Abstract
The aim of the study was to evaluate the antibacterial activity of essential oil extracted from Chrysanthemum boreale (C. boreale) on Streptococcus mutans (S. mutans). To investigate anticariogenic properties, and bacterial growth, acid production, biofilm formation, bacterial adherence of S. mutans were evaluated. Then gene expression of several virulence factors was also evaluated. C. boreale essential oil exhibited significant inhibition of bacterial growth, adherence capacity, and acid production of S. mutans at concentrations 0.1–0.5 mg/mL and 0.25–0.5 mg/mL, respectively. The safranin staining and scanning electron microscopy results showed that the biofilm formation was also inhibited. The result of live/dead staining showed the bactericidal effect. Furthermore, real-time PCR analysis showed that the gene expression of some virulence factors such as gtfB, gtfC, gtfD, gbpB, spaP, brpA, relA, and vicR of S. mutans was significantly decreased in a dose dependent manner. In GC and GC-MS analysis, seventy-two compounds were identified in the oil, representing 85.42% of the total oil. The major components were camphor (20.89%), β-caryophyllene (5.71%), α-thujone (5.46%), piperitone (5.27%), epi-sesquiphellandrene (5.16%), α-pinene (4.97%), 1,8-cineole (4.52%), β-pinene (4.45%), and camphene (4.19%). These results suggest that C. boreale essential oil may inhibit growth, adhesion, acid tolerance, and biofilm formation of S. mutans through the partial inhibition of several of these virulence factors.
1. Introduction
Dental caries, known as tooth decay or a cavity, is a plaque-related disease of teeth and slowly progressive infectious disease in the dental area [1, 2]. The dental caries disease is caused by specific types of acid-producing bacteria that cause demineralization and destruction of the teeth [3].
S. mutans are generally regarded as one of the primary pathogenic bacteria in dental caries [4]. The S. mutans adhere to the colonizer and accumulate on the tooth enamel surface by generation of extracellular polysaccharide from fermentable carbohydrates such as sucrose, by action of glucosyltransferases (GTFase) [1, 5]. The carbohydrate metabolism promotes bacteria aggregation to the tooth surface and acid production [1]. The produced acids initiate dissolution of the enamel surface of teeth subsequently leading to localized decalcification [6].
Therefore, inhibition of the growth and biofilm formation of the S. mutans is one of the strategies for prevention of dental caries. Although several antiplaque agents have been used, the attempt to search for an effective agent still continued [7, 8]. For example, some studies reported that several natural products derived herb, such as Mentha longifolia L., Aralia continentalis, and Curcuma longa L, showed the inhibitory effect of dental plaque [9–11].
C. boreale is a perennial herb with yellow flowers and belongs to the Asteraceae family. It is widely distributed in wild fields and mountains of East Asia. It is also usually have been used as tea or wine in Korea. The Chrysanthemum species herb has been reported as having potential medicinal properties including anti-inflammatory, antiviral, and antibacterial [12–14]. In previous study [15], the essential oil was extracted from C. boreale and eighty-seven constituents were identified. Furthermore, the essential oil showed antibacterial activity against several bacteria including S. mutans. However there is poorly scientific evidence about effect of essential oil from C. boreale on S. mutans causing dental plaque formation. Therefore, in this study, we examined influence of essential oil of extracted from C. boreale on the growth, acid-production, bacterial attachment, and biofilm formation of S. mutans. Furthermore, several virulence factors of S. mutans, associated with dental plaque and caries formation, were assessed, and the detailed chemical constituents of C. boreale essential oil were also analyzed by GC and GC-MS.
2. Materials and Methods
2.1. Plant Material and Essential Oil
C. boreale was collected in October, 2013, at the full flowering stage from plants grown wild in Iksan district in Korea and the aerial parts were used to isolate essential oil. The identity was confirmed by Young-Hoi Kim at the College of Environmental & Bioresource Sciences, Chonbuk National University. Voucher specimen (number: 10-24-13) has been deposited at the Herbarium of College of Environmental & Bioresource Sciences, Chonbuk National University. The aerial parts (leaves, stems, and flowers) of C. boreale (1 kg) were finely chopped. The chopped plant materials of C. boreale were placed in 5 L round-bottom flask and distilled water was added (3 L). Hydrodistillation was carried out in a Clevenger-type apparatus for 3 hours. The yield of the essential oil of C. boreale was 0.84%, based on fresh weight of the plant. The essential oil was stored in a deep freezer (−70°C) to minimize the escape of volatile compounds.
2.2. Inhibition of Bacterial Growth
S. mutans (ATCC 25175) was purchased from the American Type Culture Collection (ATCC; Rockville, MD, USA) and cultured in brain heart infusion (BHI; Difco, Detroit, MI) broth under aerobic condition at 37°C. To determine inhibitory effect of C. boreale on bacterial growth, S. mutans was cultured at 37°C in 0.95 mL of BHI broth containing 1% glucose and various concentrations of the essential oil of C. boreale. These tubes were inoculated with 0.05 mL of an overnight culture grown in BHI broth (final: 5 × 105 colony-forming units (CFU)/mL), and incubated for 24 h. Also, 0.1% of sodium fluoride (NaF) was used as a positive control. The optical density (OD) of cells was measured at 550 nm using a spectrophotometer. Three replicates were made for each concentration of the test extracts.
2.3. Acid Production
Acid production by S. mutans was examined to evaluate the effect of the essential oil of C. boreale, as described by a previous study [16]. Briefly, the C. boreale essential oil was filtered to sterilization using membrane filter with 0.2 μm pore size and added to 0.95 mL of the phenol red broth containing 1% glucose, which was then inoculated with 0.05 mL of the seed culture of S. mutans. After 24 h of cultivation, the pH was directly determined in the bacterial growth media using a pH meter (Corning Inc, Corning, NY, USA). The initial pH of BHI with various concentrations of C. boreale essential oil was also determined before inoculation of S. mutans. Each concentration of the extract was tested in triplicate.
