primary factor on strabismus risk was estimated in a Cox proportional hazard model and is displayed in five different scenarios. Within each panel (birth weight (BW) on left, gestational age (GA) on right), from left to right the models are for (1) the crude effect of the primary factor, (2) the effect adjusting for just the other main factor in an additive model, (3) the effect in a fully adjusted model, (4–5) the effects determined by the adjusted joint exposure model in subgroups defined by the other main factor. The risk of strabismus is higher for very low birth weight infants vs. mildly low birth weight infants in all cases. The effect of gestational age is negligible after controlling for birth weight. Two intervals are very wide for the rare combination of Mildly Low BW and Very Premature.
Association between strabismus and gestational age
When GA was considered alone, very premature infants were found to have a 48% increased hazard of strabismus relative to mildly premature infants (unadjusted HR=1.48, [CI, 1.15–1.90]) (Table 3, Figure 3). When we considered both GA and BW together in an additive model to determine their relative impacts, very premature infants did not have a significantly different strabismus risk relative to mildly premature infants (adjusted HR=1.12, [CI, 0.83–1.52]). After adjustment for BW and other potential confounders, there was no significant association between strabismus and GA (adjusted HR=0.98, [CI, 0.69–1.38]). Adjusting for the same covariates in a separate model, each additional week of prematurity was associated with a (statistically insignificant) 2% decreased hazard of developing strabismus (adjusted HR=0.98, [CI, 0.91–1.05]).
Interactions between birth weight and gestational age
The joint exposure model allowed more detailed comparisons of the four primary exposure groups. (eTable 3) Compared to the reference group of infants with mildly low BW and mild prematurity, (1) infants born very prematurely but still with only mildly low BW had 88% reduced risk of strabismus (adjusted HR=0.12 [CI, 0.02–0.87]); (2) infants with very low BW but still only mildly premature had 38% increased risk of strabismus (adjusted HR=1.38, [CI, 1.01–1.88]); and (3) infants with very low BW and born very premature had 75% increased risk of strabismus (adjusted HR=1.75 [CI, 1.27–2.42]). Two final comparisons of note: (a) among those with very low BW, very premature birth did not confer a statistically significant additional hazard for strabismus (adjusted HR=1.27, [CI, 0.86–1.88]), but (b) among those born very prematurely, very low BW was associated with a 14-fold increase in risk of strabismus (adjusted HR=14.4, [CI 1.99–104]) relative to those of mildly low BW (Figure 3).
Discussion
In this analysis of 38055 premature children who were followed longitudinally over time for at least 6 months, we find, after accounting for potential confounding factors, that BW appears to impact the risk of strabismus much more than GA. Premature infants who weighed <2000g at birth were found to have a 61% increased hazard of strabismus, independent of GA and other factors. By comparison, after accounting for BW and other variables, premature infants born earlier than 32 weeks had no significantly different risk of strabismus relative to others born after 32 weeks. In the joint exposure model, infants who were born with both putative risk factors, very premature with very low BW, had over a 14-fold increased risk of strabismus relative to very premature infants of mildly low BW. Of the four groups, infants with the rare (665 of 25,601, 2.5%) covariate combination of very premature but only mildly low birth weight had the lowest risk of strabismus, underscoring the relative importance of BW to that of GA.
Several studies of premature children have looked at the relationship of GA with the risk of developing strabismus without considering BW. One small longitudinal study by Schalij-Delfos that followed 99 premature children for 5 years found that those born at >32 weeks GA had a significantly lower risk of strabismus (p=0.005) relative to those born at <28 weeks and 28–32 weeks.30 Data from the Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) and the Baltimore Pediatric Eye Disease Study (BPEDS) also demonstrated that after multivariate analysis, infants with a GA <33 weeks had a considerably higher odds (OR=2.48 ([CI, 1.17–5.25]) for development of strabismus relative to those with GA of ≥33 weeks.43 Unfortunately, neither study considered BW. Since it is known that BW is correlated with GA, it is possible that the findings observed in these analyses may actually be more attributable to BW than GA.
