Abstract
The double-deficit hypothesis of dyslexia posits that both rapid naming and phonological impairments can cause reading difficulties, and that individuals who have both of these deficits show greater reading impairments compared to those with a single deficit. Despite extensive behavioral research, the brain basis of poor reading with a double-deficit has never been investigated. The goal of the study was to evaluate the double-deficit hypothesis using functional MRI. Activation patterns during a printed word rhyme judgment task in 90 children with a wide range of reading abilities showed dissociation between brain regions that were sensitive to phonological awareness (left inferior frontal and inferior parietal regions) and rapid naming (right cerebellar lobule VI). More specifically, the double-deficit group showed less activation in the fronto-parietal reading network compared to children with only a deficit in phonological awareness, who in turn showed less activation than the typically-reading group. On the other hand, the double-deficit group showed less cerebellar activation compared to children with only a rapid naming deficit, who in turn showed less activation than the typically-reading children. Functional connectivity analyses revealed that bilateral prefrontal regions were key for linking brain regions associated with phonological awareness and rapid naming, with the double-deficit group being the most aberrant in their connectivity. Our study provides the first functional neuroanatomical evidence for the double-deficit hypothesis of developmental dyslexia.
Keywords: developmental dyslexia, double deficit, reading, fMRI, phonological processing
1. Introduction
Reading provides one of the most significant gateways to knowledge (Gabrieli, 2009) and is a critical skill in modern societies. However, dyslexia affects approximately 5-17% of children, making it the most common learning disability (Shaywitz, 1998). Dyslexia is a developmental condition characterized by marked yet unexpected difficulty in learning to read despite sufficient cognitive ability, effort, and opportunity (Shaywitz & Shaywitz, 2005). Dyslexia is typically diagnosed in second or third grade (or later), once children have failed to learn to read as expected; therefore, children may be exposed to repeated academic failure before diagnosis (Fletcher et al., 2006; Shaywitz et al., 2007). Children with dyslexia can experience a host of social and emotional problems secondary to reading and associated academic difficulties (Brooks, 2001; Fletcher et al., 2006; Gerber et al., 1990), and both dyslexia and its associated negative outcomes can persist into adulthood (Raskind et al., 1999).
Despite the prevalence and severe consequences of dyslexia, its underlying causes are not yet fully clear. It is widely believed that dyslexia reflects an underlying weakness in phonological processing, specifically phonological awareness (PA; the ability to recognize and manipulate the sound structure of words) (Bradley & Bryant, 1978; Snowling et al., 1996; Wagner & Torgensen, 1987). PA is important for mapping sound-to-letter correspondences for decoding and spelling, and is associated with later reading skills such as orthographic awareness and comprehension (Torgesen et al., 1997).
Deficits in PA alone do not account for all cases of dyslexia (e.g., Lovett, Steinbach & Frijters, 2000). Rapid automatized naming (RAN) deficits are also evident in a subset of individuals with developmental dyslexia (Ackerman & Dykman, 1993; Badian, 1995; Bowers et al., 1988; Katzir et al., 2008; Scarborough, 1998). RAN, sometimes referred to as naming speed or rapid naming, is the speed with which one can name visually-presented familiar stimuli such as letters, numbers, colors and objects out loud (Denckla & Rudel, 1976), and reflects the automaticity of processes which are also important for reading (Norton & Wolf, 2012).
The double-deficit hypothesis (Wolf & Bowers, 1999) posits that RAN is an independent core deficit that can cause reading difficulties, in addition to or in the absence of the phonological processing deficits seen in many individuals with developmental dyslexia. According to this theory, impairments in either RAN or PA can cause reading difficulties, and individuals with a ‘double-deficit’ have more severe deficits in reading than those with single deficits (Wolf & Bowers, 1999). Individuals with a RAN deficit may perform in the typical range on untimed tests of word reading accuracy, but show particular impairment on timed relative to untimed reading measures (Waber et al., 2004; Wolf, Bowers & Biddle, 2000).
Some researchers hold that RAN fits under the umbrella of phonological processing skills (Wagner et al., 1993; Wagner & Torgesen, 1987); however, there are several lines of evidence suggesting that RAN and PA deficits are independent (for review see Wolf & Bowers, 1999; Norton & Wolf, 2012). Correlations between RAN and phonological tasks are modest in both typical readers and individuals with dyslexia, and RAN and PA load onto separate factors in factor analyses (Powell et al., 2007). Further, a proportion of poor readers demonstrate RAN deficits in the absence of phonological deficits (Lovett, 1987; Wolf et al., 2002).
Wolf and Bowers noted that the double-deficit hypothesis was proposed not to fully explain all reading difficulties, but rather to move the field forward in considering the possible subtypes and multiple etiologies of dyslexia. Many studies have found support for the double-deficit hypothesis in English (e.g., Compton, DeFries & Olson, 2001; King, Giess & Lombardino, 2007; Lovett et al., 2000; McBride-Chang & Manis, 1996; Miller et al., 2006) as well as in other languages (e.g., Dutch: Boets et al., 2010; Chinese: Ho et al., 2004; Greek: Papadopoulos, Georgiou & Kendeou, 2009; and Finnish: Torppa et al., 2012). A meta-analysis of the literature on the double-deficit hypothesis identified several limitations of past research including problems with inconsistencies regarding the presence of a single deficit in RAN, and the inherent problems in trying to establish the independence of two skills that are positively correlated (Vukovic & Siegel, 2006; see also Schatschneider et al., 2002). This meta-analysis emphasized the importance of further sound research before conclusions can be made about the double-deficit hypothesis, and indeed, better clinical and educational decisions could be made if the relations among phonological processing, RAN, and dyslexia were better understood.
Heretofore the functional neural mechanisms underlying the double-deficit hypothesis have never been explored, perhaps in part because the pathophysiology of dyslexia is still not fully understood. There is, however, increasing evidence to suggest that the reading difficulties experienced by individuals with dyslexia have neurobiological substrates, and that there may be observable differences in the brain basis of phonological versus RAN deficits. Functional magnetic resonance imaging (fMRI) studies have identified brain regions critical to skilled reading, and differential functioning has been observed in dyslexia in each region (reviewed in Gabrieli, 2009; Maisog et al., 2008; Richlan et al., 2009).
The brain's “reading network” is typically described as including three main regions: left hemisphere occipito-temporal, temporo-parietal, and inferior frontal areas. The occipito-temporal region encompasses the visual word form area (VWFA) of the fusiform gyrus, which is believed to support the automatic identification of printed words (Schlaggar & McCandliss, 2007). The temporo-parietal region (including the inferior parietal lobule, or IPL) is involved in phonological storage and retrieval (Vigneau et al., 2006), as well as the integration of orthography and phonology (Newman & Joanisse, 2011). Anomalous function in this brain region would be expected to compromise the phonological and phonological-to-orthographic mapping processes essential for developing successful reading. Decreased functional activation and connectivity in these left posterior brain systems (temporo-parietal and occipito-temporal regions) seems to be related to the pathophysiology of dyslexia rather than to current level of reading ability (Hoeft et al., 2006; Hoeft et al., 2007; Saygin et al., 2013). The left inferior frontal gyrus (IFG), particularly the pars triangularis (IFGtri) and opercularis (IFGop) aspects of IFG, is important for articulation and naming (Fiez & Petersen, 1998; Gaillard et al., 2001; Gaillard et al., 2003; Shankweiler et al., 2008) and phonological processing (Pugh et al., 2000; Vigneau et al., 2006). Findings regarding the IFG's role in dyslexia have been mixed, showing both hypo- and hyper-activation in poor readers (Brunswick et al., 1999; Georgiewa, 1999; Maisog et al., 2008; Richlan et al., 2009; Richlan, 2012). In contrast to the reduced connectivity among posterior reading regions, in dyslexia, connectivity to inferior frontal areas is increased (Finn et al., 2013).
Phonological processing has been repeatedly associated with inferior frontal and temporo-parietal regions of the reading network. The brain basis of naming speed however, is not yet well understood. Only one published study has asked participants to complete a rapid naming task during fMRI, and found that as compared to rest, silent rapid naming elicited a diffuse and bilateral pattern of activation (Misra et al., 2004). Perhaps in part because of the challenge of adapting RAN tasks to the MRI environment, other studies have examined how RAN skill measured outside the scanner correlates with neuroanatomical (Eckert et al., 2003; He et al., 2013) and neurofunctional patterns (Turkeltaub et al., 2003). Although many regions were related to rapid naming in these studies, commonly reported regions across studies included the left IFG and right cerebellar hemisphere. These same regions uniquely differentiate readers who have a RAN deficit from those who do not: in a study that used multivariate analyses to classify brains as belonging to a group with dyslexia or a control group, the best classifiers were IFG pars triangularis and right cerebellum (Eckert et al., 2003); importantly, 94% of the individuals correctly classified as having dyslexia had a RAN deficit. In another study, the most accurate classifier of whether an individual had dyslexia was right cerebellum (Pernet et al., 2009).