2.4. Bacterial Adherence
The effect of C. boreale essential oil on bacterial adherence was determined using hydroxyapatite beads (diameter of 80 μm; Bio-Rad, Hercules, CA, USA) in a previously described method [17]. Briefly, hydroxyapatite beads were coated with clarified human saliva and the saliva-coated hydroxyapatite beads (S-HAs) were immersed in bacterial suspension (1 × 107 CFU/mL) with various concentrations of C. boreale essential oil. To allow bacteria to be adherent, the mixture was gently agitated for 90 min at 37°C. Following this, S-HAs was rinsed to remove nonadherent bacteria and was transferred to a new tube that contained potassium phosphate buffer. The adherent S. mutans onto the S-HAs were dispersed using a sonicator (Fisher Scientific, Springfield, NJ, USA) at 50 W for 30 sec and the supernatants were spread on bacitracin (3.2 mg/mL) contained MSA plate. After 48 h of cultivation, the numbers of colonies were counted.
2.5. Biofilm Formation Assay
Biofilm formation was measured by staining with safranin [18] and observation was done by scanning electron microscopy (SEM). Briefly, various concentrations of C. boreale essential oil were added to 0.1% sucrose containing BHI broth in 35 mm polystyrene dish or 24-well plate that contained resin teeth (Endura, Shofu Inc., Kyoto, Japan). Then, the culture was created in the allotted broths by inoculating them with seed cultures of S. mutans (5 × 105 CFU/mL) and incubated for 24 h. After incubation, the supernatants were removed and the culture dish or resin teeth were rinsed with distilled water. Biofilm formation was stained with 0.1% safranin and photographed. In addition, to observe the biofilm formation using a SEM, the biofilms formed polystyrene dishes were rinsed with distilled water, fixed with 2.5% glutaraldehyde solution, and dehydrated in ethanol gradient series. Then, the samples were sputter-coated with gold and observed by SEM (JOM-6360, JEOL, Tokyo, Japan).
2.6. Confocal Laser Scanning Microscopy
To determine the bactericidal effect of C. boreale essential oil on S. mutans live and dead staining were performed. Briefly, approximately 1 × 107 CFU/mL of S. mutans was treated with various concentrations of C. boreale essential oil for 24 h at 37°C under aerobic conditions. Then the bacteria were washed with PBS and stained with LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes, Eugene, OR, USA) according to the manufacture's protocol. After 15 min of staining, the bacteria were observed using a confocal laser scanning microscopy (LSM 510, Zeiss, Germany).
2.7. Real-Time Polymerase Chain Reaction (PCR) Analysis
A real-time PCR was performed to evaluate the effect of C. boreale essential oil on gene expression of S. mutans. The subminimal inhibitory concentration (0.5–0.25 mg/mL) of the essential oil was treated. After 24 h of culture, total RNA was isolated from S. mutans using a Trizol reagent (Bibco-BRL) and cDNA was synthesized. The amplification was performed using a StepOnePlus Real-Time PCR system with QPCR SYBR Green Mixes (Applied Bio system, Foster City, CA, USA). 16S rRNA was used as an internal control. The primer pairs were described by previous report [19] and are listed in Table 1.
Table 1.
Oligonucleotide primers that were used in this study.
| Gene* | Gene description | Primer sequences (5′-3′) | |
|---|---|---|---|
| gtfB | Glucosyltransferase-I | Forward: Reverse: |
AGCAATGCAGCCAATCTACAAAT ACGAACTTTGCCGTTATTGTCA |
|
| |||
| gtfC | Glucosyltransferase-SI | Forward: Reverse: |
GGTTTAACGTCAAAATTAGCTGTATTAGC CTCAACCAACCGCCACTGTT |
|
| |||
| gtfD | Glucosyltransferase-S | Forward: Reverse: |
ACAGCAGACAGCAGCCAAGA ACTGGGTTTGCTGCGTTTG |
|
| |||
| brpA | Biofilm regulatory protein A | Forward: Reverse: |
GGAGGAGCTGCATCAGGATTC AACTCCAGCACATCCAGCAAG |
|
| |||
| spaP | Cell surface antigen SpaP | Forward: Reverse: |
GACTTTGGTAATGGTTATGCATCAA TTTGTATCAGCCGGATCAAGTG |
|
| |||
| gbpB | Secreted antigen GbpB/SagA | Forward: Reverse: |
ATGGCGGTTATGGACACGTT TTTGGCCACCTTGAACACCT |
|
| |||
| relA | GTP pyrophosphokinase | Forward: Reverse: |
ACAAAAAGGGTATCGTCCGTACAT AATCACGCTTGGTATTGCTAATTG |
|
| |||
| vicR | Response regulator | Forward: Reverse: |
TGACACGATTACAGCCTTTGATG CGTCTAGTTCTGGTAACATTAAGTCCAATA |
|
| |||
| 16S rRNA | 16S rRNA | Forward: Reverse: |
CCTACGGGAGGCAGCAGTAG CAACAGAGCTTTACGATCCGAAA |
*Based on the NCBI S. mutans genome database.
2.8. GC and GC-MS Analysis
GC analysis was performed on Hewlett-Packard (HP) model 6890 series gas chromatograph, with a flame ionization detector (FID), a split ratio of 30 : 1 using two different fused silica capillary columns, Supelcowax 10 (30 m × 0.32 mm, i.d., 0.25 μm film thickness) and SPB-1 (30 m × 0.32 mm, i.d., 0.25 μm film thickness). The temperature of the column was programmed from 50°C to 230°C at 2°C/min and then kept constant at 230°C for 30 min for Supelcowax 10 column and SPB-1 column was programmed from 40°C to 230°C at 2°C/min and then kept constant at 230°C for 20 min. The injector and detector temperatures for both analyses were 250°C, respectively. The gas carrier was nitrogen at a flow rate of 1.50 mL/min for Supelcowax 10 column and nitrogen at a flow rate of 1.20 mL/min for SPB-1 column. Peak areas were measured by electronic integration. The relative amounts of the individual components are based on the peak areas. The GC-MS was carried out on Agilent 7890A GC and Agilent 5975C mass selective detector (MSD) operating in EI mode at 70 eV, fitted a DB-Wax column (30 m × 0.25 mm, i.d., 0.25 μm film thickness) and SPB-1 column (30 m × 0.25 mm, i.d., 0.25 μm film thickness). The temperature of the column were programmed from 40°C to 230°C at 2°C/min and then kept constant at 230°C for 30 min for both analyses. The injector and interface temperatures were 250°C, respectively. The gas carrier was helium at a flow rate of 1.50 mL/min for both analyses. The identification of the chemical constituents was based on comparison of their mass spectral spectra with those of Wiley7n/NIST05 mass spectra libraries, and then the compounds of MS matching similarity ≥ 90% were selected as results. Linear retention indices were calculated for each component with the retention time of n-alkane series (C6–C26) [20] under same GC operating conditions with the sample. They were compared with their retention indices available in the literatures [21, 22] or NIST gas chromatographic retention data webbook (http://webbook.nist.gov/chemistry/) database.
