Abstract
Background
In secondary hyperparathyroidism (SHPT), enhanced parathyroid levels of transforming growth factor-α (TGFα) increase EGF receptor (EGFR) activation causing parathyroid hyperplasia, high parathyroid hormone (PTH) and also reductions in vitamin D receptor (VDR) that limit vitamin D suppression of SHPT. Since anti-EGFR therapy is not an option in human SHPT, we evaluated ADAM17 as a therapeutic target to suppress parathyroid hyperplasia because ADAM17 is required to release mature TGFα, the most potent EGFR-activating ligand.
Methods
Computer analysis of the ADAM17 promoter identified TGFα and C/EBPβ as potential regulators of the ADAM17 gene. Their regulation of ADAM17 expression, TGFα/EGFR-driven growth and parathyroid gland (PTG) enlargement were assessed in promoter–reporter assays in A431 cells and corroborated in rat and human SHPT, using erlotinib as anti-EGFR therapy to suppress TGFα signals, active vitamin D to induce C/EBPβ or the combination.
Results
While TGFα induced ADAM17-promoter activity by 2.2-fold exacerbating TGFα/EGFR-driven growth, ectopic C/EBPβ expression completely prevented this vicious synergy. Accordingly, in advanced human SHPT, parathyroid ADAM17 levels correlated directly with TGFα and inversely with C/EBPβ. Furthermore, combined erlotinib + calcitriol treatment suppressed TGFα/EGFR-cell growth and PTG enlargement more potently than erlotinib in part through calcitriol induction of C/EBPβ to inhibit ADAM17-promoter activity, mRNA and protein. Importantly, in rat SHPT, the correction of vitamin D deficiency effectively reversed the resistance to paricalcitol induction of C/EBPβ to suppress ADAM17 expression and PTG enlargement, reducing PTH by 50%.
Conclusion
In SHPT, correction of vitamin D and calcitriol deficiency induces parathyroid C/EBPβ to efficaciously attenuate the severe ADAM17/TGFα synergy, which drives PTG enlargement and high PTH.
Keywords: EGF receptor tyrosine kinase inhibitor, TGFα, transcriptional regulation, vitamin D receptor
INTRODUCTION
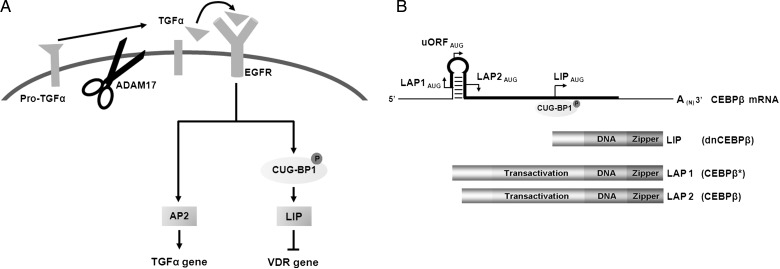
Secondary hyperparathyroidism (SHPT) is a common and serious complication of chronic kidney disease-mineral bone disorders (CKD-MBD) [1, 2]. The degree of parathyroid hyperplasia determines both the severity of SHPT and the response to therapy [3, 4]: the former by increasing the gland capacity for parathyroid hormone (PTH) synthesis and secretion [2], and the latter by reducing parathyroid levels of the vitamin D receptor (VDR), the calcium sensing receptor (CaSR) and the anti-aging protein α-klotho, a co-receptor for fibroblast growth factor 23 (FGF23) [5]. All three receptors mediate the suppression of cell growth and serum PTH by vitamin D, calcium or elevations of FGF23. Importantly, parathyroid hyperplasia precedes the reductions in parathyroid CaSR [6]. Instead, hyperplastic growth and VDR reductions occur simultaneously and are caused by EGF receptor (EGFR) activation [7]. Since the calcitriol–VDR complex induces CaSR and klotho gene expression [8, 9], the reductions in both proteins with parathyroid hyperplasia could be secondary to VDR loss. Thus, inhibition of EGFR activation should effectively attenuate the progression of SHPT and improve the response to treatment. Figure 1A summarizes the molecular link between EGFR activation, hyperplastic growth and VDR loss. In rat and human SHPT, the severity of parathyroid EGFR activation is determined by CKD-induced increases in parathyroid levels of transforming growth factor-α (TGFα, the most potent EGFR-activating ligand) [7, 10, 11], which are aggravated by high serum phosphate (P), low calcium (Ca) or vitamin D deficiency [11, 12]. Enhanced TGFα/EGFR signals induce the synthesis of activator protein-2 (AP2), a transactivator of the TGFα gene [13] and of LIP (for liver inhibitory protein), an oncogene and a transrepressor of the VDR gene [7, 14]. TGFα/EGFR-induction of CUG-BP1 phosphorylation and its binding to the third in-frame AUG codon of the single C/EBPβ mRNA [15] (Figure 1B) make LIP translation prevail over that of the two other CAAT-enhancer binding protein-β (C/EBPβ* and C/EBPβ), both potent growth suppressors [16]. LIP lacks the transactivation domain but binds C/EBP sequences on DNA with higher affinity than either C/EBPβ molecule [14]. LIP acts as an oncogene and a VDR gene suppressor by competing with C/EBPβ homodimers for DNA-binding, or by forming LIP/C/EBPβ heterodimers, transcriptionally inactive to induce growth arrest [14] and VDR gene expression [7]. In nodular hyperplasia, the worst form of human SHPT, the highest TGFα levels concur with the highest LIP content and proliferation rates, and with the lowest VDR mRNA and protein [7]. Accordingly, inhibition of EGFR activation using erlotinib, a highly specific EGFR tyrosine-kinase inhibitor, effectively prevented TGFα induction of its own gene, LIP levels, proliferation rates and reductions of VDR gene transcription, in A431 cells [7], in which growth is driven by TGFα and EGFR over-expression [17, 18] and also in established rat SHPT, in which only TGFα is enhanced [7]. In these uremic rats, a low calcitriol dose (4 ng thrice weekly) which failed to suppress PTH if given during week 4 after 5/6nephrectomy (NX) due to VDR loss became effective if preceded by erlotinib treatment during week 3 [7]. This strategy, however, was insufficient for calcitriol suppression of parathyroid hyperplasia [7].
FIGURE 1:
Pathogenesis of severe EGFR-driven growth and VDR loss. (A) TGFα/EGFR induction of AP2 and LIP synthesis exacerbate growth and VDR loss through TGFα gene upregulation and VDR gene downregulation. (B) Structure of the C/EBPβ mRNA and protein isoforms: a single C/EBPβ mRNA encodes (darker lane) for three distinct proteins C/EBPβ* (LAP1), C/EBPβ (LAP2) and dominant negative C/EBPβ (LIP) through their translation initiation (AUG) from three distinct in-frame translation sites. TGFα induces LIP translation through activation and binding of CUG-BP1 to the third in frame AUG. The protein diagram indicates transactivation, DNA-binding and leucine zipper regions.