There are several prior studies which have considered the impact of both GA and BW on strabismus risk. The largest and most recent of these is a retrospective population-based cohort study of 96842 full-term and premature Danish children born between 1996 and 2003.34 Analysis of the subset of the 1320 infants with strabismus within this cohort found an increased risk of esotropia with BW <2000g (RR=2.20, [CI 1.60–3.05]), 2000–2499g (RR=2.35, [CI 1.80–3.07]), and 2500–2999g (RR=1.29, [CI 1.04–1.58]) relative to 3000–3499g (reference group); this relationship was impacted very little after adjusting for GA. In their study, the increased risk of esotropia found with GA of 33–36 weeks compared to 37–41 weeks (reference group) was significantly diminished with adjustment for BW, although it remained statistically significant (RR=1.39, [95% CI 1.07–1.81]). While this study did not adjust for possible confounding risk factors as we did, it is notable that in both their study and ours, BW and not GA seemed to be the biggest driver of strabismus risk. Direct comparisons of these prior studies with one another and our study is challenging because of differences in how BW and GA are characterized, variation in the potential confounding risk factors adjusted for in the analyses, the types of providers evaluating the children, and the providers’ experience in diagnosing strabismus. In addition, our study included infants covered by a commercial insurance carrier; infants of lower socioeconomic status were likely underrepresented in our study population.
Nevertheless, past studies have associated low and very low BW with a negative impact on physical growth, mental development, motor performance, and balance.44,45 Therefore, it is unsurprising that we found BW to be associated with the development of strabismus, which signals impaired physical or motor development.
Aside from BW and GA, others have noted an association between method of delivery and risk of strabismus.15,25,30 In the present analysis, when we adjusted for BW and GA, we found no statistically significant difference in strabismus risk among children born by vaginal delivery versus cesarean section.
The 2012 American Academy of Ophthalmology (AAO) Preferred Practice Pattern for Pediatric Eye Evaluations offers guidelines on how frequently children should undergo ocular examinations to check for strabismus and other ocular diseases.5 Neither these guidelines, nor guidelines put forth by the American Academy of Pediatrics, recommend that clinicians carefully monitor premature infants of low BW for strabismus and amblyopia beyond the standard monitoring in the first 10 weeks of life to check for ROP.46 Based on the findings of our analysis and others, future guidelines may consider recommending that premature infants with low BW undergo periodic assessment in the first few years of life to check for strabismus.
Strengths and Limitations
Strengths of this study include the large number of premature children in the sample and the ability to follow these infants longitudinally over time, even if they changed pediatricians or eye care providers, providing they maintained the same insurance coverage. We captured care provided to prematurely born infants in communities throughout the US, not simply those receiving care at one particular academic medical center or residing in one community setting. Thus, our findings may be more generalizable than findings of some prior analyses. The data regarding BW, GA, and other parameters were obtained directly from health care providers, which may be more accurate than parental report.47 Finally, we were able to adjust for a variety of potential confounding factors.
Our study has several limitations. As with all analyses that rely on claims data, this data source lacks information on several important clinical parameters which are known to impact risk of strabismus. For example, past studies have demonstrated an association between prematurity and myopia, even outside the context of ROP.48–50 Refractive error (myopia, hyperopia, and astigmatism) and family history of strabismus can predispose patients to different forms of strabismus. With claims data, however, we are unable to capture the type or degree of refractive error of each child, or determine whether there is a known family history of strabismus. We were therefore unable to account for these factors in our models. As claims data are used primarily for billing and not research purposes, coding errors may exist and we were unable to confirm the accuracy of the strabismus diagnosis, ascertain the severity of disease, or assess the impact of BW and GA on the specific type of strabismus observed. We tried to reduce mischaracterization of children with strabismus due to miscoding by requiring ≥2 diagnostic codes for this condition on two distinct dates. The providers who were diagnosing strabismus in this analysis varied in their level of experience and training and this may have impacted our results, though unless there was differential misdiagnosis of strabismus based on BW or GA, the bias associated with the misclassification would have tended towards reducing significance and supporting the null hypothesis. Finally, it is important to point out the families of all these children had health insurance so our findings may not be generalizable to uninsured or underinsured groups, who may be at greater risk for delivering infants prematurely.51,52 Our findings may also not be generalizable to premature infants with chronic health problems, who we excluded from our study.
Conclusion
Based on the findings of this analysis and others, existing guidelines set forth by the AAP, AAO, and other organizations should be reassessed for the inclusion of low BW as a key risk factor for developing strabismus. These premature children are at increased risk for irreversible vision loss if their strabismus is not detected and treated early, and therefore need to undergo periodic evaluation.