2.9. Statistical Analysis
All experiments were performed in triplicate. Data were analyzed using the statistical package for social sciences (SPSS, Chicago, IL, USA). The data were expressed as the mean ± standard deviation (SD) values. The statistical analysis was evaluated by one-way ANOVA. Values of P < 0.05 were considered as statistically significant.
3. Results
3.1. Bacterial Growth Inhibition by C. boreale
In the study, we firstly investigated the antibacterial activity of the essential oil of C. boreale against S. mutans. The bacteria were treated with 0.05, 0.1, 0.25, and 0.5 mg/mL of C. boreale essential oil. When treated with 0.1% NaF, as a positive control, the manifested significant inhibition was shown. When treated with 0.1 mg/mL of the essential oil, the bacterial growth was significantly inhibited. In addition, significant inhibition was shown at concentrations 0.25 mg/mL and 0.5 mg/mL of essential oil in comparison to the control group (Figure 1) (P < 0.05).
Figure 1.
Effect of Chrysanthemum boreale (C. boreale) essential oil on growth of Streptococcus mutans (S. mutans). S. mutans was inoculated into BHI broth with various concentrations of C. boreale essential oil and incubated for 24 h. Antibacterial activity against S. mutans was shown in presence of C. boreale essential oil at concentration ranging from 0.1 mg/mL to 0.5 mg/mL. Each value is expressed as a mean ± standard deviation (SD). 0.1% of sodium fluoride (NaF) was used as a positive control. Significance was determined at * P < 0.05 when compared with the control.
Furthermore, the manifested significant inhibition was shown at concentrations higher than 0.25 mg/mL and 0.5 mg/mL in comparison to the control group.
3.2. Inhibition of Acid Production
To determine whether the C. boreale essential oil inhibits the acid production in S. mutans, the bacteria were cultured in the presence of various concentrations (0.05–0.5 mg/mL) of the essential oil and the pH change was measured. As shown in Table 2, the pH was significantly decreased at control group (pH 5.47 ± 0.05). However, the pH decrease was significantly inhibited at positive group (0.1% NaF, pH 7.37 ± 0.05). Although the pH decrease was not inhibited at 0.05–0.1 mg/mL of C. boreale essential oil, when treated with 0.25 mg/mL and 0.5 mg/mL of C. boreale essential oil, the pH decrease was also significantly inhibited and the inhibition levels was similar to the positive group. These results indicate that the C. boreale essential oil may inhibit the organic acid production by S. mutans.
Table 2.
Effect of essential oil of C. boreale on acid production of S. mutans.
| Conc. (mg/mL) | pH (before incubation) | pH (after incubation) |
|---|---|---|
| Control | 7.00 ± 0.00 | 5.47 ± 0.05 |
| 0.05 | 7.00 ± 0.00 | 5.43 ± 0.05 |
| 0.1 | 7.00 ± 0.00 | 5.33 ± 0.05 |
| 0.25 | 7.00 ± 0.00 | 7.33 ± 0.05* |
| 0.5 | 7.00 ± 0.00 | 7.33 ± 0.05* |
| 0.1% NaF | 7.00 ± 0.00 | 7.37 ± 0.05* |
Data (pH) are represented as mean ± standard deviation. * P < 0.05 when compared with the control group after incubation.
3.3. Inhibitory Effect of C. boreale Essential Oil on S. mutans Adherence
We tested the inhibitory effect of C. boreale essential oil on the ability of S. mutans to adhere to S-HAs. When treated with C. boreale essential oil, the S. mutans was significantly inhibited in a dose dependent manner. The adherence onto S-HAs particularly was significantly inhibited at concentration of 0.1–0.5 mg/mL of C. boreale essential oil (Figure 2).
Figure 2.
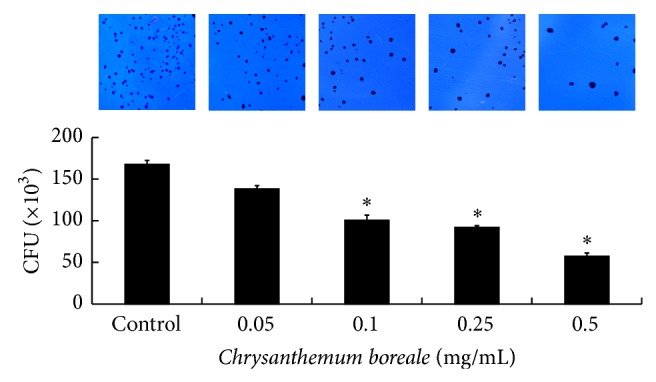
Effect of Chrysanthemum boreale (C. boreale) essential oil on colony-forming units (CFU) of Streptococcus mutans (S. mutans). S. mutans was inoculated into BHI broth with various concentrations of C. boreale essential oil and incubated for 24 h. The CFU of S. mutans that adhered to saliva-coated hydroxyapatite beads that were treated with various concentration of C. boreale essential oil are shown. When treated with 0.1–0.5 mg/mL of C. boreale essential oil, adherence was significantly repressed. Each value is expressed as a mean ± standard deviation (SD). Significance was determined at * P < 0.05 when compared with the control. 0.1% of sodium fluoride (NaF) was used as a positive control.