Though it is not commonly considered part of the “reading network,” atypical cerebellum function has been proposed as a primary cause of dyslexia (Nicolson, Fawcett & Dean, 2001). Meta-analyses of neuroimaging studies of dyslexia reveal that the right cerebellar lobule VI is associated with both structural and functional abnormalities in dyslexia (Linkersdörfer et al., 2012). Right cerebellar lobule VI plays a role in motor, linguistic, and working memory processes (Stoodley & Schmahmann, 2009), and has connections to left IPL and IFG, which may support the automaticity required for fluent reading Bernard et al., 2012). In contrast, other sections of the cerebellum connect to more dorsal or medial regions of the cerebrum.
Though studies have begun to examine the correlates of RAN, one outstanding question is whether individuals with different deficits or subtypes of dyslexia (phonological vs. rapid naming) recruit different regions of the brain during reading and reading related activities. Findings from an fMRI study involving an implicit reading task (Turkeltaub et al., 2003), in conjunction with neuroanatomical studies (He et al., 2013; Eckert et al., 2003) found that phonological processing and RAN abilities were correlated with brain patterns in anatomically distinct brain regions. Though these findings point to separable contributions of both phonological processing and RAN in the reading brain, single versus double-deficits were not examined in these studies.
The main goal of the present analyses was to examine whether there are patterns of brain activation consistent with the predictions of the double-deficit hypothesis. Data for these analyses come from a larger study focused on examining phonological processing and the relationships between behavioral measures and brain activation in children. The larger study was not focused on the double-deficit hypothesis per se, yet these data provide an opportunity to investigate whether patterns of brain activation support this theory, thus far only shown behaviorally. We compared brain activations during a word reading task with a phonological decision (rhyme judgment) among four groups of school-age readers: typical readers (Control), RAN deficit with no phonological deficit (RANdef), phonological processing deficit with no RAN deficit (PHONOdef), and double-deficit in both PA and RAN (DOUBLEdef). We were especially interested in examining whether there are brain regions and connectivity patterns that show significant dysfunction in the DOUBLEdef group compared to the single deficit groups (both PHONOdef and RANdef groups). In summary, we investigated the following research questions:
Is there support for the double-deficit hypothesis in which distinct patterns of brain activation are evident for the Control, RANdef, PHONOdef, and DOUBLEdef groups? To this end, we examined which brain areas demonstrate a phonological or rapid naming “gradient” that would be consistent with the double-deficit hypothesis. That is, for both phonology and rapid naming, in which brain areas does the control group have greater activation than children with a single deficit, and in which areas do children with a single deficit have greater activation than children with the double deficit?
Within brain areas that show a phonological or rapid naming gradient, are there post-hoc differences that might elucidate the relationship between groups? Do individual PA and RAN scores also correlate with activations in these gradient regions of interest for phonology and rapid naming, respectively, as a complement to the group gradient analysis?
Is there also evidence for differences among the four double-deficit hypothesis groups from the brain's functional connectivity?
If it is the case that the double-deficit hypothesis is invalid and that RAN is simply a form of phonological processing, then activation patterns in the RANdef, PHONOdef, DOUBLEdef group should be similar. If on the other hand, we find evidence that supports the double-deficit hypothesis, we would see different, atypical patterns in the RANdef, PHONOdef and DOUBLEdef groups, with the DOUBLEdef groups showing the greatest atypicality. For this reason, we were specifically interested in interrogating the patterns of brain activation elicited from a phonological processing task, as a way to examine whether and how the RANdef group shows abnormality during phonological processing. Accordingly, we hypothesized that during phonological processing, the DOUBLEdef group will show significant deficits in the left IFG and IPL as well as prefrontal and cerebellar regions. We also hypothesized that there will be a “phonological gradient” in the left frontoparietal reading network (IFG and IPL regions), that is, significantly reduced brain activation in the DOUBLEdef group as compared to the PHONOdef group and reduced activation in the PHONOdef group compared to the Control group. On the other hand, we hypothesized that there will also be a “rapid naming gradient” in the left IFGtri and right cerebellar lobule VI, i.e., the DOUBLEdef group will show significantly reduced brain activation compared to the RANdef group, who in turn will show reduced activation compared to the Control group.
2. Material and Methods
2.1. Participants
Participants included 90 right-handed, native English-speaking children (54 females, age 8.2 to 12.6 years). Poor readers were participants in a larger behavioral study (Torgesen et al., 2006), which enrolled third- and fifth-graders with a wide range of reading ability (initially identified by their teachers as poor readers) from public schools near Pittsburgh, Pennsylvania; good readers were recruited from the same schools. Participants were healthy and without any neurological or psychiatric disorders (e.g., brain injuries, attention deficit hyperactivity disorder [ADHD]), psychotropic medications and/or MRI contraindications (e.g., metal in their body). A subset of parents completed a questionnaire regarding their socio-economic status; among 56 parents who completed the questionnaire, the median household income was approximately $40,000. The University of Pittsburgh and Carnegie Mellon University Institutional Review Boards approved all protocols. Written informed consent and assent were collected from parents and children, respectively.
2.2. Behavioral Measures and Group Assignment
Performance on the Letters and the Numbers subtests of RAN-RAS Tests (Wolf & Denckla, 2005) was averaged to produce a composite age-based standard score (ss) that was used to determine the presence (RANdef and DOUBLEdef < 90ss) or absence (Control and PHONOdef > 90ss) of RAN deficits. Performance on the Elision and Blending Words subtests of the Comprehensive Test of Phonological Processing (CTOPP; Wagner et al., 1999) was averaged to produce a composite ss that was used to determine the presence (PHONOdef and DOUBLEdef < 90ss) or absence (Control and RANdef > 90ss) of phonological deficits. The criterion of a standard score below 90, equivalent to the 25th percentile, is frequently employed in studies of dyslexia (e.g., Foorman et al., 1997; Shaywitz et al., 2002).
Participants were assigned to one of four groups based on their behavioral scores: a non-impaired group of typical children (Control, N=39), a group of children with a deficit only in phonological awareness (PHONOdef, N=27), a group of children with a deficit only in rapid automatized naming (RANdef, N=10), and a group of children with deficits in both phonological awareness and rapid automatized naming (i.e., those with a double-deficit, DOUBLEdef, N=14) (Table 1). As substantial evidence suggests that the abilities of poor readers and the brain basis of reading are independent of IQ (see Tanaka et al., 2011), we did not consider IQ-ability discrepancy in group assignment.
Table 1.
Demographic information, standard scores, and post-hoc comparisons for each group.
| Group |
||||||
|---|---|---|---|---|---|---|
| Control [mean (SD)] | RANdef [mean (SD)] | PHON Odef [mean (SD)] | DOUBL Edef [mean (SD)] | ANOVA [F; p] | Post hoc (p) | |
| Age | 9.77 (1.0) | 9.85(1.1) | 10.51 (1.0) | 10.51 (1.1) | 3.68; 0.02 | 0.99a; 0.03b; 0.11c; 0.32d; 0.43e; 1.00f |
| Gender | 24 female/15 male | 6 female/4 male | 15 female/12 male | 9 female/5 male | χ2 (3, N=90)= 0.37, p = 0.95 | |
| Task Accuracy (percent correct) | 89.9 (11.5) | 85.0 (10.5) | 87.2 (9.5) | 81.8 (11.5) | 2.09; 0.108 | |
| IQ (PPVT)-ss | 107.2 (12.4) | 107.8 (16.0) | 93.5 (12.6) | 93.6 (9.6) | 8.88; <0.001 | 0.99a; <0.001b; 0.006c; 0.01d; 0.04e; 1.00f |
| RAN-ss | 101.8 (8.0) | 84.6 (4.4) | 102.9 (7.0) | 84.5 (4.2) | 38.35; <0.001 | <0.001a; 0.93b; <0.001c; <0.001d; 1.00e; <0.001f |
| PA (CTOPP)-ss | 104.8 (11.9) | 100.0 (11.7) | 79.0 (5.9) | 77.3 (6.6) | 51.81; <0.001 | 0.51a; <0.001b; <0.001c; <0.001d; <0.001e; 0.95f |
| Word ID-ss | 104.7 (12.4) | 100.5 (8.3) | 91.6 (6.6) | 86.5 (9.9) | 15.28; <0.001 | 0.65a; <0.001b; <0.001c; 0.09d; 0.007e; 0.43f |
| Word Attack-ss | 109.7 (13.9) | 106.9 (10.4) | 93.0 (6.1) | 90.6 (9.3) | 17.88; <0.001 | 0.89a; <0.001b; <0.001c; 0.006d; 0.003e; 0.91f |
| Passage Comp-ss | 107.1 (11.8) | 103.2 (9.4) | 94.6 (10.0) | 90.5 (11.0) | 11.51; <0.001 | 0.74a; <0.001b; <0.001c; 0.16d; 0.03e; 0.67f |
| TOWRE-SWE-ss | 101.2 (15.5) | 90.2 (4.1) | 91.0 (9.2) | 85.9 (10.3) | 7.37; <0.001 | 0.61a; 0.07b; <0.001c; 0.99d; 0.83e; 0.57f |
| TOWRE-PDE-ss | 100.9 (15.6) | 93.4 (2.8) | 86.1 (10.2) | 80.9 (8.3) | 12.77; <0.001 | 0.31a; <0.001b; <0.001c; 0.39d; 0.07e; 0.56f |
Control vs RANdef
Control vs PHONOdef
Control vs DOUBLEdef
RANdef vs PHONOdef
RANdef vs DOUBLEdef
PHONOdef vs DOUBLEdef
Note: SS=standard score, computed for age. PPVT = Peabody Picture Vocabulary Test. RAN score is a mean of RAN-RAS rapid letter naming and number naming subtest standard scores. PA (CTOPP) is a composite phonological awareness standard score derived from CTOPP Elision and Blending Words subtests. SWE=sight word efficiency. PDE=phonemic decoding efficiency (nonword reading).