Since erlotinib treatment is not an option in human SHPT, we searched for a target upstream from TGFα activation of the EGFR to prevent parathyroid gland (PTG) growth. We focused on ADAM17, the enzyme that cleaves the membrane TGFα precursor releasing mature TGFα to the circulation, thus enhancing autocrine, paracrine and endocrine TGFα/EGFR-growth [19]. In mouse chronic kidney disease (CKD), systemic inhibition of ADAM17 activity attenuated angiotensin II-induced TGFα/EGFR-driven proteinuria and damage to the renal parenchyma as effectively as global TGFα gene deletion or renal-specific EGFR inactivation [20]. However, current ADAM17 inhibitors are highly toxic to be used therapeutically in SHPT [21, 22]. To overcome this limitation, a computer analysis of the ADAM17 promoter searched for targetable regulators of ADAM17-gene expression. The identification of 13 putative C/EBP and 4 AP2 binding sites suggested that enhanced parathyroid TGFα/EGFR induction of LIP [7] and AP2 [13] could increase ADAM17-gene expression further aggravating growth rates and VDR loss. Importantly, it also revealed a potentially safe anti-ADAM17 strategy: calcitriol induction of parathyroid C/EBPβ, as demonstrated in other cell types [23–26]. Therefore, herein, in vivo, ex vivo and in vitro protocols tested the hypothesis that simultaneous treatment with erlotinib and calcitriol should enhance erlotinib potency to suppress parathyroid growth through an effective induction of C/EBPβ to inhibit ADAM17-gene expression.
In addition, because 25-hydroxyvitamin D (25D) enhances calcitriol/VDR-antiproliferative actions in various cancer cell types [27], we examined whether this 25D/calcitriol synergy could compensate for parathyroid VDR loss and induce C/EBPβ repression of the ADAM17-gene and PTG enlargement without erlotinib. To evaluate the contribution of 25D conversion to calcitriol to the 25D + active vitamin D synergy, calcitriol treatment had to be substituted by its analog, paricalcitol [28].
MATERIALS AND METHODS
Cell culture and proliferation assays
The human epidermoid carcinoma cell line A431 (ATCC) was grown in 10% Fetal Bovine Serum Dulbecco's Modified Eagles's Medium (FBS DMEM) (Invitrogen) at 37°C in 5% CO2. Either 106 (10-cm plate) or 104 cells (96-well plates) were synchronized at G0 using serum-free DMEM for 8 h and treated with erlotinib [in dimethyl sulfoxide (DMSO)], 1,25-dihydroxyvitamin D (in ethanol) or the combination in 2% FBS DMEM for 60 h followed by 1% BSA DMEM up to 84 h. The colorimetric 3-(4,5 dimethylthiazol-2-yl)2-5-diphenyl tetrasodium bromide assay kit (Chemicon International) measured A431 proliferation.
RT-PCR for human ADAM17
Total RNA was extracted using RNA-Bee (TEL-TEST) and quantified by a UV-VIS spectrophotometer (Nanodrop Technologies). First-strand cDNA was obtained from 2 μg RNA using Omniscript Reverse Transcription reagents (Qiagen). Cycling conditions for reverse transcription polymerase chain reaction (RT-PCR) for ADAM17 and cyclophilin B (CypB) were 5′ at 94°C, 40 or 24 cycles, respectively, of 30″ at 94°C, 30″ at 57 or 54°C, respectively, 45″ at 72°C, with final 5′ extension at 72°C. RT-PCR products were electrophoresed in 1% agarose gels, visualized using a transilluminator (Sigma Chemical T1202) and quantified with ImageJ. Primer sequences were: ADAM17: forward: 5-TCATTGACCACGTGAGCATC-3; reverse: 5-TCGTCCATATGTGAGTCTGTGC-3; CycB: forward: 5-GTGATCTTTGGTCTCTTCGG-3; reverse: 5-CGATGATCACATCCTTCAGG-3.
Plasmids
ADAM17-luciferase reporter
The human ADAM17-promoter fragment [−2305 to −1 before ATG] containing 13 C/EBP and 4 AP2 putative binding sites was PCR amplified with forward primer: 5-GATAAACTAATTAATCTATCC-3 and reverse primer: 5-GAGTCGGTAGCGGGGCCGGGAAC-3, subcloned into T-vector (pBluescript II), sequenced and inserted into pGL2-Basic vector (between KpnI and HindIII).
Expression vector exclusive for human C/EBPβ: the LIP's ATG (Met) was replaced by TCC (Ser) to impede the initiation of LIP translation (Figure 1). The 5′-fragment of the human mutant C/EBPβ was PCR amplified, forward primer: 5-TATGGAAGTGGCCAACTTCTAC-3 and reverse primer: 5-AGGATCCTGCGCCGCCGCCCGGCGC-3; and the human mutant C/EBPβ 3′-fragment with forward primer: 5-AGGATCCGCGGCGGGCTTCCCGTACGCG-3 and reverse primer: 5-ATCTAGACTAGCAGTGGCCGGAGGAGG-3. PCR fragments were cloned into T-vector (pBluescript II) and sequenced. 5′- and 3′-fragments were assembled with BamHI and subcloned into expression vector pcDNA3.
Promoter–reporter assays
One microgram of the human ADAM17 luciferase reporter and 0.1 μg of the β-galactosidase (βgal) expression plasmid [7] with or without 0.1 μg of the C/EBPβ expression vector were transiently transfected (Myrus Transfection reagents, using 4 μL/1 μg of DNA following manufacturer's protocols) into A431 cells, plated at a concentration of 3 × 105 cells/mL media/well. pGEM DNA was added when required to maintain the total amount of transfected DNA constant. Upon an overnight incubation, cells were treated with vehicle or TGFα 8 nM for 24 h, then lysed, and luciferase and βgal activities measured using Luciferase reporter system (Promega) and Galacto-Light (Applied Biosystems).
Protocols for rat SHPT
Female Sprague-Dawley rats (200–225 g) underwent 5/6NX as in [7] and were fed a high P diet for 4 weeks (0.9% P, 0.6% Ca; Dyets). Protocol 1: rats received from week 2 to 4, either vehicle (200 μL of DMSO), erlotinib (6 mg/kgbw, daily, in 100 μL propylene glycol as in [7]; Genentech), calcitriol (4 ng thrice weekly in 200 µL of propylene-glycol–PBS 1:1), or the combination. Protocol 2: one week after 5/6NX, rats received either vehicle, 25D (800 ng weekly) to correct vitamin D deficiency; paricalcitol (16 ng, equivalent to 4 ng calcitriol in PTH suppression [28], thrice weekly) or the combination, for 3 weeks. A group of 5/6NX rats were fed a low P diet (0.2% P; 0.5% Ca) throughout the study.
At sacrifice, blood was drawn for analytical determinations. PTG were removed and weighed (Cahn Instruments). For immunohistochemistry, glands were fixed in formalin and paraffin included. Plasma P, creatinine, ionized Ca and intact PTH levels were measured as described [7]. All animal protocols were approved by the Animal Study Committee at Washington University.
Human PTG
Cryopreserved 1–2 mm3 sections of 32 human PTG were obtained at parathyroidectomy from advanced CKD patients (Vall d'Hebron Hospital, Barcelona, Spain; kindly provided by Dr Galicia).
Immunohistochemistry
Antigen retrieval, quenching and blocking in rat PTG, was performed as described [7]. Primary antibodies against ADAM17 (Genetex, 1:50), C/EBPβ (Biolegend, 1:50, directed to an epitope in the N-terminal domain absent in LIP) and proliferating cell nuclear antigen (PCNA; Biolegend 1:500) were added overnight at 4°C, followed by biotinylated secondary antibody and streptavidin–horseradish peroxidase conjugate. Immune complexes were visualized with aminoethyl carbazole substrate–chromogen (Histostain-plus, Invitrogen). For negative controls, one section was left without primary antibody. ADAM17, C/EBPβ and PCNA were quantified in at least three sections per gland, as in [12]. A semi-quantification scoring from 1+ (low intensity) to 4+ (high intensity) evaluated PTGs from the 25D/paricalcitol protocol.