3.4. Bactericidal Effect of C. boreale Essential Oil on S. mutans
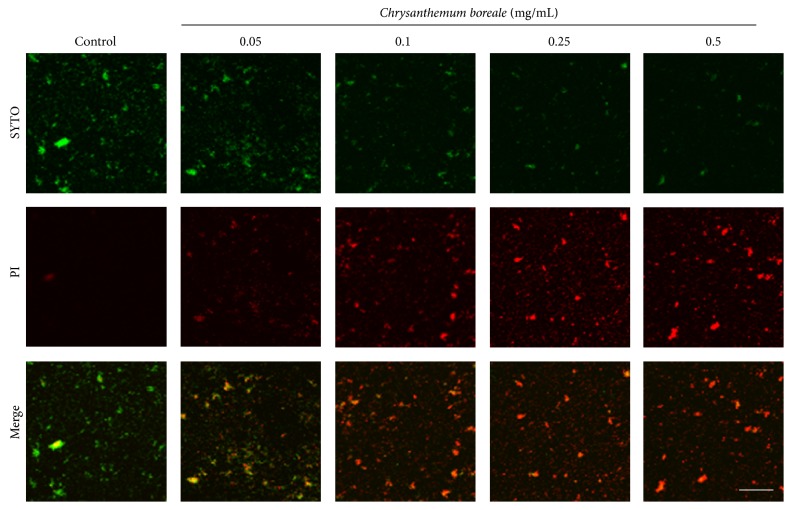
To evaluate bactericidal effect of C. boreale essential oil, S. mutans were cultured in presence of various concentrations (0.05–0.5 mg/mL) of the essential oil and stained with LIVE/DEAD BacLight Bacterial Viability Kit and they were observed using confocal laser scanning microscopy. Treatment with C. boreale essential oil decreases living bacteria (green fluorescence labeled cell stained by SYTO 9) and increases dead bacteria (red fluorescence labeled cell stained by PI) in a dose dependent manner (Figure 3). This result suggests that C. boreale essential oil has bactericidal effect on S. mutans.
Figure 3.
Bactericidal effect of Chrysanthemum boreale (C. boreale) essential oil. Cultured Streptococcus mutans (S. mutans) were treated with C. boreale essential oil and stained with LIVE/DEAD BacLight Bacterial Viability Kit. Treatment with C. boreale essential oil showed bactericidal effect on S. mutans in a dose dependent manner. Living bacteria was stained by SYTO 9 as green color and dead bacteria was stained by PI as a red color. Scale Bar = 100 μm.
3.5. Inhibitory Effect of C. boreale Essential Oil on Biofilm Formation
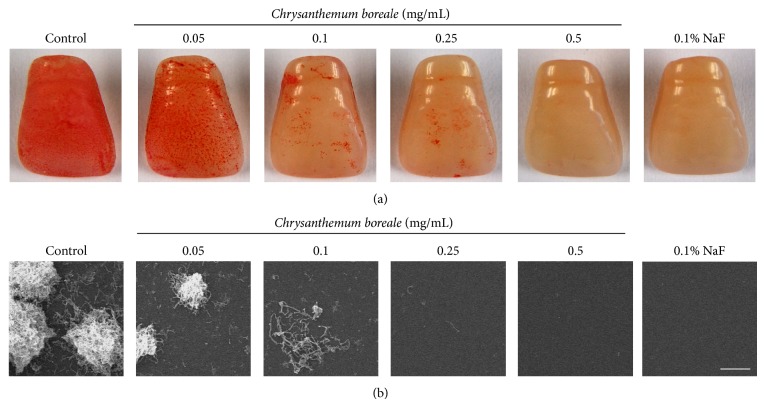
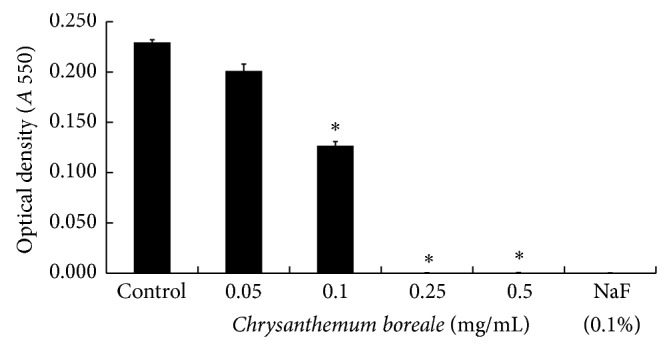
To determine whether C. boreale essential oil inhibits biofilm formation by S. mutans, the bacteria had been cultured in the presence of various concentrations of C. boreale essential oil in polystyrene dishes. As a result of safranin staining, the biofilm formation by S. mutans was significantly inhibited by treatment with C. boreale essential oil in a dose dependent manner. When treated with 0.1% NaF (positive control), complete inhibition was shown. In addition, the biofilm formation was also significantly inhibited at 0.1 mg/mL and 0.5 mg/mL of the essential oil (Figure 4). Also, we observed biofilm formation on the surface of resin teeth by safranin staining and SEM observation. Treatment with 0.05 mg/mL of C. boreale essential oil slightly inhibited biofilm formation by S. mutans and significantly inhibited at concentration 0.1–0.5 mg/mL of C. boreale essential oil (Figure 5(a)). Also the SEM image showed consistent result with safranin staining of resin teeth (Figure 5(b)).
Figure 4.
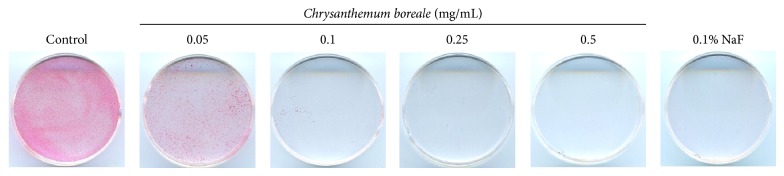
Effect of Chrysanthemum boreale (C. boreale) essential oil on biofilm formation on polystyrene dishes by Streptococcus mutans (S. mutans). S. mutans was inoculated into BHI broth with various concentrations of C. boreale essential oil and cultured for 48 h. The biofilm that formed on the polystyrene dish surface was measured by staining with 0.1% safranin. Biofilm formation was also significantly inhibited at 0.1 mg/mL and 0.5 mg/mL of the C. boreale essential oil. 0.1% of sodium fluoride (NaF) was used as a positive control.
Figure 5.
Effect of Chrysanthemum boreale (C. boreale) essential oil on biofilm formation on resin teeth surface. Streptococcus mutans (S. mutans) biofilm on resin tooth surface were incubated in various concentration of C. boreale essential oil (a). Biofilm formation was significantly inhibited at 0.05–0.25 mg/mL of C. boreale essential oil. Also biofilm formation was completely inhibited at 0.5 mg/mL of C. boreale essential oil. Scanning electron microscopy image of S. mutans biofilm formation on resin tooth surfaces (b). 0.1% of sodium fluoride (NaF) was used as a positive control. Scale bar represents 25 μm.