All participants also completed subtests of the Woodcock Reading Mastery Test-Revised/Normative Update (WRMT-R/NU) including Word Identification (Word ID), Passage Comprehension, and Word Attack to assess reading achievement, as well as the Peabody Picture Vocabulary Test (PPVT-3) to assess estimated IQ. PPVT is highly correlated (r = 0.90) with full-scale IQ scores from other measures such as the WISC (Dunn and Dunn, 1997).
2.3. fMRI Task Design
A block-design word-rhyme task, with alternating rhyme and rest conditions, was used in the fMRI scanner to assess brain activation associated with reading ability (described in detail in Hoeft et al., 2006; Hoeft et al., 2007; Tanaka et al., 2011). During the rhyme condition, participants read two visually presented words and judged whether they rhymed (e.g., bait, gate) or not (e.g., price, miss), indicating each response with a right- or left-handed button press, respectively. Word pairs were selected so that the visual appearance of the last letters of the two words could not be used to determine whether they rhymed. Stimuli were balanced for word frequency, number of letters, and syllables between rhyme and non-rhyme trials and across blocks. Each 6s trial consisted of a 4s presentation of two words followed by a 2s fixation cross. Each task block consisted of a 2s cue period followed by five trials (32s total). During rest blocks, participants saw a fixation cross on the screen for 15s. The entire scan was 234s (including two practice trials at the beginning) and consisted of four rhyme blocks and five rest blocks.
This word rhyming task was designed to elicit robust activation in phonological and reading regions; however, it requires us to interpret our findings in light of both reading and phonological processing, without being able to explicitly separate the two. In order to determine the specificity of our findings from the phonological reading task, another fMRI task of word reading with semantic processing was used as a control task (see details in Supplemental Text). The task was identical to that of phonological processing, except it asked participants to decide whether the two words belonged to the same semantic category. During the semantic condition, participants judged whether or not two visually presented words were both living (e.g., dog, boy) or not (e.g., desk, cat), and indicated each response with a right- or left-handed button press.
2.4. Image Acquisition
The fMRI imaging was performed at the Brain Imaging Research Center (CMU and University of Pittsburgh) with a 3.0 Tesla Siemens Allegra scanner (Siemens Medical, Malvern, PA). A T2*-weighted gradient echo, resonant echo planar pulse sequence sensitive to blood oxygen level-dependent contrast was used with the following acquisition parameters: repetition time (TR) 1,000ms, time to echo (TE) 30ms, flip-angle 60°, field of view (FOV) 20 × 20cm, matrix size 64 × 64, axial-oblique plane with 16 slices, and slice-thickness of 6mm with a 1-mm gap. In addition, a T1-weighted 3D-MPRAGE with the following parameters was acquired for registration purposes: TR = 2,000ms, TE = 3.34ms, flip-angle = 7°, dimensions = 256 × 256 × 160, axial plane, voxel-size = 1 × 1 × 1 mm.
2.5. fMRI Data Processing
Statistical analysis was performed with statistical parametric mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK). After image reconstruction, each participant's data were realigned to a reference volume and corrected for motion using both SPM and in-house tools including ArtRepair (http://www.nitrc.org/projects/art_repair/). Data were spatially normalized using normalization parameters obtained from the children's segmented gray matter images of high resolution T1 MRI normalized to standard template and applied to the mean functional image. Resultant images were resampled to 2×2×2mm voxels in Montreal Neurological Institute (MNI) stereotaxic space. Spatial smoothing was done with a 8mm Gaussian filter. Each participant's data were high pass filtered at 97s, and analyzed using a fixed effects model examining the task (rhyme); rest was not modeled and was included as implicit baseline.
2.6. fMRI Analyses
Phonological gradient
Conjunction analyses were performed (conjunction null method as in Nichols et al., 2005) with a random effects model (Friston et al., 1999) using the rhyme vs. rest contrast images to identify brain regions that showed both significantly greater activation for the Control group compared to the PHONOdef group, and significantly greater activation for the PHONOdef group compared to the DOUBLEdef group. We excluded the RANdef readers from the analysis as even though these individuals showed phonological awareness performance within typical range because we did not have specific hypothesis as to how the deficit in RAN processing would affect the results.
Analyses were performed in two ways: first, at a stringent corrected threshold within regions of interest (ROIs), and second, on the whole brain at a lower threshold. Two ROIs were combined to form one mask used for ROI analyses: (1) left inferior frontal gyrus (pars triangularis, pars opercularis), and (2) left temporo-parietal (IPL). These regions were selected because they were identified in previous neuroimaging reports as being involved in phonological processing and showed differences between typical readers and readers with dyslexia (Maisog et al., 2008; Meyler et al., 2007; Richlan et al., 2009; Shaywitz et al., 1998). Further, these were the only left-hemisphere regions related to dyslexia in a previous study using the same task (Hoeft et al., 2006).
Rapid naming gradient
Conjunction analysis was performed similar to the phonological gradient analysis, examining brain regions that showed significantly greater activation for the Control group compared to the RANdef group, and significantly greater activation for the RANdef group compared to the DOUBLEdef group. Similar to the phonological gradient analyses above, we excluded the PHONOdef readers from the analysis.
As in the phonological gradient, we examined ROIs in brain regions that show consistent differences related to RAN ability measured outside the scanner (Eckert et al., 2003; Turkeltaub et al., 2003) and have been shown to play a prominent role in dyslexia (Eckert et al., 2003; Linkersdörfer et al., 2012; Pernet et al., 2009). A mask was created from two regions defined as (1) left IFG (pars triangularis, pars opercularis), and (2) right cerebellar vermis lobule VI.
Gradient and whole-brain analysis methods and thresholds
ROIs were created using Automated Anatomical Labeling (AAL) in the WFU PickAtlas toolbox (http://fmri.wfubmc.edu/software/PickAtlas). For both the Phonological and rapid naming gradient conjunction analyses, a voxel-wise statistical threshold of p=0.05 for voxel-height followed by p=0.05 corrected for family-wise error (FWE) post small-volume correction (SVC) was applied on the overall mask. The more exploratory whole-brain analysis was conducted at a weaker threshold of p=0.001 uncorrected, extent threshold (ET)=0 voxels to examine specificity of our findings beyond the pre-defined ROIs.
For both gradient analyses, we further extracted contrast estimates from the clusters found to be significant and examined correlation with brain activation and PA and RAN scores. Statistical images were overlaid onto the MRIcroN template image for 3D viewing (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Peak coordinates of brain regions with significant effects were converted from MNI to Talairach space using the mni2tal function (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml). Brain regions were identified from these X, Y, Z coordinates using Talairach Daemon (http://www.talairach.org/daemon.html) and confirmed with the Talairach atlas (Talairach & Tournoux, 1988).
2.7. Functional Connectivity Analysis
Functional connectivity analysis was performed using the CONN Functional Connectivity Toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012; http://www.nitrc.org/projects/conn). These data were band-pass filtered (0.008-0.09 Hz), corrected for physiological noise and motion using an aCompCor strategy (Behzadi et al., 2007). A Hanning window was applied to the task blocks to examine task-specific functional connectivity. Similar strategies were applied to examine the resting block periods. ROIs were defined as 10mm diameter spheres centered at peaks of clusters that showed significant Phonological and RAN gradients, i.e., left IFG, IPL and right cerebellar lobule VI. Whole-brain analyses rather than ROI analyses were performed, as the nature of the analysis was more exploratory. Therefore, in the between-group comparisons, a stringent statistical threshold of p=0.01 for peak voxel and p=0.05 FWE corrected for cluster extent for the whole brain, was used. This more stringent threshold was designed to minimize type 1 errors across our analyses of multiple seed regions. Between-group analyses were performed using the same analytical strategies as in the main analyses above.