For human PTG, upon an overnight exposure with primary antibody [ADAM17 (Abcam 1:200); TGFα (GF10, Calbiochem 1:20); VDR (Millipore 1:200) and C/EBPβ (Biolegend 1:100)], sections were washed, the appropriate Alexa-fluor secondary antibodies and Hoechst added for 1 h and quantified with ImageJ.
Western blots
A431 cells: whole cell and nuclear extracts were obtained using 1% radio immunoprecipitation assay buffer/protease inhibitor cocktail (Roche), or Nuclear Extract Kit (Active Motif), respectively. Protein concentration was quantified by Bio-Rad Protein Assay (Bio-Rad Laboratories). Protein was resolved by either 10% or 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride (Immobilon-P transfer membrane; Millipore) membranes. After probing overnight at 4°C with primary antibodies [total C/EBPβ (Santa Cruz 1:1000, directed to an epitope in the C-terminal domain common to the 3 isoforms), ADAM17 (Genetex 1:1000)] blots were visualized by enhanced chemiluminescence (SuperSignal West Pico; Pierce), and quantified with ImageJ.
Statistical analyses
ANOVA assessed statistical differences among all experimental groups, with Bonferroni tests (or unpaired t-test analysis when indicated) to compare selected pair of groups.
RESULTS
Calcitriol/erlotinib synergy to suppress PTG growth involves induction of C/EBPβ
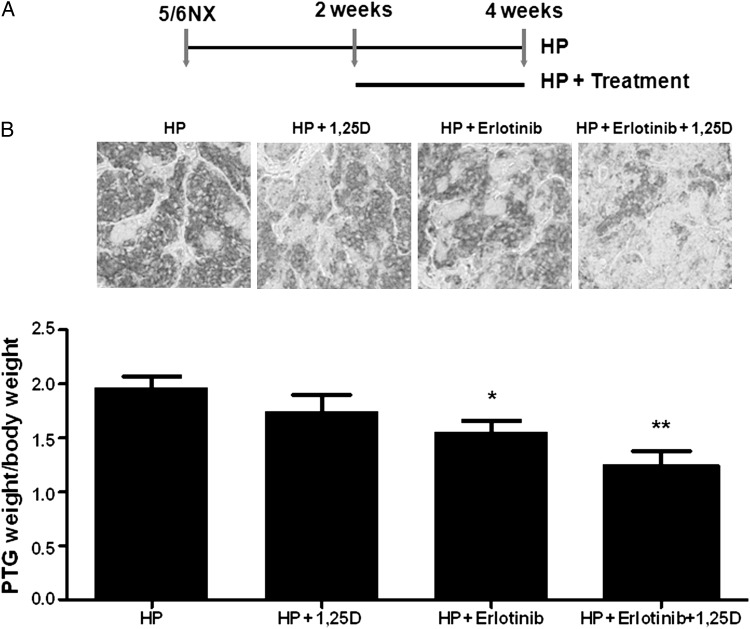
Figure 2A and Table 1 show that, in rat established SHPT, treatment from week 2 to 4 after 5/6NX with erlotinib and calcitriol (at a selected dose, ineffective to suppress PTG enlargement to mimic calcitriol resistance due to VDR loss) had a higher potency than erlotinib alone in inhibiting proliferation, as measured by parathyroid PCNA and PTG weight/bw, and in enhancing PTH suppression by calcitriol from 16 to 34.5%. This higher potency was unrelated to differences in serum creatinine, Ca and P levels, and associated directly with the doubling of parathyroid C/EBPβ protein (P < 0.05) and with marked reductions in parathyroid ADAM17 immunostaining (Figure 2B). Neither monotherapy enhanced parathyroid C/EBPβ above the levels in untreated controls. To overcome the limitation in estimating the parathyroid C/EBPβ/LIP ratio immunohistochemically, as LIP antibodies recognize C/EBPβ (see Figure 1B), the contribution of increases in the C/EBPβ/LIP ratio to suppress ADAM17-gene expression was examined in A431 cells, upon reproducing the down-regulation of TGFα/EGFR-growth by erlotinib and calcitriol observed in rat SHPT.
FIGURE 2:
The higher potency of the erlotinib + calcitriol combination compared with erlotinib alone to suppress PTG enlargement in rat SHPT associates with marked reductions in parathyroid ADAM17. (A) Scheme of the experimental protocol. (B) Top: representative immunostaining for parathyroid ADAM17 expression. Magnification ×200; bottom: bars and error bars represent the mean ± SEM of PTG weight/body weight from 5/6NX rats fed a high P diet (HP) and receiving vehicle (HP), or calcitriol (HP + 1,25D); erlotinib (HP + ertotinib), or the combination (HP + erlotinib + 1,25D), at the concentrations specified in the ‘Methods’ section. * and ** indicate P < 0.05 and P < 0.01 versus HP.
Table 1.
Serum chemistry, body weight and changes in parathyroid features with treatment
| Vehicle (n = 7) | Erlotinib (n = 8) | 1,25D (n = 7) | Erlotinib + 1,25D (n = 6) | |
|---|---|---|---|---|
| ICa (mg/dL) | 4.56 ± 0.08 | 4.68 ± 0.12 | 4.71 ± 0.07 | 4.76 ± 0.14 |
| Creatinine (mg/dL) | 1.38 ± 0.12 | 1.20 ± 0.12 | 1.20 ± 0.08 | 1.14 ± 0.10 |
| Total Ca (mg/dL) | 9.95 ± 0.16 | 10.32 ± 0.20 | 10.6 ± 0.10 | 10.43 ± 0.23 |
| P (mg/dL) | 7.74 ± 0.63 | 8.06 ± 0.64 | 7.16 ± 0.48 | 7.66 ± 0.63 |
| PTH (pg/mL) | 427.6 ± 120.0 | 414.9 ± 116.0 | 359.6 ± 62.5 | 280.0 ± 82.2 |
| Body weight (g) | 271.0 ± 4.8 | 272.4 ± 3.7 | 277.1 ± 4.7 | 272.0 ± 5.4 |
| PCNA (+nuclei/area) | 0.42 ± 0.08 (N = 12) | 0.21 ± 0.03 (N = 14)* | 0.28 ± 0.07 (N = 9) | 0.14 ± 0.05 (N = 8)*** |
| CEBPβ (IOD/area) | 36.6 ± 5.3 | 37.7 ± 6.3 | 39.4 ± 5.5 | 56.6 ± 4.7* |
Values indicate the mean ± SEM in 5/6NX rats fed a high P (HP) diet and treated with vehicle, erlotinib (6 mg/kg bw, daily, i.p. in 100 μL DMSO), calcitriol (1,25D, 4 ng thrice weekly in 200 μL of propylene glycol–PBS 1:1) or the combination, from week 2 to week 4 after 5/6NX.
ICa, ionized calcium; PTH, parathyroid hormone; PCNA, proliferating cell nuclear antigen, where n is the number of rats and N is the number of PTGs examined.
*, **and ***indicate P < 0.05; P < 0.01 and P < 0.001 versus HP.