3.6. Inhibitory Effect of C. boreale Essential Oil on Expression of Virulence Factor
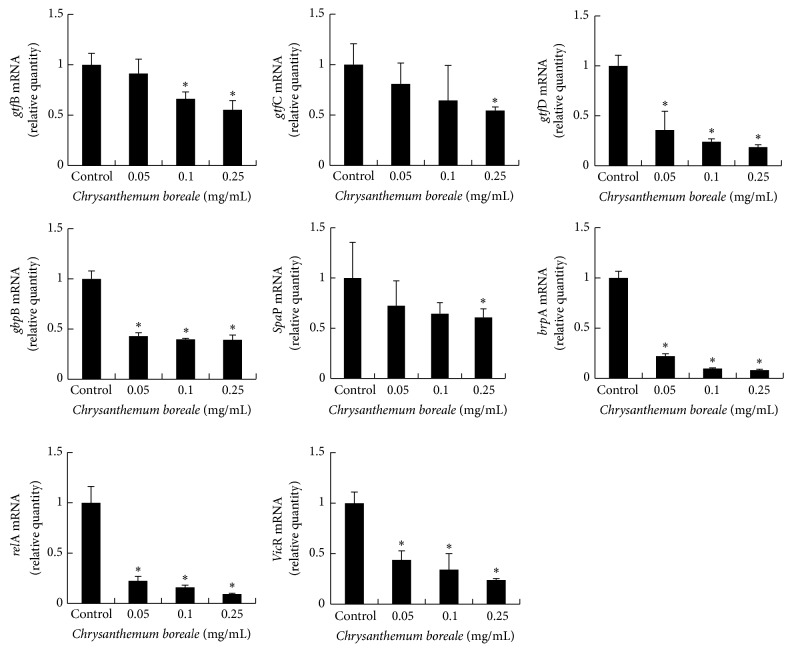
To assess the effect of C. boreale essential oil on the gene expression of virulence factors, S. mutans was cultured in presence of 0.05–0.25 mg/mL of C. boreale essential oil and the gene expressions of virulence factors were evaluated by real-time PCR (Figure 6). After treatment with C. boreale essential oil, firstly genetic expression of gtfB, gtfC, and gtfD, which encode GTFase B, C, and D proteins, respectively, was evaluated. The expression of gtfB was significantly decreased when S. mutans was treated with 0.1 mg/mL and 0.25 mg of C. boreale essential oil and gtfC was significantly decreased at 0.25 mg/mL of C. boreale essential oil. However, the expression of gtfD was significantly decreased by C. boreale essential oil at concentration of 0.05–0.25 mg/mL. The expression of SpaP and gbpB, which contribute to bacterial adherence, was also decreased at 0.25 mg/mL and 0.05–0.25 mg/mL of C. boreale essential oil, respectively. The expression of brpA and relA, which are related with acid tolerance and vicR, which is associated with regulating the expression of gbpB, gtfB, gtfC, and gtfD, was also decreased by C. boreale essential oil treatment at the concentration of 0.05–0.25 mg/mL.
Figure 6.
Real-time PCR analysis of mRNA expressions of several virulence factor genes. Streptococcus mutans (S. mutans) was cultured and treated with various concentrations of Chrysanthemum boreale (C. boreale) essential oil and real-time PCR analysis was performed as described in the Materials and Methods. gtfB, gtfC, and gtfD were significantly inhibited at 0.1–0.25 mg/mL, 0.25 mg/mL, and 0.05–0.25 mg/mL of C. boreale essential oil, respectively. In addition, gbpB, brpA, relA, and vicR expressions were significantly inhibited at 0.05–0.25 mg/mL. The expression of spaP was significantly inhibited at 0.25 mg/mL of C. boreale essential oil. Each value is expressed as a mean ± standard deviation. Significance was determined at * P < 0.05 when compared with the control.
3.7. GC and GC-MS Analysis
The chemical composition of C. boreale essential oil identified by GC and GC-MS analysis was shown in Table 3 together with their major constituent. Seventy-two compounds were identified in the oil, representing 85.42% of the total oil. All unidentified compounds were minor components. The major components were camphor (20.89%), β-caryophyllene (5.71%), α-thujone (5.46%), piperitone (5.27%), epi-sesquiphellandrene (5.16%), α-pinene (4.97%), 1,8-cineole (4.52%), β-pinene (4.45%), and camphene (4.19%) (Table 3).
Table 3.
GC and GC-MS analysis of the essential oil isolated from C. boreale.