3. Results
3.1. Demographic and Behavioral Results
Demographic variables
Descriptive statistics for demographic characteristics and behavioral scores and ANOVA results are reported in Table 1. Group differences were computed via univariate ANOVAs, all F(3,86), with post-hoc comparisons using Tukey's HSD test. Initial demographic comparisons were made among the groups for gender and age. Comparisons were not needed for handedness as all participants were right-handed. There were no significant differences in the gender distribution of the four groups. ANOVA and post-hoc comparisons revealed that the PHONOdef group was significantly older than the Control group, but there were no other significant age differences among groups. The Control and RANdef groups scored higher than PHONOdef and DOUBLEdef groups on the PPVT, which was used as a proxy for IQ. A nonverbal IQ measure was not collected in these children; however, PPVT and full-scale IQ scores on the WISC are highly correlated at r = 0.90 (Dunn & Dunn, 1997). To ensure that IQ differences did not impact our brain imaging results, we computed partial correlations controlling for age and IQ (see Results and Supplementary Table 1). There were no significant differences among the groups for accuracy on the in-scanner rhyme judgment task.
Reading measures
As expected, the Control group performed significantly better than the PHONOdef and DOUBLEdef groups on standard measures of untimed reading accuracy, including WRMT-R Word Attack (phonological decoding), Word ID (single word reading), and Passage Comprehension (reading comprehension) (Table 1). The RANdef group also significantly outperformed PHONOdef and DOUBLEdef groups on all three of these untimed reading subtests, whereas reading achievement did not differ significantly between the Control and RANdef groups on untimed accuracy measures. The PHONOdef group's reading scores were higher than DOUBLEdef scores, but no comparison reached significance. Pairwise comparisons between these two groups demonstrated medium effect size for single-word reading and passage comprehension, whereas the effect size was small for PA and decoding (Word Attack) measures (effect size of all statistics reported in Table 2).
Table 2.
Effect sizes (Cohen's d) for pairwise comparisons.
| Control vs. RANdef | Control vs. PHONOdef | Control vs. DOUBLEdef | RANdef vs. PHONOdef | RANdef vs. DOUBLEdef | PHONOdef vs. DOUBLEdef | |
|---|---|---|---|---|---|---|
| Age | −0.33 | −0.78 | −0.76 | −0.45 | −0.43 | 0.01 |
| Task Accuracy |
0.43 | 0.25 | 0.70 | −0.23 | 0.29 | 0.53 |
| IQ | −0.05 | 1.10 | 1.15 | 1.06 | 1.13 | −0.01 |
| RAN | 2.31 | −0.14 | 2.39 | −2.83 | 0.01 | 2.95 |
| CTOPP | 0.40 | 2.60 | 2.54 | 2.70 | 2.51 | 0.28 |
| Word ID- ss |
0.36 | 1.25 | 1.54 | 1.26 | 1.50 | 0.65 |
| Word Attack-ss |
0.21 | 1.46 | 1.48 | 1.86 | 1.67 | 0.33 |
| Passage Comp-ss |
0.35 | 1.12 | 1.44 | 0.87 | 1.23 | 0.40 |
| TOWRE SWE-ss |
0.78 | 0.76 | 1.07 | −0.10 | 0.52 | 0.54 |
| TOWRE PDE-ss |
0.53 | 1.08 | 1.42 | 0.81 | 1.90 | 0.55 |
The Control group scored significantly better than the DOUBLEdef group on both timed reading measures, and better than the PHONOdef group on the TOWRE Phonemic Decoding Efficiency measure (see Table 1 for statistics and Table 2 for effect sizes). RANdef and PHONOdef groups did not differ significantly on the timed reading measures. The control group outperformed the RANdef group on the two measures (by an average of 0.73 SD for SWE, and 0.50 SD for PDE), but these effects were not significant. However, whereas the RANdef group performed in the average range on untimed measures of reading, they scored significantly lower on timed as compared to untimed measures of word reading, as predicted by the double-deficit hypothesis (paired samples t-test for SWE vs. Word ID, t(90)=2.75, p=0.007; for PDE vs. Word Attack, t(89)=10.18, p<0.001).
In-scanner motion
Across groups, motion artifacts were identified in 4.9% of timepoints (SD=8.1). The four groups did not differ in the number of fMRI timepoints with motion artifiacts (independent samples Kruskal-Wallis test, p=0.29).
3.2. fMRI Results: Gradient Effect for Phonological Processing
Brain activation patterns during phonological processing compared to resting fixation in all participants as a group showed a typical activation pattern including the left temporo-parietal, bilateral occipito-temporal and bilateral inferior frontal regions (left > right).
Gradient analysis in ROI mask
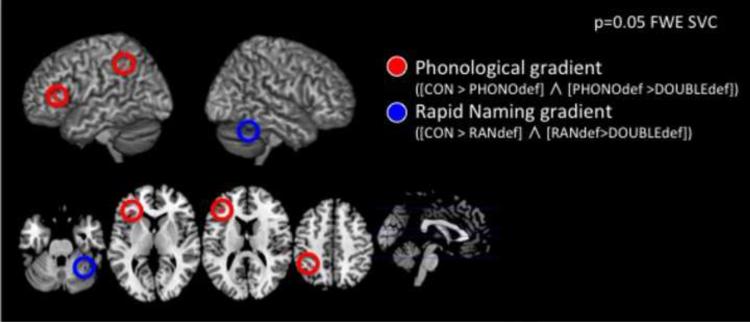
Conjunction analysis examining brain regions that showed significantly greater activation in both the Control group compared to the PHONOdef group, and the PHONOdef group compared to the DOUBLEdef group showed significant effects in the left IPL (Talairach coordinates X=−38, Y=−44, Z=45, p=0.041 corrected, z=3.46) and left IFG (Talairach coordinates X=−36, Y=33, Z=11, p=0.015 corrected, z=3.95) (Fig. 1a,b red).
Figure 1. Brain activation differences between groups.
a. Brain regions in red show reduced activation in both children with deficits in phonological awareness (PA, PHONOdef) compared to controls (CON) and children with double-deficit (DOUBLEdef) compared to PHONOdef (Controls > PHONOdef > DOUBLEdef). Statistical threshold was set at p = 0.05 family-wise error (FWE) corrected after small volume correction (SVC). Brain regions in blue show reduced activation in both children with deficits in rapid naming (RANdef) compared to controls (CON) and children with double-deficit (DOUBLEdef) compared to RANdef.
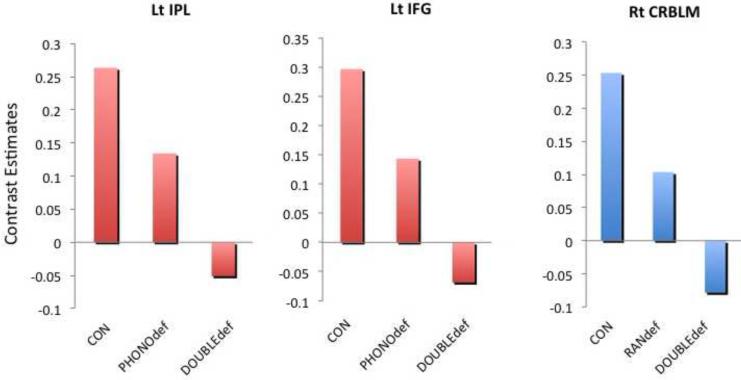
b. Mean average contrast estimates of each cluster in Figure 1a was extracted for each child and plotted (left inferior parietal lobule = Lt IPL, left inferior frontal gyrus = Lt IFG, right cerebellum= Rt CRBLM).
Group ROI analysis
When the mean average parameter estimates were extracted from the two clusters (left IPL and IFG), there were no significant differences between the Control group and the RANdef group in the left IPL (t (47)=1.13, p=0.26) and left IFG (t (47)=0.56, p=0.58), further confirming the specificity of the phonological gradient effect in these regions.