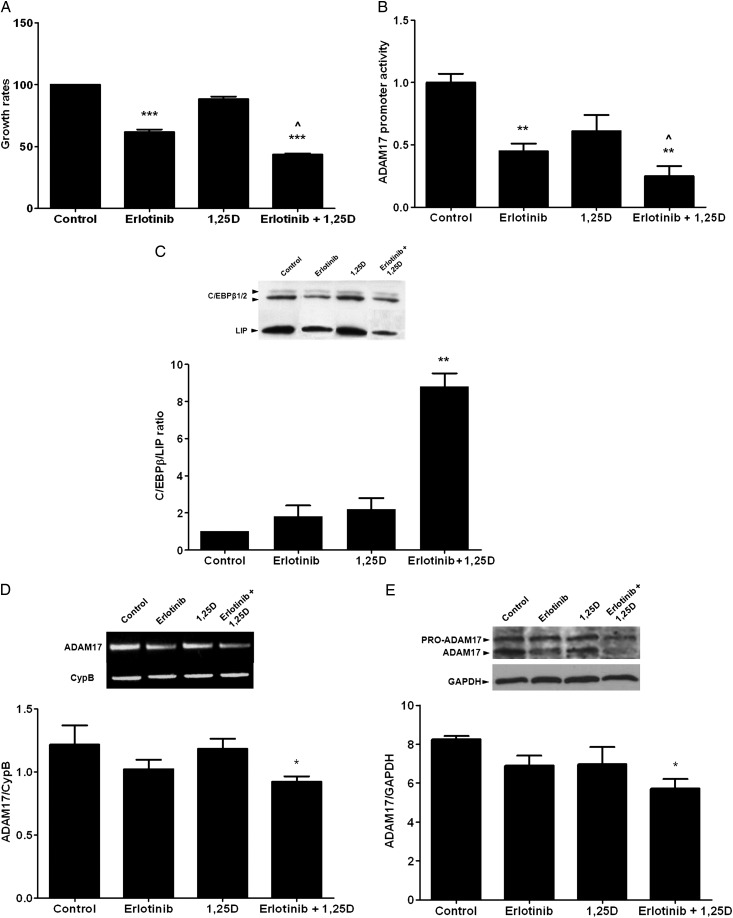
Calcitriol/erlotinib synergy to inhibit TGFα/EGFR-growth involves the induction of C/EBPβ/LIP ratio to suppress ADAM17-gene expression
The combination of erlotinib (0.25 µM) + calcitriol (10−7 M, a dose ineffective to suppress A431 growth), inhibited proliferation more potently than erlotinib alone (Figure 3A), in part through a higher suppression of ADAM17-promoter activity (Figure 3B). Only erlotinib + calcitriol treatment, which increased nuclear C/EBPβ/LIP ratio by 9-fold (Figure 3C), reduced ADAM17 mRNA and protein expression (Figure 3D and E).
FIGURE 3:
TGFα and vitamin D regulation of ADAM17 expression. (A) Growth rates (MTT assay) of A431 cells treated for 84 h with vehicle (control), erlotinib 0.25 μM, calcitriol (1,25D) 100 nM or the combination (erlotinib + 1,25D). Bars and error bars represent the mean ± SEM from three independent experiments. ** and *** indicate P < 0.01 and P < 0.001 versus controls; ^ indicates P < 0.05 versus erlotinib. (B) Promoter–reporter assay of luciferase activity driven by the human ADAM17 promoter, corrected by βgal, in A431 cells treated as in (A). Bars and error bars represent the mean ± SEM of at least triplicate determinations per condition from two independent experiments. ** and ^ indicate P < 0.01 versus control and P < 0.05 versus erlotinib. (C) Top: representative western blot analysis of nuclear LAP1/2 and LIP expression in A431 cells treated as in (A); bottom: nuclear C/EBPβ (LAP1/2): LIP ratios, in A431 cells treated as in (A). Bars and error bars represent the mean ± SEM from at least four independent experiments; ** indicates P < 0.01 versus controls. (D, E) Top panels: representative RT-PCR for ADAM17 (D) and the loading control CypB and western blot analysis (E) of pro-ADAM17 and active ADAM17 protein expression and the loading control GAPDH in A431 cells treated as in (A). Bottom panels: densitometric analysis of ADAM17 mRNA expression corrected for the housekeeping gene CypB (D) and of ADAM17 protein expression corrected for GAPDH as the loading control (E) in A431 cells treated as in (A). Bars and error bars represent the mean ± SEM from at least two independent experiments. * indicates P < 0.05 versus controls.
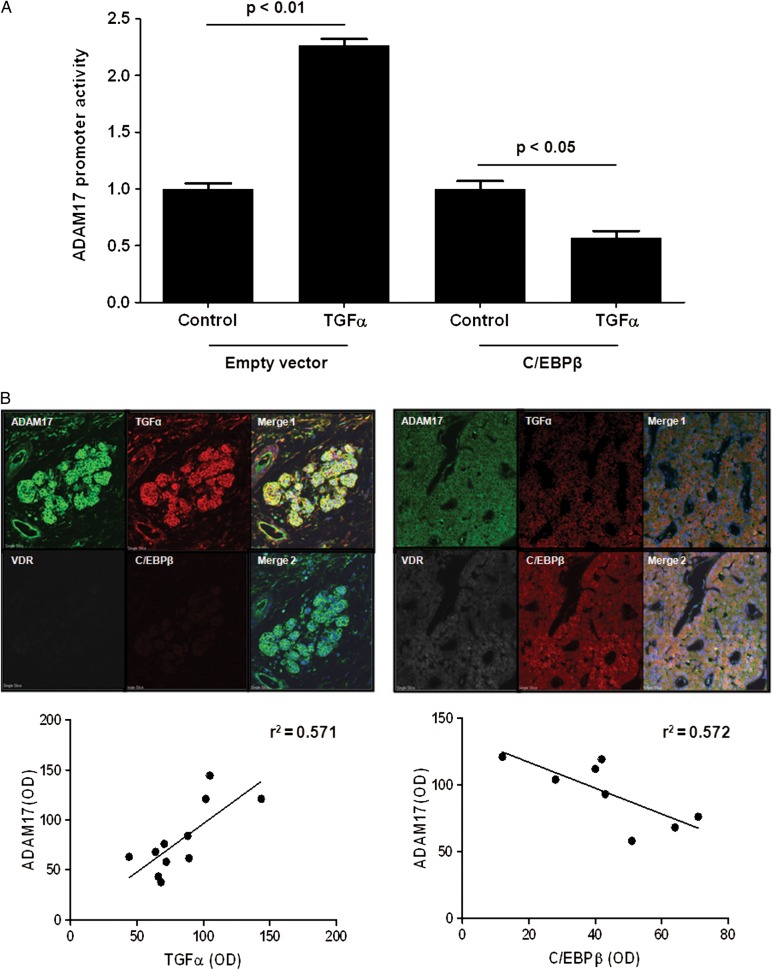
ADAM17-gene expression is induced by TGFα and suppressed by C/EBPβ
Treatment of A431 cells with 8 nM TGFα for 48 h caused a 2.2-fold increase in ADAM17-promoter activity (Figure 4A), which was reduced by 43% through ectopic expression of C/EBPβ.