| Peak no.a |
Components | Retention index | Peak area (%)d |
|
|---|---|---|---|---|
| Polarb | Apolarc | |||
| Monoterpene hydrocarbons | (19.66) | |||
| 1 | Tricyclene | 1009 | 920 | 0.18 |
| 2 | α-Pinene | 1027 | 933 | 4.97 |
| 3 | α-Thujene | 1030 | 925 | 0.23 |
| 4 | Camphene | 1070 | 945 | 4.19 |
| 5 | β-Pinene | 1110 | 970 | 4.45 |
| 6 | Sabinene | 1123 | 966 | 0.61 |
| 10 | Myrcene | 1167 | 984 | 0.92 |
| 11 | α-Terpinene | 1181 | 1007 | 0.48 |
| 13 | Limonene | 1199 | 1020 | 0.65 |
| 16 | cis-β-Ocimene | 1239 | 1029 | 0.87 |
| 17 | γ-Terpinene | 1249 | 1050 | 0.47 |
| 18 | p-Cymene | 1276 | 1022 | 1.34 |
| 19 | Terpinolene | 1286 | 1078 | 0.30 |
| Oxygenated monoterpenes | (47.23) | |||
| 12 | 2,3-Dehydro-1,8-cineole | 1191 | 976 | 0.05 |
| 14 | 1,8-Cineole | 1212 | 1020 | 4.52 |
| 22 | α-Thujone | 1427 | 1090 | 5.46 |
| 23 | β-Thujone | 1441 | 1096 | 1.04 |
| 28 | Camphor | 1518 | 1124 | 20.89 |
| 30 | Linalool | 1537 | 1101 | 0.10 |
| 31 | trans-Sabinene hydrate | 1562 | 1051 | 0.40 |
| 32 | trans-Chrysanthenyl acetate | 1569 | 1186 | 0.46 |
| 33 | Bornyl acetate | 1576 | 1268 | 0.69 |
| 35 | Terpinen-4-ol | 1599 | 1165 | 0.72 |
| 36 | Lavandulyl acetate | 1608 | — | 0.08 |
| 37 | Myrtenal | 1621 | 1167 | 0.15 |
| 38 | Umbellulone | 1639 | 1149 | 0.28 |
| 39 | Pinocarveol | 1651 | 1124 | 0.29 |
| 40 | p-Mentha-1,5-dien-8-ol | 1662 | 1169 | 0.60 |
| 43 | α-Terpineol | 1697 | 1174 | 0.38 |
| 44 | Borneol | 1701 | 1146 | 1.94 |
| 48 | Piperitone | 1725 | 1224 | 5.27 |
| 49 | Carvone | 1731 | 1212 | 1.14 |
| 50 | cis-Chrysanthenol | 1761 | 1157 | 2.07 |
| 53 | Myrtenol | 1795 | 1179 | 0.32 |
| 54 | trans-Carveol | 1833 | 1181 | 0.09 |
| 55 | Geraniol | 1852 | — | 0.07 |
| 56 | Geranyl acetone | 1854 | — | 0.08 |
| 57 | cis-Carveol | 1860 | 1196 | 0.14 |
| Sesquiterpene hydrocarbons | 12.51 | |||
| 25 | α-Guaiene | 1470 | — | 0.09 |
| 27 | α-Copaene | 1493 | 1370 | 0.32 |
| 29 | Berkheyaradulen | 1527 | 1377 | 0.19 |
| 34 | β-Caryophyllene | 1590 | 1412 | 5.71 |
| 41 | cis-β-Farnesene | 1671 | — | 0.38 |
| 42 | β-Selinene | 1676 | 1488 | 0.15 |
| 45 | epi-Sesquiphellandrene | 1707 | — | 5.16 |
| 46 | Widdrene | 1710 | — | 0.09 |
| 47 | Zingiberene | 1714 | 1496 | 0.42 |
| 51 | ar-Curcumene | 1780 | 1484 | 0.28 |
| Oxygenated Sesquiterpenes | (4.44) | |||
| 58 | Caryophyllene oxide | 1975 | 1561 | 2.08 |
| 59 | Viridiflorol | 2042 | 1569 | 0.10 |
| 60 | Nerolidol | 2049 | 1555 | 0.38 |
| 61 | Elemol | 2072 | — | 0.40 |
| 62 | Spathulenol | 2118 | 1563 | 0.42 |
| 64 | Torreyol | 2167 | 1606 | 0.15 |
| 67 | epi-Globulol | 2217 | 1589 | 0.44 |
| 68 | Farnesol | 2244 | 1688 | 0.03 |
| 70 | α-Costol | 2301 | — | 0.29 |
| 71 | Caryophyllenol II | 2349 | — | 0.07 |
| 72 | Farnesol (isomer) | 2353 | 1704 | 0.08 |
| Others | (1.58) | |||
| 5 | n-Hexanal | 1087 | 835 | 0.07 |
| 8 | Butyl benzene | 1126 | 938 | 0.11 |
| 9 | 2-Methylpropylbenzene | 1133 | 1050 | 0.14 |
| 15 | 2-Pentyl furan | 1236 | 981 | 0.04 |
| 20 | 6-Methyl-5-hepten-2-one | 1341 | — | 0.06 |
| 21 | n-Hexanol | 1356 | 882 | 0.10 |
| 24 | 1-Octen-3-ol | 1454 | 966 | 0.21 |
| 26 | 2,2,4-Trimethyl-2-cyclohexene carbaldehyde | 1474 | 1040 | 0.04 |
| 52 | Methyl salicylate | 1789 | 1169 | 0.07 |
| 63 | Eugenol | 2164 | 1327 | 0.29 |
| 65 | Thymol | 2185 | 1275 | 0.34 |
| 66 | Carvacrol | 2212 | 1275 | 0.05 |
| 69 | Decanoic acid | 2263 | — | 0.06 |
|
| ||||
| Total identified | (85.42) | |||
aNumbering refers to the elution order on Supelcowax 10 column.
bRetention index on polar Supelcowax 10 column.
cRetention index on apolar SPB-1 column.
dPeak area percentage is based on polar Supelcowax 10 column, and values represent averages of three determinations.
4. Discussion
C. boreale are frequently used as a tea or wine in oriental medicine and their medicinal effects such as anti-inflammatory, ant-viral, and antibacterial have been reported [12–14]. Previously, we reported that the C. boreale essential oils were extracted and it was identified that the essential oils were composed of eighty-seven constituents where major components were camphor, α-thujone, cis-chrysanthenol, 1,8-cineole, α-pinnen, and β-caryophyllene. Furthermore, the essential oil exhibited the inhibitory effect on growth of several bacteria including S. mutans [15]. However, there is no report on its potential effect on the cariogenic properties such as bacterial growth, adherence, biofilm formation, and acid production.
To evaluate anticariogenic properties of C. boreale essential oil, S. mutans was used because the bacteria is considered as a major bacterium for the formation of dental caries [5, 23]. Our results showed that growth of S. mutans was suppressed by treatment with C. boreale essential oil. Furthermore, the live/dead staining results also showed that C. boreale essential oil has an antibactericidal effect against S. mutans. These results suggested that C. boreale essential oil has a potential for anticariogenic effect because the inhibition of the growth of S. mutans is one of the strategies for prevention of dental caries.
In dental plaque formation, pH is one of the major causes because low pH leads to demineralized tooth enamel and favors the occurrence of the dental caries. S. mutans can metabolize dietary sugars and produce organic acid and the produced acids and it is induced by acidic environment in the mouth [24]. Therefore, the alternation of pH is used as an indicator to determine the effect of anticariogenic agents. In this study, C. boreale essential oil inhibited the decrease of pH induced by S. mutans and the result suggests that C. boreale essential oil may be inhibiting dental caries through inhibition of acid production by S. mutans.