Supplementary Material
Acknowledgments
Grant support: W.K. Kellogg Foundation; National Eye Institute K23 Mentored Clinician Scientist Award (1K23EY019511) (JDS); Research to Prevent Blindness Physician Scientist Award (JDS) and Lew R. Wasserman Merit Award (DCM).
Footnotes
Joshua D. Stein, MD, MS, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors have no proprietary or commercial interest in any material discussed in this manuscript
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Robaei D, Rose KA, Kifley A, Cosstick M, Ip JM, Mitchell P. Factors Associated with Childhood Strabismus: Findings from a Population-Based Study. Ophthalmology. 2006;113:1146–53. doi: 10.1016/j.ophtha.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Williams C, Northstone K, Howard M, et al. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008;92:959–64. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, Tielsch JM. Prevalence of Amblyopia and Strabismus in White and African American Children Aged 6 through 71 Months: The Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116:2128–34. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months: the multi-ethnic pediatric eye disease study. Ophthalmology. 2008;115:1229–36. doi: 10.1016/j.ophtha.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Ophthalmology. Preferred Practice Patterns: Esotropia and Exotropia. San Francisco, CA: American Academy of Ophthalmology; 2012. Preferred Practice Patterns Committee. Pediatric Ophthalmology/Strabismus Panel. [Google Scholar]
- 6.Wen G, McKean-Cowdin R, Varma R, et al. Multi-ethnic Pediatric Eye Disease Study Group. General health-related quality of life in preschool children with strabismus or amblyopia. Ophthalmology. 2011;118:574–80. doi: 10.1016/j.ophtha.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolchin JG, Lederman ME. Congenital (infantile) esotropia: psychiatric aspects. J Pediatr Ophthalmol Strabismus. 1978;15:160–3. doi: 10.3928/0191-3913-19780501-10. [DOI] [PubMed] [Google Scholar]
- 8.Satterfield D, Keltner JL, Morrison TL. Psychosocial aspects of strabismus study. Arch Ophthalmol. 1993;111:1100–5. doi: 10.1001/archopht.1993.01090080096024. [DOI] [PubMed] [Google Scholar]
- 9.Arthur BW, Smith JT, Scott WE. Long-term stability of alignment in the monofixation syndrome. J Pediatr Ophthalmol Strabismus. 1989;26:224–31. doi: 10.3928/0191-3913-19890901-05. [DOI] [PubMed] [Google Scholar]
- 10.Birch EE, Fawcett S, Stager DR. Why does early surgical alignment improve stereoacuity outcomes in infantile esotropia? J AAPOS. 2000;4:10–4. doi: 10.1016/s1091-8531(00)90005-3. [DOI] [PubMed] [Google Scholar]
- 11.Joint Writing Committee for the Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study Groups. Risk factors associated with childhood strabismus: the Multi-Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology. 2011;118:2251–61. doi: 10.1016/j.ophtha.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobson V, Sebris SL. Longitudinal study of acuity and stereopsis in infants with or at-risk for esotropia. Invest Ophthalmol Vis Sci. 1989;30:1146–58. [PubMed] [Google Scholar]
- 13.Parikh V, Shugart YY, Doheny KF, et al. A strabismus susceptibility locus on chromosome 7p. Proc Natl Acad Sci U S A. 2003;100(21):12283–12288. doi: 10.1073/pnas.2035118100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice A, Nsengimana J, Simmons IG, et al. Replication of the recessive STBMS1 locus but with dominant inheritance. Invest Ophthalmol Vis Sci. 2009;50(7):3210–3217. doi: 10.1167/iovs.07-1631. [DOI] [PubMed] [Google Scholar]
- 15.Chew E, Remaley NA, Tamboli A, Zhao J, Podgor MJ, Klebanoff M. Risk factors for esotropia and exotropia. Arch Ophthalmol. 1994;112(10):1349–1355. doi: 10.1001/archopht.1994.01090220099030. [DOI] [PubMed] [Google Scholar]
- 16.Hakim RB, Tielsch JM. Maternal cigarette smoking during pregnancy: a risk factor for childhood strabismus. Arch Ophthalmol. 1992;110(10):1459–1462. doi: 10.1001/archopht.1992.01080220121033. [DOI] [PubMed] [Google Scholar]
