Abstract
The traditional herb Plumula Nelumbinis is widely used in the world because it has many biological activities, such as anti-inflammation, antioxidant, antihypertension, and butyrylcholinesterase inhibition. However, the action of Plumula Nelumbinis on airway smooth muscle (ASM) relaxation has not been investigated. A chloroform extract of Plumula Nelumbinis (CEPN) was prepared, which completely inhibited precontraction induced by high K+ in a concentration-dependent manner in mouse tracheal rings, but it had no effect on resting tension. CEPN also blocked voltage-dependent L-type Ca2+ channel- (VDCC-) mediated currents. In addition, ACh-induced precontraction was also completely blocked by CEPN and partially inhibited by nifedipine or pyrazole 3. Besides, CEPN partially reduced ACh-activated nonselective cation channel (NSCC) currents. Taken together, our data demonstrate that CEPN blocked VDCC and NSCC to inhibit Ca2+ influx, resulting in relaxation of precontracted ASM. This finding indicates that CEPN would be a candidate of new potent bronchodilators.
1. Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are highly prevalent diseases that currently affect more than 300 million individuals worldwide. Excessive airway obstruction is a cardinal symptom in asthma and COPD [1–3]. Airway smooth muscle cells (ASMCs), one important cell type in the respiratory system, contribute to the symptoms of these airway obstructive diseases [4]. Excessive contraction of ASMCs can narrow the airway lumen, which limits gas exchange and threatens the lives of asthmatics and COPD patients [5, 6]. Therefore, bronchodilators, such as β2 adrenergic agonists, are standard medicines that are widely used for the pharmacological management of these diseases [7, 8]. However, the currently available bronchodilators have serious side effects; thus, the development of novel effective and safe bronchodilators is an important task for asthma and COPD therapy.
Some of traditional herbs have long been used in the treatment of asthma and COPD because they can effectively relax ASM contraction [9–11]. This inspired us to identify novel bronchodilators from the traditional herbs. This study investigated whether an extract of Plumula Nelumbinis relaxed precontracted ASM induced by high K+ and ACh and the underlying mechanism.
Plumula Nelumbinis is the green germ of a mature lotus (Nelumbo nucifera Gaertn) seed [12, 13]. This traditional medicine was documented in a well-known materia medica “Bencao Gangmu” by Shizhen Li during the Ming dynasty (1596). Plumula Nelumbinis has many pharmacological and physiological activities, including anti-inflammation [14, 15], antioxidant [16], antihypertension [17], and butyrylcholinesterase inhibition [18]. Several active ingredients in Plumula Nelumbinis exhibit beneficial biological activities on smooth muscle: neferine markedly inhibits angiotensin II-stimulated proliferation, reduces 45Ca-influx induced by phenylephrine in vascular smooth muscle [19, 20], and relaxes corpus cavernosum smooth muscle cells [21], and isoliensinine possesses antiproliferative effects on coronary arterial smooth muscle cells [22, 23]. However, the effects of Plumula Nelumbinis extract on ASMC tension have not been studied previously.
The present study investigated the relaxation effects of a chloroform extract of Plumula Nelumbinis (CEPN) on mouse ASM precontraction induced by high K+ and ACh. The results show that CEPN inhibited VDCCs and NSCCs, which then resulted in relaxation of precontracted ASM.
2. Materials and Methods
2.1. Plant Material
Plumula Nelumbinis, germs of Nelumbo nucifera Gaertn seeds, were collected in Honghu City, Hubei Province, China, in October 2013, and were identified by Professor Dr. Ding-rong Wan, College of Pharmacy, South-Central University for Nationalities. A voucher specimen (SCUN201310006) is deposited at the Herbarium of the College of Pharmacy, South-Central University for Nationalities, China.
2.2. Extraction and Isolation
Plumula Nelumbinis (1 Kg) were air-dried, milled into powder, and extracted at room temperature with 95% ethanol (3 × 4 L, 2 h each). Extracts were centrifuged, and the supernatants were collected. The ethanol extract (193 g) was next evaporated to dryness under reduced pressure using a rotary evaporator and immersed in a 2% HCl solution (500 mL). Residues were extracted with petroleum ether (3 × 300 mL, 4 h each) to remove lipids. Ammonia adjusted the pH of the filtrate to 10, and the crude sample was extracted with chloroform (5 × 300 mL, 4 h each). The chloroform extract was evaporated under reduced pressure, and the extraction yield was 0.71% of the raw material dry weight. The dried chloroform extract of Plumula Nelumbinis (CEPN) was dissolved in 3% DMSO for the experiments.