Furthermore, in the creation of dental plaque process, synthesized extracellular glucan by S. mutans is generally regarded as being a major factor [25]. Glucans induce bacterial adherence and result in the formation of dental biofilm [26]. Herein, we examined whether C. boreale essential oil can inhibit the ability of S. mutans to adhere to S-HAs. Our result showed that C. boreale essential oil significantly inhibited bacterial adhesion. In addition, biofilm formation by S. mutans was also inhibited by treatment with C. boreale essential oil cultured both on polystyrene dishes and on surface of resin teeth. These results suggested that C. boreale essential oil directly inhibits the attachment and biofilm formation by S. mutans.
Several virulence factors of S. mutans are associated with cariogenicity such as bacterial adhesion [27], biofilm formation [28], and acid tolerance [29]. In this study, to evaluate correlation between inhibitory effect by C. boreale essential oil and virulence factors expression, we determined the mRNA expression level of several virulence factors such as gtfB, gtfC, gtfD, gbpB, spaP, brpA, relA, and vicR, using a real-time PCR analysis. Firstly we evaluated the gene expression level of gtfB, gtfC, and gtfD, which encode the glucosyltransferases (GTFase) B, C, and D. GTFase are recognized as essential virulence factor because these enzymes synthesize glucan from sucrose; the synthesized glucans provide binding site for bacterial adhesion [27]. Besides GTFase virulence factors, gbpB, which encodes surface-associated glucan binding protein (GBP), are also a required factor for bacterial adhesion because the protein mediates interaction between cell surface and glucan [30]. Furthermore, S. mutans expressed spaP gene which encodes SpaP protein and the protein contributes adhesion of S. mutans [31, 32]. In this study, C. boreale essential oil significantly inhibited the transcription level of gtfB, gtfC, gtfD, gbpB, and spaP. In biofilm formation process by S. mutans, brpA and relA gene play critical roles. brpA is associated with biofilm regulation [28] and relA gene plays a major role in several processes including biofilm formation, glucose uptake, and acid tolerance [33, 34]. Also vicR gene is reportedas a regulatory gene of other virulence factors such as gbpB, gtfB, gtfC, and gtfD [19]. Based on our results of real-time PCR, the expression of brpA, relA, and vicR was also repressed when treated with C. boreale essential oil. The chemical constituents of C. boreale were analyzed with GC and GC-MS. Seventy-two compounds were identified in the oil, representing 85.42% of the total oil. All unidentified compounds were minor components. The major components were camphor (20.89%), β-caryophyllene (5.71%), α-thujone (5.46%), piperitone (5.27%), epi-sesquiphellandrene (5.16%), α-pinene (4.97%), 1,8-cineole (4.52%), β-pinene (4.45%), and camphene (4.19%). Some previous results reported that the essential oils from C. coronarium and C. indicum contain monoterpene hydrocarbons and oxygenated monoterpenes such as α-pinene, β-pinene, camphene, 1,8-cineole, α-thujone, camphor, and sesquiterpene hydrocarbon β-caryophyllene as major components and these components contribute to antimicrobial and antifungal properties of the oil [35, 36].
5. Conclusion
This study has proved that C. boreale essential oil exhibited significant inhibition of bacterial growth, adherence capacity, and acid production of S. mutans. Furthermore, C. boreale essential oil also inhibited the transcription level of several virulence factors such as gtfB, gtfC, gtfD, gbpB, spaP, brpA, relA, and vicR of S. mutans. In GC and GC-MS analysis, the major components were camphor, β-caryophyllene, α-thujone, piperitone, epi-sesquiphellandrene, α-pinene, 1,8-cineole, β-pinene, and camphene. Therefore, C. boreale essential oil appears to be a promising new agent that may prevent dental caries.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (no. 2013R1A1A4A03011203).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Hamada S., Torii M. Interaction of glucosyltransferase from Streptococcus mutans with various glucans. Journal of General Microbiology. 1980;116(1):51–59. doi: 10.1099/00221287-116-1-51. [DOI] [PubMed] [Google Scholar]
- 2.Wang X.-Y., Zhang Q., Chen Z. A possible role of LIM mineralization protein 1 in tertiary dentinogenesis of dental caries treatment. Medical Hypotheses. 2007;69(3):584–586. doi: 10.1016/j.mehy.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 3.Oong E. M., Griffin S. O., Kohn W. G., Gooch B. F., Caufield P. W. The effect of dental sealants on bacteria levels in caries lesions: a review of the evidence. Journal of the American Dental Association. 2008;139(3):271–278. doi: 10.14219/jada.archive.2008.0156. [DOI] [PubMed] [Google Scholar]
- 4.Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiological Reviews. 1986;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiater A., Choma A., Szczodrak J. Insoluble glucans synthesized by cariogenic streptococci: a structural study. Journal of Basic Microbiology. 1999;39(4):265–273. doi: 10.1002/(sici)1521-4028(199909)39:4x0003C;265::aid-jobm265x003E;3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Hardie J. M. Oral microbiology: current concepts in the microbiology of dental caries and periodontal disease. British Dental Journal. 1992;172(7):271–278. doi: 10.1038/sj.bdj.4807849. [DOI] [PubMed] [Google Scholar]
- 7.Pasquantonio G., Greco C., Prenna M., et al. Antibacterial activity and anti-biofilm effect of chitosan against strains of Streptococcus mutans isolated in dental plaque. International Journal of Immunopathology and Pharmacology. 2008;21(4):993–997. doi: 10.1177/039463200802100424. [DOI] [PubMed] [Google Scholar]