Correlation between ROI activation and PA skills
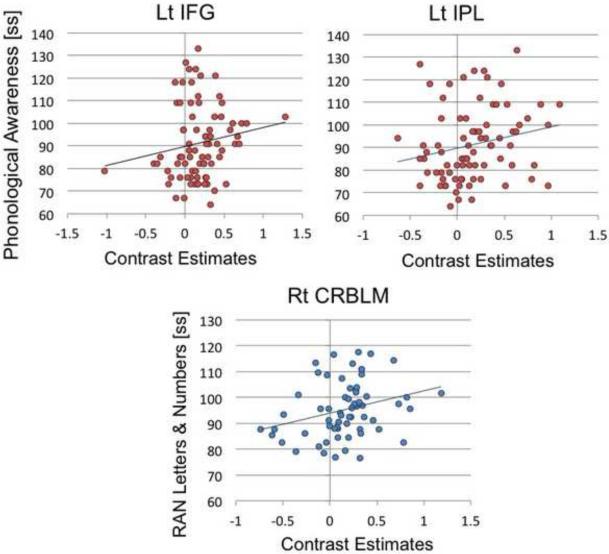
Correlation analyses partially supported the results from the conjunction analysis. The left IPL showed significant positive association between parameter estimates and PA skills (N=80 excluding the RANdef group: r=0.25, p=0.025, Fig. 2; N=90 including all 4 groups: r=0.25, p=0.017). The left IFG however, did not reach significance with regard to association between brain activation and PA skills (N=80 excluding the RANdef group: r=0.15, p=0.15, Fig. 2; N=90 including all 4 groups: r=0.20, p=0.065). Partial correlations between PA scores and activation in these ROIs controlling for RAN, age, and verbal IQ all showed similar results (see Supplemental Table 1.)
Figure 2. Associations between brain activation and reading-related measures in clusters identified in Figure 1.
Association between brain activation in the left inferior frontal gyrus (Lt IFG) and phonological awareness scores (p=0.15; left), between brain activation in the left inferior parietal lobule (p=0.025; Left IPL) and phonological awareness scores (p=0.017; middle), and between brain activation in the right cerebellum (Right CRBLM) and rapid automatized naming (RAN) scores (p=0.025; right).
Whole-brain analysis
When results of conjunction analysis were examined at a more lenient threshold across the whole brain, the only additional regions that showed the phonological gradient effect were observed in left precentral gyrus (PreCG), and additional clusters in the left IPL and IFG (Table 3, sFig. 1 red).
Table 3.
Areas of activation for whole-brain gradient analyses.
| Brain Region | Brodmann Area | Talairach Coordinates | T score | Cluster Size (voxels) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Phonological Gradient (Control > PHONOdef > DOUBLEdef) | ||||||
| Left Inferior Frontal Gyrus | 46, 47 | −36 | 34 | 11 | 3.85 | 147 |
| −34 | 31 | 4 | 3.59 | |||
| Left Precentral Gyrus | 6 | −40 | −6 | 44 | 3.75 | 63 |
| Left Inferior Parietal Lobule | 40 | −38 | −44 | 45 | 3.46 | 19 |
| Left Inferior Parietal Lobule | 40 | −63 | −28 | 31 | 3.39 | 12 |
| Left Inferior Frontal Gyrus | −38 | 5 | 31 | 3.19 | 4 | |
| Rapid Naming Gradient (Control > RANdef > DOUBLEdef) | ||||||
| Left Inferior Parietal Lobule | 28, 40 | −61 | −28 | 34 | 3.44 | 28 |
| −55 | −33 | 41 | 3.19 | |||
| Right Cerebellar Lobule VI, Culmen | -- | 36 | −44 | −26 | 3.35 | 7 |
3.3. fMRI Results: Gradient Effect for Rapid Naming
Gradient analysis in ROI-mask
We performed a conjunction analysis to determine whether our hypothesized regions showed a rapid naming gradient, that is: greater activation in both the Control group compared to the RANdef group, and the RANdef group compared to DOUBLEdef group. A significant gradient effect was identified in the right cerebellar lobule VI (Talairach coordinates X=36, Y=−53, Z=−21, p=0.044 corrected, z=3.35) (Fig. 1a,b blue). No significant gradient effect was observed in the left IFG.
Group ROI analysis
Examination of the extracted mean parameter estimates from the right cerebellar lobule VI showed no significant difference between the Control and the PHONOdef groups, further confirming the rapid naming gradient effect in this region t(64)=0.039, p=0.97).
Correlation between ROI activation and RAN skills
Supporting the results from the conjunction analysis, brain activation in the right cerebellar (right CRBLM) lobule VI showed a significant positive association between parameter estimates and RAN skills (N=63 excluding the PHONOdef group: r=0.28, p=0.025, Fig. 2; N=90 including all 4 groups: r=0.23, p=0.031). Partial correlations between RAN scores and activation in the ROI controlling for PA, age, and verbal IQ all showed similar results (see Supplemental Table 1).
Whole-brain analysis
When results of the conjunction analysis were examined across the whole brain at a more lenient threshold of p=0.001 uncorrected, ET=0 voxels, the only additional region that showed the rapid naming gradient effect was observed in the left IPL, overlapping with the second left IPL cluster that also showed a phonological gradient effect at this threshold (Table 3, sFig. 1 blue).
3.4. Functional Connectivity Analyses
Within-group analyses
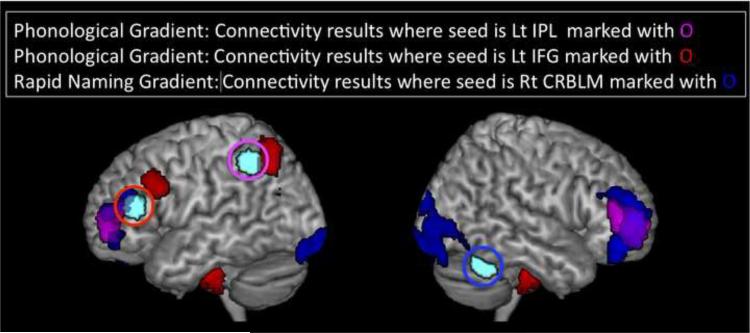
We examined functional connectivity within each group using seed regions that showed gradient effects in our fMRI analyses. The left IPL seed mainly showed associations with bilateral IFG and PreCG as well as temporal regions in the Control group (sFig. 2a). The left IFG seed connected with bilateral PreCG, IPL and temporal regions, heavily overlapping with the left IPL connectivity results in the Control group (sFig. 2b). On the other hand, seed-based connectivity analysis of the right cerebellum lobule VI from the rapid naming gradient showed very different patterns. The right cerebellum time-series during the task condition primarily correlated with that of bilateral occipital cortices and dorsolateral prefrontal cortex (DLPFC) (sFig. 2c). Within group analyses for other groups showed similar patterns (sFig. 3).
Between-group analyses
We also observed significant between-group differences in functional connectivity patterns. When the seed was placed in the left IPL region derived from the phonological gradient, functional connectivity with bilateral DLPFC was significantly reduced in groups with deficits in phonological processing, i.e., in the PHONOdef and DOUBLEdef compared to the Control and RANdef groups (Fig. 3, Table 4). Similarly, when the seed was placed in the left IFG, functional connectivity with left PreCG and IPL was significantly reduced in the groups with a deficit in phonological processing, i.e., PHONOdef and DOUBLEdef compared to the Control and RANdef groups, (Fig. 3, Table 4). When the seed was placed in the right cerebellar lobule VI identified in the rapid naming gradient, functional connectivity with bilateral DLPFC and occipital regions was significantly reduced in the groups with a deficit in rapid naming, i.e., in the RANdef and DOUBLEdef compared to the Control and PHONOdef groups (Fig. 3, Table 4).
Figure 3. Between group differences in functional connectivity.
Open circles indicate seeds used for functional connectivity where the peak coordinates were derived from Figure 1. Red regions showed activation for CON and RANdef > PHONOdef and DOUBLEdef in functional connectivity during task blocks when a seed was placed in the left inferior frontal gyrus (Lt IFG), violet regions indicate regions that showed CON and RANdef > PHONOdef and DOUBLEdef when a seed was placed in the left inferior parietal lobule (Lt IPL), and blue regions indicate regions that showed CON and PHONOdef > RANdef and DOUBLEdef when a seed was placed in the right cerebellar lobule VI (Right CRBLM). There are large overlaps in bilateral PFC with the left IFG and Right CRBLM functional connectivity networks during task blocks. Seed regions are marked with large cyan spheres for visual display only; the actual seeds were 10mm diameter.
Table 4.