2.3. Reagents
Nifedipine, acetylcholine chloride (ACh), niflumic acid (NA), tetraethylammonium chloride (TEA), and pyrazole 3 (Pyr3) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China).
2.4. Animals
Sexually mature male BALB/c mice were purchased from the Hubei Provincial Center for Disease Control and Prevention (Wuhan, China). Mice were housed at room temperature (20–25°C) and constant humidity (50–60%) under a 12 h light-dark cycle in an SPF grade laboratory. The animal study was performed according to the guidelines of the Institutional Animal Care and Use Committee of the South-Central University for Nationalities (Wuhan, China) and approved by the Animal Care and Ethics Committee of the South-Central University for Nationalities (QHL-2, 02-03-2012).
2.5. ASM Contraction Measurement
Mouse ASM contraction was measured in tracheal rings [24, 25]. Mice were sacrificed using an intraperitoneal injection of sodium pentobarbital (150 mg/kg), and tracheae were isolated and quickly transferred to ice cold PSS (composition in mM: NaCl 135, KCl 5, MgCl2 1, CaCl2 2, HEPES 10, glucose 10, pH 7.4). Connective tissues were removed, and small rings (~5 mm) were cut from the bottom of tracheae. Each ring was mounted with a preload of 0.5 g in an organ bath with a 10 mL capacity containing PSS bubbled with 95% O2-5% CO2 at 37°C. Tracheal rings were equilibrated for 60 min, precontracted with high K+ (80 mM) or ACh (10−4 M), washed, and rested 3 times. Experiments were performed following an additional 30 min rest.
2.6. Isolation of Single ASMCs
Mouse ASMCs were isolated as previous method [25, 26]. Briefly, tracheae were isolated as described above and digested in an ice-cold low-Ca2+ physiological saline solution (LCPSS) (composition in mM: NaCl 135, KCl 5, MgSO4 1, glucose 10, HEPES 10, CaCl2 0.1, pH 7.4) containing 1 mg/mL papain, 0.5 mg/mL dithioerythritol, and 1 mg/mL bovine serum albumin (BSA) at 37°C for 20 min. Tissues were transferred to LCPSS containing 1 mg/mL collagenase H, 1 mg/mL dithiothreitol, and 1 mg/mL BSA and incubated at 37°C for 20 min. Tissues were washed and gently triturated in LCPSS to yield single ASMCs for experiments.
2.7. Measurement of VDCC Currents
VDCC currents were measured using Ba2+ as the charge carrier and an EPC-10 patch-clamp amplifier (HEKA, Lambrecht, Germany). The pipette solution contained the following (in mM): CsCl 130, EGTA 10, MgCl2 4, Mg-ATP 4, HEPES 10, TEA 10, and pH 7.2 (adjusted with CsOH). The composition of the bath solution was (in mM) NaCl 105, CsCl 6, BaCl2 27.5, glucose 11, HEPES 10, TEA-Cl 10, NA 0.1, and pH 7.4 (adjusted with NaOH). ASMCs were patched and held at −70 mV. Currents were measured following depolarization for 1000 ms from −70 to +30 mV in 10 mV increments every 10 s.
2.8. Measurement of NSCC Currents
The pipette solution contained the following chemicals for the measurement of NSCC currents (in mM): CsCl 18, cesium acetate 108, MgCl2 1.2, HEPES 10, EGTA 3, CaCl2 1, and pH 7.2 (adjusted with Tris). The free Ca2+ concentration was approximately 70 nM, as calculated using WEBMAXC STANDARD. The bath solution was PSS without K+ containing 10 µM nifedipine, 100 µM NA, and 10 mM TEA to block VDCC, Cl−, and K+ currents, respectively. ACh-induced NSCC currents were recorded with a ramp using a perforated whole-cell configuration with a holding potential of −60 mV. The ramp was performed over 500 ms from −80 to +60 mV.
2.9. Statistical Analysis
Statistical analysis was performed with Student's t-test using Origin 9.0 software (OriginLab, Northampton, USA). Statistical significance was defined as P < 0.05. Data are expressed as the means ± SEM.