- 8.Pan P. H., Finnegan M. B., Sturdivant L., Barnett M. L. Comparative antimicrobial activity of an essential oil and an amine fluoride/stannous fluoride mouthrinse in vitro. Journal of Clinical Periodontology. 1999;26(7):474–476. doi: 10.1034/j.1600-051x.1999.260710.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Bayati F. A. Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Annals of Clinical Microbiology and Antimicrobials. 2009;8, article 20 doi: 10.1186/1476-0711-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K.-H., Kim B.-S., Keum K.-S., et al. Essential oil of Curcuma longa inhibits Streptococcus mutans biofilm formation. Journal of Food Science. 2011;76(9):H226–H230. doi: 10.1111/j.1750-3841.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.-H., Seo B.-R., Kim H.-Y., et al. Inhibitory effect of Aralia continentalis on the cariogenic properties of Streptococcus mutans . Journal of Ethnopharmacology. 2011;137(2):979–984. doi: 10.1016/j.jep.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Lee D. Y., Choi G., Yoon T., Cheon M. S., Choo B. K., Kim H. K. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. Journal of Ethnopharmacology. 2009;123(1):149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Hu C.-Q., Chen K. E., Shi Q., Kilkuskie R. E., Cheng Y.-C., Lee K.-H. Anti-aids agents, 10. Acacetin-7-O-β-D-galactopyranoside, an anti-HIV principle from Chrysanthemum morifolium and a structure-activity correlation with some related flavonoids. Journal of Natural Products. 1994;57(1):42–51. doi: 10.1021/np50103a006. [DOI] [PubMed] [Google Scholar]
- 14.Shafaghat A., Sadeghi H., Oji K. Composition and antibacterial activity of essential oils from leaf, stem and root of Chrysanthemum parthenium (L.) Bernh. from Iran. Natural Product Communications. 2009;4(6):859–860. [PubMed] [Google Scholar]
- 15.Kim K. J., Kim Y. H., Yu H. H., et al. Antibacterial activity and chemical composition of essential oil of Chrysanthemum boreale . Planta Medica. 2003;69(3):274–277. doi: 10.1055/s-2003-38479. [DOI] [PubMed] [Google Scholar]
- 16.Nakahara K., Kawabata S., Ono H., et al. Inhibitory effect of oolong tea polyphenols on glucosyltransferases of mutans Streptococci . Applied and Environmental Microbiology. 1993;59(4):968–973. doi: 10.1128/aem.59.4.968-973.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T.-H., Bae G.-S., Oh H.-J., et al. 2′,4′,6′-tris(methoxymethoxy) chalcone (TMMC) attenuates the severity of cerulein-induced acute pancreatitis and associated lung injury. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2011;301(4):G694–G706. doi: 10.1152/ajpgi.00210.2010. [DOI] [PubMed] [Google Scholar]
- 18.Jeong S.-I., Kim B.-S., Keum K.-S., et al. Kaurenoic acid from Aralia continentalis inhibits biofilm formation of Streptococcus mutans . Evidence-Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/160592.160592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shemesh M., Tam A., Steinberg D. Expression of biofilm-associated genes of Streptococcus mutans in response to glucose and sucrose. Journal of Medical Microbiology. 2007;56(11):1528–1535. doi: 10.1099/jmm.0.47146-0. [DOI] [PubMed] [Google Scholar]
- 20.van den Dool H., Kratz P. D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. Journal of Chromatography A. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 21.Jennings W., Shibamoto T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. New York, NY, USA: Academic Press; 1981. [Google Scholar]
- 22.Babushok V. I., Linstrom P. J., Zenkevich I. G. Retention indices for frequently reported compounds of plant essential oils. Journal of Physical and Chemical Reference Data. 2011;40(4) doi: 10.1063/1.3653552.043101 [DOI] [Google Scholar]
- 23.Salam M. A., Matsumoto N., Matin K., et al. Establishment of an animal model using recombinant NOD.B10.D2 mice to study initial adhesion of oral streptococci. Clinical and Diagnostic Laboratory Immunology. 2004;11(2):379–386. doi: 10.1128/cdli.11.2.379-386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köhler B., Birkhed D., Olsson S. Acid production by human strains of Streptococcus mutans and Streptococcus sobrinus . Caries Research. 1995;29(5):402–406. doi: 10.1159/000262099. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annual Review of Microbiology. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- 26.Marsh P. D., Bradshaw D. J. Dental plaque as a biofilm. Journal of Industrial Microbiology. 1995;15(3):169–175. doi: 10.1007/BF01569822. [DOI] [PubMed] [Google Scholar]
- 27.Aoki H., Shiroza T., Hayakawa H., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glycosyltransferase gene coding for insoluble glucan synthesis. Infection and Immunity. 1986;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg D., Moreinos D., Featherstone J., Shemesh M., Feuerstein O. Genetic and physiological effects of noncoherent visible light combined with hydrogen peroxide on Streptococcus mutans in biofilm. Antimicrobial Agents and Chemotherapy. 2008;52(7):2626–2631. doi: 10.1128/aac.01666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton I. R., Buckley N. D. Adaptation by Streptococcus mutans to acid tolerance. Oral microbiology and immunology. 1991;6(2):65–71. doi: 10.1111/j.1399-302x.1991.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 30.Banas J. A. Virulence properties of Streptococcus mutans . Frontiers in Bioscience. 2004;9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson H. F., Lamont R. J. Streptococcal adhesion and colonization. Critical Reviews in Oral Biology and Medicine. 1997;8(2):175–200. doi: 10.1177/10454411970080020601. [DOI] [PubMed] [Google Scholar]
- 32.Napimoga M. H., Höfling J. F., Klein M. I., Kamiya R. U., Gonçalves R. B. Tansmission, diversity and virulence factors of Sreptococcus mutans genotypes. Journal of oral science. 2005;47(2):59–64. doi: 10.2334/josnusd.47.59. [DOI] [PubMed] [Google Scholar]
- 33.Xu X., Zhou X. D., Wu C. D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans . Antimicrobial Agents and Chemotherapy. 2011;55(3):1229–1236. doi: 10.1128/aac.01016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemos J. A. C., Brown T. A., Jr., Burne R. A. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans . Infection and Immunity. 2004;72(3):1431–1440. doi: 10.1128/iai.72.3.1431-1440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Castellanos P. P., Bishop C. D., Pascual-Villalobos M. J. Antifungal activity of the essential oil of flowerheads of garland chrysanthemum (Chrysanthemum coronarium) against agricultural pathogens. Phytochemistry. 2001;57(1):99–102. doi: 10.1016/s0031-9422(00)00461-1. [DOI] [PubMed] [Google Scholar]
- 36.Jung E.-K. Chemical composition and antimicrobial activity of the essential oil of Chrysanthemum indicum against oral bacteria. Journal of Bacteriology and Virology. 2009;39(2):61–69. doi: 10.4167/jbv.2009.39.2.61. [DOI] [Google Scholar]