Between-group functional connectivity results
| Brain Region | Brodmann Area | Talairach Coordinates | T score | Cluster Size (voxels) | p value (FWE) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Seed: Left Inferior Frontal Gyrus (IFG) | |||||||
| Left Superior, Inferior Parietal Lobules | 7 | −34 | −61 | 58 | 4.22 | 912 | 0.003 |
| −34 | −53 | 63 | 3.94 | ||||
| −34 | −58 | 47 | 3.71 | ||||
| Left Middle Frontal Gyrus | 9, 46 | −34 | 11 | 29 | 3.98 | 763 | 0.008 |
| −46 | 19 | 25 | 3.69 | ||||
| −53 | 25 | 30 | 3.21 | ||||
| Seed: Left Inferior Parietal Lobule (IPL) | |||||||
| Right Superior, Middle Frontal Gyri | 10 | 34 | 64 | −7 | 4.31 | 1247 | <0.001 |
| 40 | 60 | −10 | 4.11 | ||||
| 36 | 54 | −4 | 3.99 | ||||
| Left Middle Frontal Gyrus | 10 | −32 | 43 | 7 | 4.09 | 1104 | 0.001 |
| −32 | 55 | 6 | 3.84 | ||||
| −34 | 52 | −8 | 3.58 | ||||
| Seed: Right Cerebellum Lobule VI (CRBLM) | |||||||
| Right Middle Frontal Gyrus | 10, 11 | 34 | 58 | 3 | 4.38 | 803 | 0.009 |
| 36 | 58 | −13 | 3.54 | ||||
| 38 | 46 | −16 | 3.29 | ||||
| Right Middle, Inferior Occipital Gyri, Cuneus | 18, 19 | 36 | −87 | 4 | 3.82 | 1592 | <0.001 |
| 32 | −88 | −12 | 3.73 | ||||
| 16 | −94 | 23 | 3.67 | ||||
| Left Middle, Superior Frontal Gyri | 10 | −32 | 42 | 15 | 3.45 | 587 | 0.047 |
| −32 | 55 | 10 | 3.42 | ||||
| −32 | 39 | 0 | 3.30 | ||||
| Left Lingual, Middle Occipital Gyri | 17, 18, 19 | −10 | −93 | −2 | 3.40 | 1028 | 0.002 |
| −10 | −98 | −10 | 3.33 | ||||
| −34 | −91 | 8 | 3.22 | ||||
3.5. Specificity of phonological and rapid naming gradients
Whole-brain analyses based on a similar printed word semantic decision task, rather than rhyme decision, revealed no effects of a phonological gradient in the left IFG and left IPL regions (p>0.05 corrected). Likewise, when whole brain analysis was performed on the semantic fMRI task, there were no effects of a rapid naming gradient in the right cerebellum. However, when the left IFG, left IPL and right cerebellum regions defined from the rhyme task results were applied to the semantic task, we found that in all of these regions, the DOUBLEdef group showed significantly reduced activation compared to the PHONOdef group, whereas the PHONOdef group showed no significant difference in semantic activation compared to the Control group.
4. Discussion
This study examined functional brain correlates of word reading and phonological processing in children with a wide range of PA and RAN abilities. Performance on standardized reading tests and brain activation profiles during an in-scanner word reading and rhyme judgment task that recruited phonological processing skills were compared among four groups of readers (typical readers [Control], those with a rapid naming deficit only [RANdef], a phonological processing deficit only [PHONOdef] and those with a double-deficit in both PA and RAN [DOUBLEdef]).
Behavioral measures revealed that the group of children with deficits in both PA and RAN skills (i.e., children with a double-deficit, DOUBLEdef) scored the lowest on all standardized measures of reading ability administered here. These measures included untimed tests of Word ID (single word reading), Word Attack (phonological decoding), and Passage Comprehension of the WRMT-R, as well as timed Sight Word and Phonological Decoding Efficiency subtests of the TOWRE. The differences in reading ability between the DOUBLEdef and the phonology-impaired group (PHONOdef) were not significant, and the effect sizes were small to medium (Cohen's d from 0.33 to 0.65). The lack of significant behavioral differences between the single deficit groups and the DOUBLEdef groups in our sample could be due to the large variance in reading scores and our small sample size relative to the larger samples of most strictly behavioral studies. Previous behavioral studies found mixed results about the relative impairment from one versus two deficits. Individuals with a double deficit often do not show greater impairments in reading compared to individuals with a single phonological deficit (Sunseth & Bowers, 2002; Vaessen et al., 2009; Vukovic et al., 2004). Though children in the DOUBLEdef group had two deficits, it is not the case that multiple deficits always lead to the lowest performance; for example, children with low IQ and dyslexia do not perform worse on reading measures than children with typical IQ and dyslexia (Tanaka et al., 2011). On the other hand, some research finds evidence of greater impairments in reading among individuals with a double deficit than those with either deficit alone (Arns et al., 2007; Wolf & Bowers, 1999), consistent with our finding that the DOUBLEdef group scored significantly lower than the RANdef group on untimed measures of reading.
Although the Control group significantly outperformed both PHONOdef and DOUBLEdef groups, reading scores did not differ significantly between the Control and RANdef groups. However, the RANdef group outperformed both PHONOdef and DOUBLEdef groups on untimed measures of single word reading. These findings are consistent with literature that suggests that RAN impairments primarily manifest in tasks when rapid integration of reading-related processes are required (Norton & Wolf, 2012). Further, overall, this pattern of findings supports the behavioral predictions made by the double-deficit hypothesis.
Analyses of fMRI data during a visual-word reading and rhyme judgment task revealed gradients of activation specific to both phonological and rapid naming ability, suggesting unique neural bases for impairment in PA and RAN. We found a phonological gradient effect by examining brain regions that showed significantly reduced activation in PHONOdef as compared to Control and in DOUBLEdef as compared to PHONOdef groups (i.e., Control > PHONOdef > DOUBLEdef). This gradient effect was found in the left IPL and IFG for the masked ROI analysis, as well as in the whole-brain analysis. The IPL was also significantly correlated with PA scores in the whole brain analysis. These findings are consistent with previous results linking left IPL and IFG and phonological impairments in dyslexia (reviewed in Gabrieli, 2009, Maisog et al., 2008; Richlan et al., 2009; Richlan, 2012; Shaywitz and Shaywitz, 2008). The fact that the significant correlation between brain activation and PA skills was found in the left IPL but did not reach significance in the left IFG may suggest that the left IPL is more directly involved in the skill of identifying, representing and manipulating sound units, while the left IFG is more heavily recruited for general phonological processing skills beyond PA (such as matching sound units to the articulation of printed letters and words).
Although there were no significant behavioral differences between PHONOdef and DOUBLEdef groups, the degree of activation for these groups was different. Whereas the PHONOdef group showed reduced positive activation in IPL and IFG relative to the Control group, the DOUBLEdef group showed negative activation in these areas of the reading network during the printed word rhyme task. This finding of particularly severe impairment in the DOUBLEdef group converges with extant behavioral evidence of most severe reading impairment found in those with double as opposed to single deficits. We also conducted an ROI analysis in the regions that showed a phonological gradient effect, which were all identified without the inclusion of the RANdef group. Extracted ROI values in the IPL and IFG areas for the RANdef group were similar to Controls, not similar to the the DOUBLEdef group, suggesting that a more severe phonological impairment, rather than a co-occurring RAN deficit, may lead to this reduced activation in regions related to phonology.
The fMRI analyses of rapid naming skills revealed a specific role for the cerebellum. The right cerebellar lobule VI, but not the left IFG or other regions, showed a gradient effect for rapid naming in the ROI-based conjunction analysis. Whole-brain correlations for the entire sample mirror this pattern, showing again that the right cerebellum is associated with rapid naming ability across a continuum of scores. The findings in the cerebellum were also relatively specific, as the whole-brain analysis conducted at a weaker threshold showed only the cerebellum and the left IPL, which is thought to have a top-down effect integrating phonological and orthographic processes for reading (Richlan, 2012). Further, the specificity of the relationship between the right cerebellar lobule VI and RAN is supported by the lack of significant difference between the Control group and the PHONOdef group in functional activation of the right cerebellum and the significant correlation between average parameter estimates and RAN skills in this region. Thus, we found that even during a task that involves visual word reading and rhyme judgment, and not rapid naming, RAN ability is associated with wholly different regions than those observed for the phonological gradient.
The finding that rapid naming deficits are associated with the cerebellum is consistent with previous literature suggesting a cerebellar deficit in dyslexia (Nicolson et al., 2001), and finding that individuals with dyslexia have both structural alterations and reduced functional activation of the cerebellum relative to typical readers (Linkersdörfer et al., 2012). There is also support for the involvement of the right cerebellum including lobule VI in a variety of language processes, including phonology, semantics, word generation, verbal fluency and automaticity (Nicolson & Fawcett, 2011; Stoodley & Schmahmann, 2009). Our findings regarding the areas related to a rapid naming gradient are also in line with behavioral and brain evidence that PA and RAN skills have both unique and shared effects on reading. In our whole-brain analyses, two regions of the left IPL showed a phonological gradient, and the RAN gradient analysis revealed an area overlapping with one of these clusters. Behavioral studies predicting reading find that PA and RAN have some shared variance, but that each skill also exerts independent influence on reading ability, and that the relations between PA, RAN, and reading change with age and the specific skills measured. For example, one study (Schatschneider et al., 2002) found that in first grade, both RAN and PA each independently accounted for 13% of the variance for an untimed word recognition task, yet by second grade, PA accounted for 19% compared to only 5% for rapid naming. On the other hand, RAN did a significantly better job of accounting for the variance in reading efficiency, as measured by the TOWRE at both grade levels. Therefore, perhaps rapid naming is best understood as a skill that contributes to reading ability in ways that are both partially overlapping with (Wagner et al., 1997) and distinct from phonological awareness abilities (Wolf & Bowers, 1999).