- 17.Cregg M, Woodhouse JM, Stewart RE, et al. Development of refractive error and strabismus in children with Down syndrome. Invest Ophthalmol Vis Sci. 2003;44:1023–30. doi: 10.1167/iovs.01-0131. [DOI] [PubMed] [Google Scholar]
- 18.Haugen OH, Hovding G. Strabismus and binocular function in children with Down syndrome. A population-based, longitudinal study. Acta Ophthalmol Scand. 2001;79:133–9. doi: 10.1034/j.1600-0420.2001.079002133.x. [DOI] [PubMed] [Google Scholar]
- 19.Mohney BG, Erie JC, Hodge DO, Jacobsen SJ. Congenital esotropia in Olmsted County, Minnesota. Ophthalmology. 1998;105:846–50. doi: 10.1016/S0161-6420(98)95024-2. [DOI] [PubMed] [Google Scholar]
- 20.Khan SH, Nischal KK, Dean F, et al. Visual outcomes and amblyogenic risk factors in craniosynostotic syndromes: a review of 141 cases. Br J Ophthalmol. 2003;87:999–1003. doi: 10.1136/bjo.87.8.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khong JJ, Anderson P, Gray TL, et al. Ophthalmic findings in apert syndrome prior to craniofacial surgery. Am J Ophthalmol. 2006;142:328–30. doi: 10.1016/j.ajo.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Bruce BB, Biousse V, Dean AL, Newman NJ. Neurologic and ophthalmic manifestations of fetal alcohol syndrome. Rev Neurol Dis. 2009;6:13–20. [PubMed] [Google Scholar]
- 23.Bremer DL, Palmer EA, Fellows RR, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Strabismus in premature infants in the first year of life. Arch Ophthalmol. 1998;116(3):329–333. doi: 10.1001/archopht.116.3.329. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor AR, Stephenson TJ, Johnson A, Tobin MJ, Ratib S, Fielder AR. Strabismus in children of birth weight less than 1701 g. Arch Ophthalmol. 2002;120(6):767–773. doi: 10.1001/archopht.120.6.767. [DOI] [PubMed] [Google Scholar]
- 25.Pathai S, Cumberland PM, Rahi JS. Prevalence of and early-life influences on childhood strabismus: findings from the Millennium Cohort Study. Arch Pediatr Adolesc Med. 2010;164(3):250–257. doi: 10.1001/archpediatrics.2009.297. [DOI] [PubMed] [Google Scholar]
- 26.Coats DK, Avilla CW, Paysse EA, Sprunger DT, Steinkuller PG, Somaiya M. Early-onset refractive accommodative estropia. J AAPOS. 1998;2:275–8. doi: 10.1016/s1091-8531(98)90083-0. [DOI] [PubMed] [Google Scholar]
- 27.Repka MX, Summers CG, Palmer EA, Dobson V, Tung B, Davis B Cryotherapy for Retinopathy of Prematurity Cooperative Group. The incidence of ophthalmologic interventions in children with birth weights less than 1251 grams: results through 5 1/2 years. Ophthalmology. 1998;105:1621–7. doi: 10.1016/s0161-6420(98)99028-5. [DOI] [PubMed] [Google Scholar]
- 28.Holmstrom G, el Azazi M, Kugelberg U. Ophthalmological follow up of preterm infants: a population based prospective study of visual acuity and strabismus. Br J Ophthalmol. 1999;83:143–50. doi: 10.1136/bjo.83.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennefather PM, Clarke MP, Strong NP, Cottrell DG, Dutton J, Tin W. Risk factors for strabismus in children born before 32 weeks’ gestation. Br J Ophthalmol. 1999;83:514–8. doi: 10.1136/bjo.83.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maconachie GD, Gottlob I, McLean RJ. Risk Factors and Genetics in Common Comitant Strabismus: A Systematic Review of the Literature. JAMA Ophthalmol. 2013;131(9):1179–86. doi: 10.1001/jamaophthalmol.2013.4001. [DOI] [PubMed] [Google Scholar]
- 31.Schalij-Delfos NE, de Graaf ME, Treffers WF, Engel J, Cats BP. Long term follow up of premature infants: detection of strabismus, amblyopia, and refractive errors. Br J Ophthalmol. 2000;84(9):963–7. doi: 10.1136/bjo.84.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vander Veen DK, Coats DK, Dobson V, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Prevalence and course of strabismus in the first year of life for infants with prethreshold retinopathy of prematurity: findings from the Early Treatment for Retinopathy of Prematurity study. Arch Ophthalmol. 2006;124(6):766–73. doi: 10.1001/archopht.124.6.766. [DOI] [PubMed] [Google Scholar]