FIGURE 4:
Increased parathyroid TGFα and ADAM17 concur with low VDR and C/EBPβ in human hyperplastic glands from SHPT. (A) Promoter–reporter assay of human ADAM17-promoter activity, corrected by βgal, in A431 cells treated with vehicle (control) or TGFα 8 nM (TGFα), and transfected with empty vector (control + empty vector) or with a C/EBPβ expression vector. Bars and error bars represent the mean ± SEM from six and two independent experiments with triplicate determinations each, for TGFα treatment or upon ectopic C/EBPβ expression, respectively. Results are expressed as % of respective controls. (B) Top panels: representative immunofluorescence of ADAM17, TGFα, VDR and C/EBPβ expression and co-localization in two distinct areas from a single patient. Merge 1: Hoechst (blue), ADAM17 (green); TGFα (red); Merge 2: Hoechst (blue); VDR (gray); C/EBPβ (red); ADAM17 (green). Magnification ×40. Bottom panel: (left) correlation between parathyroid ADAM17 and TGFα in 11 nodules from the PTGs from four patients: r2 = 0.571, P = 0.007. Bottom panel (right): correlation between parathyroid ADAM17 and C/EBPβ in eight nodules from the PTGs from six patients: r2 = 0.572, P = 0.03.
Accordingly, immunofluorescent analysis of human hyperplastic glands (Figure 4B, top) demonstrated that the highest ADAM17 levels co-localized with the highest cell membrane TGFα expression, both whole and ADAM17-cleaved TGFα precursors (left). Instead, the lowest ADAM17 and TGFα expression concurred with the highest nuclear C/EBPβ and VDR content (right). Furthermore, Figure 4B (bottom panels) shows that parathyroid ADAM17 strongly correlated directly with TGFα (r2 = 0.571, P < 0.007) and inversely with C/EBPβ (r2 = 0.572, P < 0.03).
Next, we examined whether, in rat SHPT, 25D enhancement of calcitriol/VDR-antiproliferative actions sufficed to induce parathyroid C/EBPβ to suppress ADAM17 and PTG enlargement despite VDR loss. To measure whether 25D conversion to calcitriol contributes to the 25D/calcitriol synergy, paricalcitol replaced calcitriol treatment [28].
25-Hydroxy vitamin D + paricalcitol suppresses parathyroid ADAM17 and gland enlargement in rat SHPT as effectively as erlotinib + calcitriol
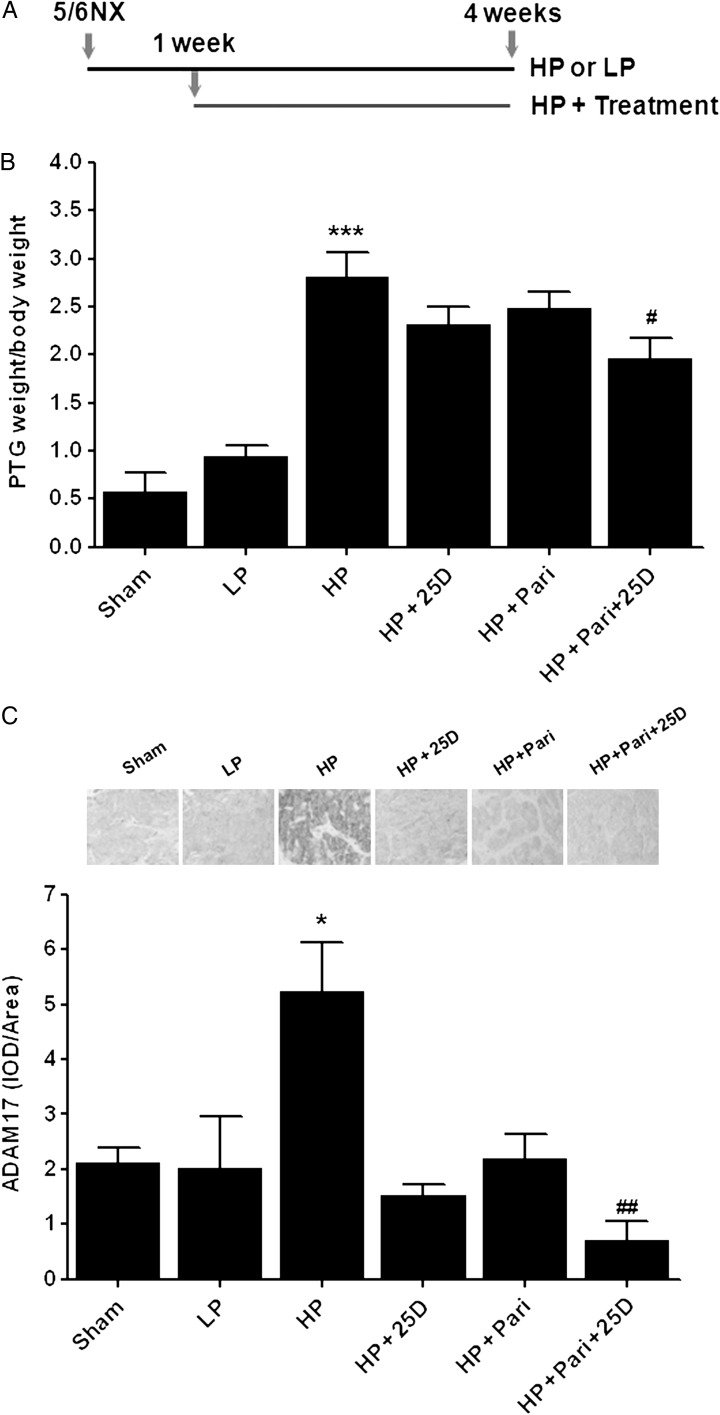
Figure 5 shows that despite similar serum creatinine, P, total and ionized Ca among all experimental groups (Table 2), and also despite the normalization of serum 25D in rats receiving 25D, only rats receiving the 25D + paricalcitol combination from week 1 after 5/6NX could prevent further PTG enlargement reducing PTH by 50%. This reduction in PTG enlargement associated with a marked reduction (7.5-fold) of parathyroid ADAM17 protein and a 2-fold increase in parathyroid C/EBPβ over untreated controls, a potency similar to that of dietary P restriction (1.95-fold P < 0.01). Serum calcitriol was similar in rats receiving combined 25D + paricalcitol compared with those receiving paricalcitol alone. Thus, normalization of serum 25D sufficed to reverse the resistance to a low dose of paricalcitol in doubling C/EBPβ and in suppressing ADAM17 protein and PTG enlargement.
FIGURE 5:
25-hydroxyvitamin D enhances paricalcitol efficacy to suppress parathyroid ADAM17 and gland enlargement in rat SHPT. (A) Scheme of the experimental protocol in rats fed either a high P (HP) or a low P diet (LP) throughout the 4 weeks after 5/6NX or sham operation and treated with vehicle, 25-hydroxyvitamin D (HP + 25D); paricalcitol (HP + Pari) or the combination (HP + Pari + 25D) at the concentrations specified in the ‘Methods’ section, starting 1 week after NX. (B) Bars and error bars represent the mean ± SEM of PTG weight/body weight in rats treated as in (A). (C) Top: representative immunostaining for parathyroid ADAM17 expression; bottom: bars and error bars represent the mean ± SEM of parathyroid ADAM17 expression estimated by the quantification of ADAM17 immunostaining per area (IOD/area), respectively, from sham operated (Sham) or 5/6NX rats treated as indicated. * and *** indicate P < 0.05 or P < 0.001, respectively, versus LP; # and ## indicate P < 0.05 or P < 0.01, respectively, versus HP.
Table 2.