Functional connectivity analyses further confirmed the separation of networks related to PA and RAN ability within the reading circuit. Left fronto-parietal networks (specificially, bilateral prefrontal and left inferior frontal) were sensitive to PA ability and showed significant reduction in the DOUBLEdef group compared to others. The right cerebellar-bilateral prefrontal network was sensitive to rapid naming ability and also showed significant reduction in the DOUBLEdef group compared to others. The overlapping bilateral DLPFC regions found in functional connectivity analyses examining both phonological processing (with seed placed in left IPL) and rapid naming gradients (with seed placed in right cerebellar lobule VI) suggest that bilateral prefrontal regions may be key regions linking these brain systems. Prefrontal cortico-cerebellar loops (Jissendi et al., 2008) have not been robustly examined in humans. However, functional connectivity analyses suggest that cerebellar lobule VI is functionally related to prefrontal and premotor cortical areas (Hayter et al., 2007), and inferior frontal and temporo-parietal regions (Buckner et al., 2011). Our functional connectivity data corroborate the findings from functional task activation in confirming the unique neural patterns in groups with and without RAN and PA deficits, as well as highlight the important role that connectivity among regions may play in determining aspects of reading ability. Brain connectivity differences may elucidate the roles of reading networks in different groups defined by the double-deficit hypothesis; an EEG study with Dutch children found different patterns of correlation among electrode site coherence during rest for phoneme deletion versus rapid letter naming abilities (Arns et al., 2007).
In sum, this is the first known neuroimaging study including children with single and double deficits to find evidence of dissociation in brain systems subserving phonological and rapid naming processing, although the findings are in line with previous research that shows unique brain activations associated with PA versus RAN abilities (Turkeltaub et al., 2003). Dissociations in brain activations were observed in multiple analyses, including functional activation in ROIs and the whole brain, as well as functional connectivity. Our study suggests that children with a double deficit have the greatest reduction in brain activation in regions important for both rapid naming skills and phonological awareness, even when compared with children who have single deficits. These findings have implications both for the design of future neuroimaging studies and also for the design of assessment batteries, diagnostic criteria, and educational interventions, as children meeting different criteria for reading impairment also show differential brain activation and behavioral profiles.
Our study has several limitations. First, the functional brain differences were found by comparing activation of a printed word rhyming task to a rest condition; thus, we cannot separate the brain activation for reading versus phonological processing, per se. Alternate functional tasks and contrasts (such as an in-scanner RAN task, a pure word reading task, or a phonological task with either visually or acoustically presented sounds or symbols) might have revealed additional differences between the four groups. Examining the brain activation for an in-scanner rapid naming task in future research will allow us to better understand RAN deficits. Though we can't say with certainty whether the group differences in brain activation are related to phonological processing or reading, and we did not investigate RAN task here, our pattern of differences among phonological, RAN, and double-deficit subtypes provides novel insight into the ongoing debate over the double-deficit hypothesis.
Second, the sample size (and unequal group numbers) might have affected our ability to detect effects in other brain regions. In the future, large datasets representing all groups in this study that both follow children prospectively and account for environmental differences known to be important predictors of reading skills (such as socio-economic status and reading activity in the home) are required. Future studies might also consider different criteria for group membership, such as limiting the deficit groups to individuals with poor reading, or excluding poor readers without RAN or phonological deficits from the control group. Continued studies will allow us to further understand developmental dyslexia, a disorder affecting a significant percentage of our nation's children (and adults), and whose deleterious effects may be lessened with continued multidisciplinary research.
Supplementary Material
Highlights.
- Unique fMRI activation patterns for phonological, RAN & double deficits in dyslexia
- Phonological deficits associated with left inferior frontal and parietal regions
- RAN deficits associated with right cerebellar lobule VI
- Functional connectivity is differentially affected by phonological vs. RAN deficits
- First functional neuroanatomical evidence for double-deficit hypothesis of dyslexia
ACKNOWLEDGMENTS
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants K23HD054720, R01HD067254, R01HD065794, P01HD001994, Flora Family Foundation, UCSF Catalyst Award, UCSF Resource Allocation Program, Brain & Behavior Research Foundation Young Investigator Award, Stanford University Lucile Packard Foundation for Children's Health, Spectrum Child Health & Clinical and Translational Science Award, and the Dyslexia Foundation to FH. Funding by William and Flora Hewlett Foundation, Richard King Mellon Foundation, Ellison Medical Foundation, Massachusetts Institute of Technology Class of 1976 Funds for Dyslexia Research, Bard and Julie Richmond through the Martin Richmond Memorial Fund was provided to JDEG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackerman PT, Dykman RA. Phonological processes, confrontational naming, and immediate memory in dyslexia. Journal of Learning Disabilities. 1993;26(9):597–609. doi: 10.1177/002221949302600910. [DOI] [PubMed] [Google Scholar]
- Arns M, Peters S, Breteler R, Verhoeven L. Different brain activation patterns in dyslexic children: evidence from EEG power and coherence patterns for the double-deficit theory of dyslexia. Journal of Integrative Neuroscience. 2007;6(1):175–190. doi: 10.1142/s0219635207001404. [DOI] [PubMed] [Google Scholar]
- Badian N. Predicting reading ability over the long term: The changing roles of letter naming, phonological awareness and orthographic processing. Annals of Dyslexia. 1995;45(1):79–96. doi: 10.1007/BF02648213. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Peltier SJ. Resting state cortico-cerebellar functional connectivity networks: A comparison of anatomical and self-organizing map approaches. Frontiers in Neuroanatomy. 2012;6 doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, De Smedt B, Cleuren L, Vandewalle E, Wouters J, Ghesquiere P. Towards a further characterization of phonological and literacy problems in Dutch-speaking children with dyslexia. British Journal of Developmental Psychology. 2010;28:5–31. doi: 10.1348/026151010x485223. [DOI] [PubMed] [Google Scholar]
- Bowers PG, Steffy R, Tate E. Comparison of the effects of IQ control methods on memory and naming speed predictors of reading disability. Reading Research Quarterly. 1988;23(3):304–319. [Google Scholar]
- Bradley L, Bryant PE. Difficulties in auditory organisation as a possible cause of reading backwardness. Nature. 1978;271:746–747. doi: 10.1038/271746a0. [DOI] [PubMed] [Google Scholar]
- Brooks RB. Fostering motivation, hope and resilience in children with learning disorders. Annals of Dyslexia. 2001;51(1):9–20. [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton D, DeFries J, Olson R. Are RAN and phonological awareness deficits additive in children with reading disabilities? Dyslexia. 2001;7(3):125–149. doi: 10.1002/dys.198. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Rapid automatized naming (R.A.N): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. 3rd ed. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Eckert M. Neuroanatomical markers for dyslexia: a review of dyslexia structural imaging studies. The Neuroscientist. 2004;10(4):362–371. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126(2):482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences USA. 1998;95(3):914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, Constable RT. Disruption of Functional Networks in Dyslexia: A Whole-Brain, Data-Driven Analysis of Connectivity. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.031. doi:10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs LS, Barnes M. Learning disabilities: from identification to intervention. Guilford Press; New York: 2006. [Google Scholar]
- Foorman BR, Francis DJ, Shaywitz SE, Shaywitz BA, Fletcher JM. The case for early reading intervention. In: Blachman B, editor. Foundations of reading acquisition and dyslexia: Implications for early intervention. Psychology Press; London: 1997. pp. 243–264. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009;325(5938):280–283. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo LM, Ibrahim Z, Sachs BC, Xu B. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003;60(1):94–100. doi: 10.1212/wnl.60.1.94. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, et al. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;57(1):47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- Georgiewa P. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10(16):3459. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Gerber PJ, Schneiders CA, Paradise LV, Reiff HB, Ginsberg RJ, Popp PA. Persisting problems of adults with learning disabilities: Self-reported comparisons from their school-age and adult years. Journal of Learning Disabilities. 1990;23(9):570–573. doi: 10.1177/002221949002300907. [DOI] [PubMed] [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N. Cerebellar contributions to working memory. NeuroImage. 2007;36(3):943–954. doi: 10.1016/j.neuroimage.2007.03.011. [DOI] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Chen C, Lu Z-L, Dong Q. Decoding the neuroanatomical basis of reading ability: a multivoxel morphometric study. The Journal of Neuroscience. 2013;33(31):12835–43. doi: 10.1523/JNEUROSCI.0449-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CS-H, Chan DW-O, Lee S-H, Tsang S-M, Luan VH. Cognitive profiling and preliminary subtyping in Chinese developmental dyslexia. Cognition. 2004;91(1):43–75. doi: 10.1016/s0010-0277(03)00163-x. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Gabrieli JDE. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. The Journal of Neuroscience. 2006;26(42):10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences USA. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jissendi P, Baudry S, Baleriaux D. Diffusion tensor imaging (DTI) and tractography of the cerebellar projections to prefrontal and posterior parietal cortices: a study at 3T. Journal of Neuroradiology. 2008;35(1):42–50. doi: 10.1016/j.neurad.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Katzir T, Kim YS, Wolf M, Morris R, Lovett MW. The varieties of pathways to dysfluent reading: Comparing subtypes of children with dyslexia at letter, word, and connected text levels of reading. Journal of Learning Disabilities. 2008;41(1):47–66. doi: 10.1177/0022219407311325. [DOI] [PubMed] [Google Scholar]