- 33.Torp-Pedersen T, Boyd HA, Poulsen G, Haargaard B, Wohlfahrt J, Holmes JM, Melbye M. Perinatal risk factors for strabismus. Int J Epidemiol. 2010;39(5):1229–39. doi: 10.1093/ije/dyq092. [DOI] [PubMed] [Google Scholar]
- 34.Stein JD, Talwar N, Laverne AM, Nan B, Lichter PR. Trends in use of ancillary glaucoma tests for patients with open-angle glaucoma from 2001 to 2009. Ophthalmology. 2012;119(4):748–58. doi: 10.1016/j.ophtha.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein JD, Kim DS, Niziol LM, Talwar N, Nan B, Musch DC, Richards JE. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118(6):1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119(10):2074–81. doi: 10.1016/j.ophtha.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International Classification of Diseases. Physician ICD-9-CM 2006. Vol. 1. Chicago, IL: AMA Press; 2006. 9th revision, Clinical Modification. [Google Scholar]
- 38.Hughes JS, Averill RF, Eisenhandler J, Goldfield NI, Muldoon J, Neff JM, Gay JC. Clinical Risk Groups (CRGs): a classification system for risk-adjusted capitation-based payment and health care management. Med Care. 2004;42:81–90. doi: 10.1097/01.mlr.0000102367.93252.70. [DOI] [PubMed] [Google Scholar]
- 39.CPT 2006: Current Procedural Terminology. Chicago, IL: AMA Press; 2006. Professional ed. [Google Scholar]
- 40.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53(282):457–81. [Google Scholar]
- 41.Cox DR. Regression Models and Life-Tables. J Royal Statist Soc, Series B. 1972;34(2):187–220. [Google Scholar]
- 42.Smirnov NV. Tables for estimating the goodness of fit of empirical distributions. Annals of Mathematical Statistics. 1948;19:279. [Google Scholar]
- 43.Cotter SA, Varma R, Tarczy-Hornoch K, et al. The joint writing committee for the Multi-ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study Group. Risk factors associated with childhood strabismus. Ophthalmology. 2011;118:2251–61. doi: 10.1016/j.ophtha.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natalucci G, Schneider M, Werner H, Caflisch JA, Bucher HU, Jenni OG, Latal B. Development of neuromotor functions in very low birth weight children from six to 10 years of age: patterns of change. Acta Paediatr. 2013;102(8):809–14. doi: 10.1111/apa.12271. [DOI] [PubMed] [Google Scholar]
- 45.Datar A, Jacknowitz A. Birth weight effects on children’s mental, motor, and physical development: evidence from twins data. Matern Child Health J. 2009;13(6):780–94. doi: 10.1007/s10995-009-0461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Academy of Pediatrics. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2013;131(1):189–95. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 47.Patty LE, Wu S, Torres M, Varma R LALES Group, Doheny Eye Institute/Keck-USC School of Medicine. Validity of self-reported diagnosis and treatment among Latinos in the Los Angeles Latino Eye Study. Poster session 516. Correlates and Outcomes of Eye Diseases. Association of Research and Vision in Ophthalmology; May 6, 2010; Fort Lauderdale, Florida. [Google Scholar]
- 48.Page JM, Schneeweiss S, White HEA, Harvey P. Ocular sequelae in Premature Infants. Pediatrics. 1993;92(6):787–790. [PubMed] [Google Scholar]
- 49.Theng JTS, Wong TY, Ling Y. Refractive errors and Strabismus in Premature Asian infants with and without Retinopathy of Prematurity. Singapore Med J. 2000;41(8):393–397. [PubMed] [Google Scholar]
- 50.Repka MX. Ophthalmological Problems of the Premature Infant. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:249–57. doi: 10.1002/mrdd.10045. [DOI] [PubMed] [Google Scholar]
- 51.Räisänen S, Gissler M, Saari J, Kramer M, Heinonen S. Contribution of Risk Factors to Extremely, Very and Moderately Preterm Births – Register-Based Analysis of 1,390,742 Singleton Births. PLoS ONE. 2013;8(4):e60660. doi: 10.1371/journal.pone.0060660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng G, Thompson ME, Hall GB. Pathways of neighbourhood-level socio-economic determinants of adverse birth outcomes. International Journal of Health Geographics. 2013;12:32. doi: 10.1186/1476-072X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