Serum chemistries and parathyroid C/EBPβ content with treatment
| Sham | LP | HP | HP + 25D | HP + Pari | HP + 25D + Pari | |
|---|---|---|---|---|---|---|
| Creatinine (mg/dl) | 0.73 ± 0.05 | 1.65 ± 0.26 | 1.38 ± 0.11 | 1.53 ± 0.14 | 1.52 ± 0.10 | 1.46 ± 0.17 |
| Total Ca (mg/dl) | 9.86 ± 0.11 | 10.56 ± 0.22 | 9.82 ± 0.15 | 9.68 ± 0.40 | 9.70 ± 0.26 | 9.63 ± 0.26 |
| ICa (mg/dl) | 4.85 ± 0.05 | 4.84 ± 0.07 | 4.47 ± 0.13 | 4.39 ± 0.17 | 4.35 ± 0.14 | 4.42 ± 0.17 |
| P (mg/dl) | 5.14 ± 0.27 | 5.13 ± 0.39 | 8.12 ± 0.92 | 7.78 ± 1.25 | 9.03 ± 0.68 | 8.08 ± 1.00 |
| PTH (pg/mL) | 91.5 ± 34.3 | 272.3 ± 58.0 | 3,555.7 ± 776.4 | 3,562 ± 151 | 3098.2 ± 437 | 1,550 ± 819 |
| 25D (ng/mL) | 32.2 ± 2.2 | 22.1 ± 6.8 | 16.6 ± 4.0 | 27.4 ± 7.4 | 17.9 ± 3.3 | 33.1 ± 9.8 |
| 1,25D (pg/mL) | 125.3 ± 17.8 | 83.6 ± 29.7 | 94.3 ± 27.9 | 89.7 ± 16.7 | 29.7 ± 7.6 | 37.8 ± 3.3 |
| C/EBPβ (score) | – | 3.25 ± 0.25 (4) | 1.67 ± 0.33** (3) | 2.0 ± 1.0 (3) | 2.0 ± 0.58 (4) | 3.0 ± 0.41^ (4) |
Values indicate the mean ± SEM in at least 5 5/6NX rats fed either a low P (LP) or a high P (HP) diet and treated with vehicle (HP), 25D (800 ng once weekly, i.p. in 200 μL of propylene glycol–PBS 1:1); paricalcitol (Pari, 16 ng thrice weekly in 200 μL of propylene glycol–PBS 1:1), or the combination, from week 1 to week 4 after 5/6NX.
ICa, ionized calcium; PTH, parathyroid hormone; 25D, 25-hydroxyvitamin D; 1,25D, 1,25-dihydroxyvitamin D; C/EBPβ, parathyroid levels of C/EBPβ protein, where (number) indicates the number of PTGs examined.
**Indicates P < 0.01 versus LP and ^indicates P < 0.06 versus HP.
DISCUSSION
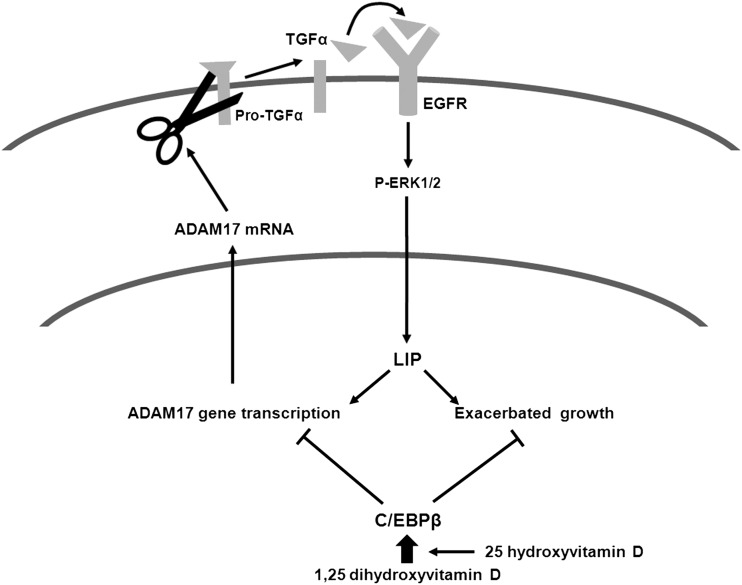
This work presents ADAM17 as a critical target upstream from EGFR activation to improve current vitamin D strategies to treat SHPT (Figure 6), as it identified: (i) TGFα induction of ADAM17-promoter activity as the initiator of a powerful ADAM17-dependent autocrine loop that aggravates the already enhanced TGFα/EGFR-growth signals; (ii) C/EBPβ trans-repression of the ADAM17 gene as a mechanism by which active vitamin D therapy enhances the efficacy of anti-EGFR therapy and (iii) a 25D/paricalcitol synergy that induces parathyroid C/EBPβ and attenuates the increases in parathyroid ADAM17 causing PTG enlargement, as efficacious as combined erlotinib + calcitriol treatment.
FIGURE 6:
Molecular bases for ADAM17/TGFα induction of severe parathyroid hyperplasia in SHPT and its suppression by vitamin D. TGFα induction of ADAM17-gene transcription reinforces the vicious autocrine loop of enhanced ADAM17 release of TGFα and TGFα activation of the EGFR to induce LIP-driven growth exacerbation and ADAM17-gene transcription in A431 cells and in rat and human SH. 1,25-dihydroxyvitamin D efficacy to enhance C/EBPβ synthesis contributes to inhibit ADAM17-gene expression. 25D enhances 1,25-dihydroxy vitamin D actions induction of C/EBPβ to suppress ADAM17-gene expression.
TGFα upregulation of ADAM17-gene expression provides a previously unrecognized pathogenic mechanism that aggravates the severe growth patterns associated with ADAM17/TGFα co-expression. Specifically, ADAM17 activity is essential to release mature TGFα, the stronger EGFR activator. In fact, the ADAM17 null mouse dies perinatally due to severe developmental defects in the morphogenesis of epithelial organs including the PTG [29]. Undoubtedly, in human SHPT, ADAM17 activity is mandatory for the strong association between TGFα content and proliferation rates [7]. Furthermore, TGFα activation of the EGFR also increases ADAM17 stability [30], the phosphorylation required for ADAM17 translocation to the plasma membrane for activity [31, 32] and causes a 2.2-fold increase in ADAM17-gene expression. Clearly, TGFα induction of ADAM17-promoter activity and post-transcriptional modifications generate an ADAM17/TGFα synergy that may contribute to nodule formation, as over-expression of LIP in normal mouse mammary glands is sufficient to induce hyperproliferation and tumorigenesis [33]. The presence of several AP2 and C/EBP putative binding sites in the human ADAM17 promoter suggested that anti-EGFR therapy should prevent both TGFα induction of parathyroid AP2 [13] and LIP [7] and consequently, ADAM17-gene upregulation. Since LIP antagonizes C/EBPβ actions, we examined whether part of C/EBPβ antiproliferative actions involved ADAM17-gene suppression. We found that, in A431 cells, in which not only TGFα but also EGFR is overexpressed [17], ectopic C/EBPβ expression prevented the doubling of ADAM17-promoter activity induced by TGFα and reduced ADAM17-gene expression by 43%. This finding suggested that calcitriol induction of C/EBPβ [23–26] could provide a safe anti-ADAM17 strategy to break the deleterious ADAM17/TGFα synergy upstream from EGFR activation. The demonstration in hyperplastic PTG from patients with advanced CKD that parathyroid ADAM17 content correlated directly with TGFα and inversely with C/EBPβ content led us to evaluate, in rat CKD, the efficacy of simultaneous anti-EGFR and anti-ADAM17 treatment in attenuating PTG hyperplasia. In established rat SHPT, in which only TGFα, but not EGFR, is overexpressed [7], simultaneous treatment with erlotinib, a suppressor of LIP synthesis, and calcitriol, an inducer of C/EBPβ expression, from week 2 to week 4 after 5/6NX, is more effective than erlotinib alone in attenuating further increases in PTG enlargement and serum PTH, despite similar serum levels of creatinine, Ca and P. The higher suppression of parathyroid cell growth by the erlotinib + calcitriol combination associated directly with the doubling of parathyroid C/EBPβ content, and with marked reductions in ADAM17 protein, supporting but not proving, the transcriptional control of the ADAM17 gene by increases in parathyroid C/EBPβ.