- King W, Giess S, Lombardino L. Subtyping of children with developmental dyslexia via bootstrap aggregated clustering and the gap statistic: Comparison with the double deficit hypothesis. International Journal of Language & Communication Disorders. 2007;42(1):77–95. doi: 10.1080/13682820600806680. [DOI] [PubMed] [Google Scholar]
- Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: an ALE meta-analysis. PLoS ONE. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett MW. A developmental approach to reading disability: Accuracy and speed criteria of normal and deficient reading skill. Child Development. 1987;58(1):234–260. [PubMed] [Google Scholar]
- Lovett MW, Steinbach KA, Frijters JC. Remediating the core deficits of developmental reading disability: A double-deficit perspective. Journal of Learning Disabilities. 2000;33(4):334–358. doi: 10.1177/002221940003300406. [DOI] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- McBride-Chang C, Manis FR. Structural invariance in the associations of naming speed, phonological awareness, and verbal reasoning in good and poor readers: A test of the double deficit hypothesis. Reading and Writing. 1996;8(4):323–339. [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, et al. Brain activation during sentence comprehension among good and poor readers. Cerebral Cortex. 2007;17(12):2780–2787. doi: 10.1093/cercor/bhm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Miller SR, Bloom JS, Jones L, Lindstrom W, Craggs J, et al. Testing the double-deficit hypothesis in an adult sample. Annals of Dyslexia. 2006;56(1):83–102. doi: 10.1007/s11881-006-0004-4. [DOI] [PubMed] [Google Scholar]
- Misra M, Katzir T, Wolf M, Poldrack R. Neural systems for rapid automatized naming in skilled readers: Unraveling the RAN-reading relationship. Scientific Studies of Reading. 2004;8(3):241–256. [Google Scholar]
- Newman RL, Joanisse MF. Modulation of brain regions involved in word recognition by homophonous stimuli: an fMRI study. Brain Research. 2011;1367:250–264. doi: 10.1016/j.brainres.2010.09.089. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex; a journal devoted to the study of the nervous system and behavior. 2011;47(1):117–127. doi: 10.1016/j.cortex.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: Implications for understanding and treatment of reading disabilities. Annual Review of Psychology. 2012;63(1):427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- Papadopoulos TC, Georgiou GK, Kendeou P. Investigating the double-deficit hypothesis in Greek: Findings from a longitudinal study. Journal of Learning Disabilities. 2009;42(6):528–547. doi: 10.1177/0022219409338745. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brian classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience. 2009;10:67. doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D, Stainthorp R, Stuart M, Garwood H, Quinlan P. An experimental comparison between rival theories of rapid automatized naming performance and its relationship to reading. Journal of Experimental Child Psychology. 2007;98:46–68. doi: 10.1016/j.jecp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Raskind MH, Goldberg RJ, Higgins EL, Herman KL. Patterns of change and predictors of success in individuals with learning disabilities: Results from a twenty-year longitudinal study. Learning Disabilities: Research & Practice. 1999;14:35–50. [Google Scholar]
- Richlan F. Developmental dyslexia: dysfunction of a left hemisphere reading network. Frontiers in Human Neuroscience. 2012;6(120) doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin Z, Norton ES, Osher D, Beach SD, Cyr AB, Ozernov-Palchik O, Yendiki A, Fischl B, Gaab N, Gabrieli JDE. Tracking the roots of reading ability: White matter volume and integrity correlate with phonological awareness in pre-reading kindergarten children. The Journal of Neuroscience. 2013;33(33) doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough H. Predicting the future achievement of second graders with reading disabilities: Contributions of phonemic awareness, verbal memory, rapid naming, and IQ. Annals of Dyslexia. 1998;48(1):115–136. [Google Scholar]
- Schatschneider C, Carlson CD, Francis DJ, Foorman BR, Fletcher JM. Relationship of rapid automatized naming and phonological awareness in early reading development: implications for the double-deficit hypothesis. Journal of Learning Disabilities. 2002;35(3):245–256. doi: 10.1177/002221940203500306. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Mencl WE, Braze D, Tabor W, Pugh KR, Fulbright RK. Reading differences and brain: cortical integration of speech and print in sentence processing varies with reader skill. Developmental Neuropsychology. 2008;33(6):745–775. doi: 10.1080/87565640802418688. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. New England Journal of Medicine. 1998;338(5):307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Gruen JR, Shaywitz BA. Management of dyslexia, its rationale, and underlying neurobiology. Pediatric Clinics of North America. 2007;54:609–623. doi: 10.1016/j.pcl.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability). Biological Psychiatry. 2005;57(11):1301–1309. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: The neurobiology of reading and dyslexia. Developmental Psychopathology. 2008;20(4):1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95(5):2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Goulandris N, Defty N. A longitudinal study of reading development in dyslexic children. Journal of Educational Psychology. 1996;88(4):653–669. [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Sunseth K, Bowers PG. Rapid naming and phonemic awareness: Contributions to reading, spelling, and orthographic knowledge. Scientific Studies of Reading. 2002;6(4):401–429. [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tanaka H, Black JM, Hulme C, Stanley LM, Kesler SR, Whitfield-Gabrieli S, et al. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychological Science. 2011;22(11):1442–1451. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, et al. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proceedings of the National Academy of Sciences USA. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen J, Myers D, Schirm A, Stuart E, Vartivarian S,W,M, et al. Closing the reading gap: First year findings from a randomized trial of four reading interventions for striving readers. Volume II. [March 30, 2012];National Assessment of Title I: Interim Report to Congress. 2006 from http://www2.ed.gov/rschstat/eval/disadv/title1interimreport/index.html.
- Torgesen JK, Wagner RK, Rashotte CA, Burgess S, Hecht S. Contributions of phonological awareness and rapid automatic naming ability to the growth of word-reading skills in second- to fifth-grade children. Scientific Studies of Reading. 1997;1(2):161–185. [Google Scholar]
- Torppa M, Georgiou G, Salmi P, Eklund K, Lyytinen H. Examining the double-deficit hypothesis in an orthographically consistent language. Scientific Studies of Reading. 2012;16(4):287–315. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6(6):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vaessen A, Gerretsen P, Blomert L. Naming problems do not reflect a second independent core deficit in dyslexia: Double deficits explored. Journal of Experimental Child Psychology. 2009;103(2):202–221. doi: 10.1016/j.jecp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vukovic RK, Siegel LS. The double-deficit hypothesis: a comprehensive analysis of the evidence. Journal of Learning Disabilities. 2006;39(1):25–47. doi: 10.1177/00222194060390010401. [DOI] [PubMed] [Google Scholar]
- Vukovic RK, Wilson AM, Nash KK. Naming speed deficits in adults with reading disabilities: a test of the double-deficit hypothesis. Journal of Learning Disabilities. 2004;37(5):440–450. doi: 10.1177/00222194040370050601. [DOI] [PubMed] [Google Scholar]
- Waber DP, Forbes PW, Wolff PH, Weiler MD. Neurodevelopmental characteristics of children with learning impairments classified according to the double-deficit hypothesis. Journal of Learning Disabilities. 2004;37(5):451–461. doi: 10.1177/00222194040370050701. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological awareness and its Causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- Wagner RK, Torgesen JK, Laughon P, Simmons K, Rashotte CA. Development of young readers' phonological processing abilities. Journal of Educational Psychology. 1993;85(1):83–103. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA, Hecht SA. Changing relations between phonological processing abilities and word-level reading as children develop from beginning to skilled readers: a 5-year longitudinal study. Developmental Psychology. 1997;33:468–479. doi: 10.1037//0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing. Pro-Ed; Austin, TX: 1999. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91(3):415–438. [Google Scholar]
- Wolf M, Bowers PG, Biddle K. Naming-speed processes, timing, and reading: a conceptual review. Journal of Learning Disabilities. 2000;33(4):387–407. doi: 10.1177/002221940003300409. [DOI] [PubMed] [Google Scholar]
- Wolf M, Denckla MB. Rapid Automatized Naming and Rapid Alternating Stimulus Tests. Pro-Ed; Austin, TX: 2005. [Google Scholar]
- Wolf M, O'Rourke AG, Gidney C, Lovett M, Cirino P, Morris R. The second deficit: An investigation of the independence of phonological and naming-speed deficits in developmental dyslexia. Reading and Writing. 2002;15:43–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.