Next, studies in A431 cells conclusively demonstrated that inhibition of the ADAM17 gene mediated, at least in part, the higher potency of the erlotinib/calcitriol combination over that of erlotinib alone to inhibit TGFα/EGFR-driven growth. This improved outcome associated with a 9-fold elevation in the C/EBPβ + LIP ratio, which resulted in significantly reduced ADAM17 mRNA and protein expression.
Because anti-EGFR is not a choice in human SHPT, and current ADAM17 inhibitors are highly toxic [21, 22], we also examined in rat SHPT whether 25D enhancement of calcitriol/VDR-antiproliferative actions [27] could safely substitute for erlotinib reversal of the resistance to active vitamin D suppression of PTG growth caused by VDR loss. Intraperitoneal administration of 25D to normalize serum vitamin D levels starting 1 week after 5/6NX, effectively reversed the resistance to 16 ng of paricalcitol in inducing C/EBPβ and suppressing ADAM17 expression and PTG enlargement, with a potency similar to either combined erlotinib + calcitriol treatment or P restriction, resulting in a 50% PTH reduction.
The mild elevations in serum 1,25D in rats treated with combined 25D + paricalcitol compared with those receiving paricalcitol alone suggested that local conversion of 25D to calcitriol could partially account for the synergy. However, direct activation of VDR-antiproliferative actions by 25D should not be ruled out, as 25D can directly activate the VDR and also synergize with calcitriol in cells from the 1α-hydroxylase null mouse [34] or upon specific inhibition of 25D conversion to calcitriol [35].
This 25D/active vitamin D synergy is translationally relevant: first, it unraveled a novel mechanism supporting the recommendation by Kidney Disease: Improving Global Outcomes guidelines to correct vitamin D deficiency to improve outcomes in SHPT at all CKD stages. Second, it provided a potentially safe alternative for patients whose high serum calcium and phosphate levels preclude escalating the dose of paricalcitol to suppress PTH [28, 36, 37]. Third, it demonstrated that the correction of vitamin D deficiency in CKD patients that are not receiving active vitamin D therapy may not suffice to reach sufficient 25D levels within parathyroid cells to compensate for VDR reductions and effectively attenuate ADAM17/TGFα-driven hyperplasia. Indeed, in CKD stage 3–4, vitamin D supplementation that normalized serum 25D and 1,25D levels was ineffective in suppressing PTH [38–41]. Mechanistically, in hemodialysis patients, CKD-induced reductions in 25D uptake by circulating monocytes is corrected by calcitriol [42]. Similar defective 25D uptake may occur in the PTG due to CKD-induced decreases in the endocytic receptor megalin [43], highly expressed in the parathyroids [44]. Because active vitamin D induces megalin [45], combined 25D + active vitamin D could enhance both parathyroid 25D uptake and 25D/active vitamin D synergy for ADAM17 suppression. A similar synergy for 25D uptake by proximal tubular cells from the glomerular ultrafiltrate should help maintain serum 25D levels [46].
An important consideration before the clinical application of this 25D/active vitamin D synergy to treat human SHPT is that in these pre-clinical studies, nutritional and active vitamin D were administered intraperitoneally. The oral formulations more commonly used in CKD patients could increase the risk for hypercalcemic and hyperphosphatemic episodes as a result of synergic 25D/active vitamin D interactions enhancing intestinal calcium and phosphate absorption. Therefore, the safe transfer of this synergy to the bedside requires the identification of noninvasive markers of parathyroid ADAM17 inhibition. Measurements of reductions in circulating mature TGFα levels in response to vitamin D therapy may not be accurate, as they reflect both parathyroid and renal ADAM17 activity in CKD [20, 47]. Importantly, the results from the 25D + active vitamin D protocols also suggested that simple measurements of the ability of vitamin D supplementation alone or in combination with low doses of active vitamin D (either calcitriol or paricalcitol), to effectively normalize serum 25D above 30 ng/mL and reduce PTH by 50%, could help personalize nutritional and active vitamin D interventions to reach the appropriate parathyroid 25D/active vitamin D synergy for ADAM17 inhibition. This is a mandatory first step prior to the much needed prospective, well-powered clinical studies.
ACKNOWLEDGEMENTS
This work was supported by the following grants to A.D.: RO1 DK062713 from NIDDK; CEDAR (Center for D-receptor Activation Research); Abbott Pharmaceuticals, FIS PI11/00259 from Institutos de Salud Carlos III, Spanish Government and the Barnes Jewish Auxilary Chapter. The processing for serum chemistries was partly supported by Core C of the O'Brien Center for renal chemistries, Grant P30DK079333.
REFERENCES
- 1.Moe SM, Drueke T, Lameire N, et al. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM. The hyperparathyroidism of chronic renal failure: a disorder of growth. Kidney Int. 1997;52:3–9. doi: 10.1038/ki.1997.297. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda N, Tanaka H, Tominaga Y, et al. Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92:1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokumoto M, Tsuruya K, Fukuda K, et al. Reduced p21, p27 and vitamin D receptor in the nodular hyperplasia in patients with advanced secondary hyperparathyroidism. Kidney Int. 2002;62:1196–1207. doi: 10.1111/j.1523-1755.2002.kid585.x. [DOI] [PubMed] [Google Scholar]
- 5.Silver J, Rodriguez M, Slatopolsky E. FGF23 and PTH—double agents at the heart of CKD. Nephrol Dial Transplant. 2012;27:1715–1720. doi: 10.1093/ndt/gfs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritter CS, Finch JL, Slatopolsky EA, et al. Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int. 2001;60:1737–1744. doi: 10.1046/j.1523-1755.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 7.Arcidiacono MV, Sato T, Alvarez-Hernandez D, et al. EGFR activation increases parathyroid hyperplasia and calcitriol resistance in kidney disease. J Am Soc Nephrol. 2008;19:310–320. doi: 10.1681/ASN.2007040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 9.Forster RE, Jurutka PW, Hsieh JC, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–562. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gogusev J, Duchambon P, Stoermann-Chopard C, et al. De novo expression of transforming growth factor-alpha in parathyroid gland tissue of patients with primary or secondary uraemic hyperparathyroidism. Nephrol Dial Transplant. 1996;11:2155–2162. doi: 10.1093/oxfordjournals.ndt.a027131. [DOI] [PubMed] [Google Scholar]
- 11.Cozzolino M, Lu Y, Sato T, et al. A critical role for enhanced TGF-alpha and EGFR expression in the initiation of parathyroid hyperplasia in experimental kidney disease. Am J Physiol Renal Physiol. 2005;289:F1096–F1102. doi: 10.1152/ajprenal.00167.2005. [DOI] [PubMed] [Google Scholar]
- 12.Dusso AS, Pavlopoulos T, Naumovich L, et al. p21(WAF1) and transforming growth factor-alpha mediate dietary phosphate regulation of parathyroid cell growth. Kidney Int. 2001;59:855–865. doi: 10.1046/j.1523-1755.2001.059003855.x. [DOI] [PubMed] [Google Scholar]
- 13.Arcidiacono MV, Cozzolino M, Spiegel N, et al. Activator protein 2alpha mediates parathyroid TGF-alpha self-induction in secondary hyperparathyroidism. J Am Soc Nephrol. 2008;19:1919–1928. doi: 10.1681/ASN.2007111216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365(Pt 3):561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldwin BR, Timchenko NA, Zahnow CA. Epidermal growth factor receptor stimulation activates the RNA binding protein CUG-BP1 and increases expression of C/EBPbeta-LIP in mammary epithelial cells. Mol Cell Biol. 2004;24:3682–3691. doi: 10.1128/MCB.24.9.3682-3691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raught B, Gingras AC, James A, et al. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- 17.Derynck R, Goeddel DV, Ullrich A, et al. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987;47:707–712. [PubMed] [Google Scholar]
- 18.Santon JB, Cronin MT, MacLeod CL, et al. Effects of epidermal growth factor receptor concentration on tumorigenicity of A431 cells in nude mice. Cancer Res. 1986;46:4701–4705. [PubMed] [Google Scholar]
- 19.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lautrette A, Li S, Alili R, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 21.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 22.Zhou BB, Fridman JS, Liu X, et al. ADAM proteases, ErbB pathways and cancer. Expert Opin Investig Drugs. 2005;14:591–606. doi: 10.1517/13543784.14.6.591. [DOI] [PubMed] [Google Scholar]
- 23.Dhawan P, Peng X, Sutton AL, et al. Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase. Mol Cell Biol. 2005;25:472–487. doi: 10.1128/MCB.25.1.472-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutierrez S, Javed A, Tennant DK, et al. CCAAT/enhancer-binding proteins (C/EBP) beta and delta activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J Biol Chem. 2002;277:1316–1323. doi: 10.1074/jbc.M106611200. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y, Studzinski GP. Retinoblastoma protein and CCAAT/enhancer-binding protein beta are required for 1,25-dihydroxyvitamin D3-induced monocytic differentiation of HL60 cells. Cancer Res. 2004;64:370–377. doi: 10.1158/0008-5472.can-03-3029. [DOI] [PubMed] [Google Scholar]
- 26.Esteban L, Vidal M, Dusso A. 1alpha-Hydroxylase transactivation by gamma-interferon in murine macrophages requires enhanced C/EBPbeta expression and activation. J Steroid Biochem Mol Biol. 2004;89–90:131–137. doi: 10.1016/j.jsbmb.2004.03.092. [DOI] [PubMed] [Google Scholar]
- 27.Lou YR, Molnar F, Perakyla M, et al. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol. 2010;118:162–170. doi: 10.1016/j.jsbmb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Martin KJ, Gonzalez EA, Gellens ME, et al. Therapy of secondary hyperparathyroidism with 19-nor-1alpha,25-dihydroxyvitamin D2. Am J Kidney Dis. 1998;32(2 Suppl 2):S61–S66. doi: 10.1053/ajkd.1998.v32.pm9808145. [DOI] [PubMed] [Google Scholar]
- 29.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 30.Santiago-Josefat B, Esselens C, Bech-Serra JJ, et al. Post-transcriptional up-regulation of ADAM17 upon epidermal growth factor receptor activation and in breast tumors. J Biol Chem. 2007;282:8325–8331. doi: 10.1074/jbc.M608826200. [DOI] [PubMed] [Google Scholar]
- 31.Obeid D, Nguyen J, Lesavre P, et al. Differential regulation of tumor necrosis factor-alpha-converting enzyme and angiotensin-converting enzyme by type I and II interferons in human normal and leukemic myeloid cells. Oncogene. 2007;26:102–110. doi: 10.1038/sj.onc.1209779. [DOI] [PubMed] [Google Scholar]
- 32.Fan H, Derynck R. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahnow CA, Cardiff RD, Laucirica R, et al. A role for CCAAT/enhancer binding protein beta-liver-enriched inhibitory protein in mammary epithelial cell proliferation. Cancer Res. 2001;61:261–269. [PubMed] [Google Scholar]
- 34.Hoenderop JG, van der Kemp AW, Urben CM, et al. Effects of vitamin D compounds on renal and intestinal Ca2+ transport proteins in 25-hydroxyvitamin D3–1alpha-hydroxylase knockout mice. Kidney Int. 2004;66:1082–1089. doi: 10.1111/j.1523-1755.2004.00858.x. [DOI] [PubMed] [Google Scholar]
- 35.Munetsuna E, Nakabayashi S, Kawanami R, et al. Mechanism of the anti-proliferative action of 25-hydroxy-19-nor-vitamin D(3) in human prostate cells. J Mol Endocrinol. 2011;47:209–218. doi: 10.1530/JME-11-0008. [DOI] [PubMed] [Google Scholar]
- 36.Dusso A, Gonzalez EA, Martin KJ. Vitamin D in chronic kidney disease. Best Pract Res Clin Endocrinol Metab. 2011;25:647–655. doi: 10.1016/j.beem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham J, Locatelli F, Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. doi: 10.2215/CJN.06040710. [DOI] [PubMed] [Google Scholar]
- 38.Moe SM, Saifullah A, LaClair RE, et al. A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:299–306. doi: 10.2215/CJN.07131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Aly Z, Qazi RA, Gonzalez EA, et al. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50:59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Zisman AL, Hristova M, Ho LT, et al. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 41.Chandra P, Binongo JN, Ziegler TR, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14:10–17. doi: 10.4158/EP.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallieni M, Kamimura S, Ahmed A, et al. Kinetics of monocyte 1 alpha-hydroxylase in renal failure. Am J Physiol. 1995;268(4 Pt 2):F746–F753. doi: 10.1152/ajprenal.1995.268.4.F746. [DOI] [PubMed] [Google Scholar]
- 43.Takemoto F, Shinki T, Yokoyama K, et al. Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int. 2003;64:414–420. doi: 10.1046/j.1523-1755.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 44.Knutson A, Hellman P, Akerstrom G, et al. Characterization of the human Megalin/LRP-2 promoter in vitro and in primary parathyroid cells. DNA Cell Biol. 1998;17:551–560. doi: 10.1089/dna.1998.17.551. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, Yu WR, Carling T, et al. Regulation of gp330/megalin expression by vitamins A and D. Eur J Clin Invest. 1998;28:100–107. doi: 10.1046/j.1365-2362.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 46.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int. 2011;79:715–729. doi: 10.1038/ki.2010.543. [DOI] [PubMed] [Google Scholar]
- 47.Melenhorst WB, Visser L, Timmer A, et al. ADAM17 upregulation in human renal disease: a role in modulating TGF-alpha availability? Am J Physiol Renal Physiol. 2009;297:F781–F790. doi: 10.1152/ajprenal.90610.2008. [DOI] [PubMed] [Google Scholar]