3. Results
3.1. CEPN Inhibits High K+-Induced Precontraction
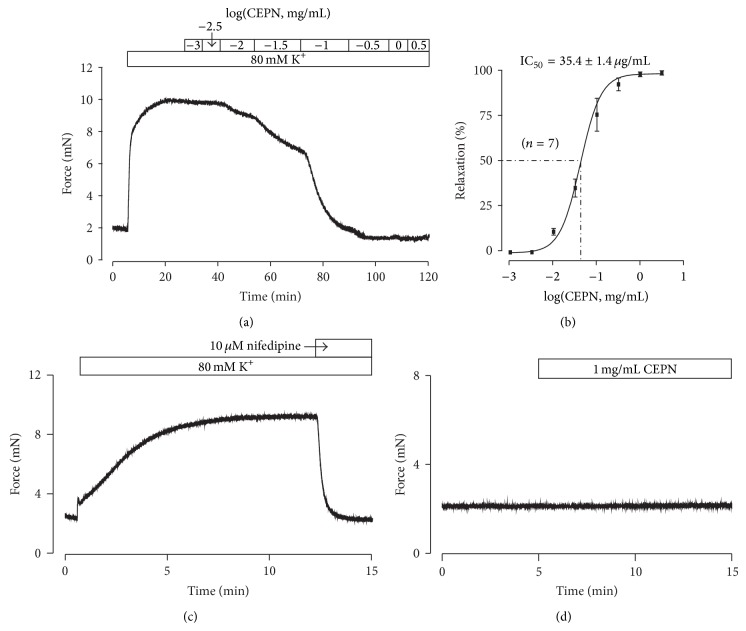
We observed the effects of CEPN on high K+-induced ASM precontraction to investigate whether CEPN relaxed ASM. High K+ (80 mM) induced contraction in a mouse tracheal ring, and CEPN (1 µg–3.16 mg/mL) was cumulatively added to the organ bath. ASM contraction was gradually reduced to baseline (Figure 1(a)). CEPN was then removed and the high K+-induced contraction will restore to 26.7 ± 6.2% (n = 6) after 30 min. The results from 7 experiments yielded a half-maximal inhibition (IC50) of 35.4 ± 1.4 µg/mL and a maximal relaxation of 103.4 ± 1.3% (Figure 1(b)). It is well known that high K+ induces depolarization resulting in the activation of VDCCs, which allows a Ca2+ influx to trigger contraction [27, 28]. This pathway was confirmed using a selective blocker of VDCCs, nifedipine (10 µM), which completely blocked 80 mM K+-induced contractions (Figure 1(c)). CEPN inhibited precontraction, but it did not alter resting tension in 4 tracheal rings (Figure 1(d)). These data indicate that CEPN relaxed high K+-precontracted ASM via VDCC inhibition.
Figure 1.
Relaxant effects of CEPN on high K+-induced precontraction. (a) High K+ induced a steady-state contraction in a mouse tracheal ring, which was inhibited by CEPN in a concentration-dependent manner. (b) Dose-relaxation curve of CEPN based on the results of 7 different experiments shown in (a). (c) High K+-induced precontraction was completely blocked by nifedipine. This experiment was performed in 8 tracheal rings from 8 mice, and the result was reproducible across experiments. (d) CEPN had no effect on resting tension in 4 rings. These results indicate that the CEPN-induced relaxation might result from blockade of VDCCs.
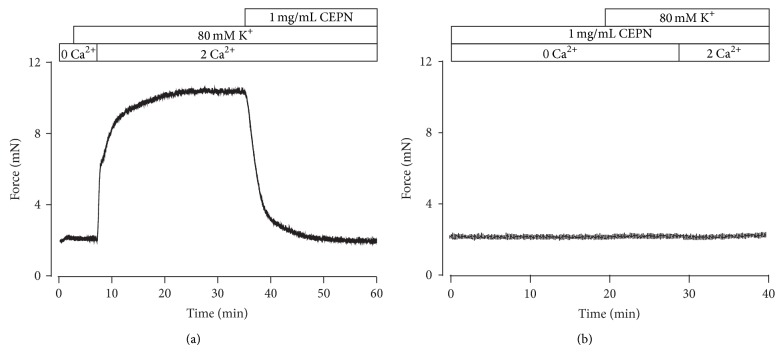
The above results suggest that the relaxation induced by CEPN might be due to the termination of VDCC-mediated Ca2+ influx. This hypothesis was examined in the following experiments. Figure 2(a) shows that high K+ did not induce contraction under Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA); however, contraction immediately occurred following Ca2+ restoration (2 mM), and CEPN (1 mg/mL) completely inhibited contraction. However, a Ca2+ restoration-induced contraction was not observed in the presence of 1 mg/mL CEPN (Figure 2(b)). This result suggests that CEPN-evoked relaxation of high K+-induced precontraction was completely dependent on the inhibition on VDCC-mediated Ca2+ influx.
Figure 2.
CEPN blocks high K+-evoked Ca2+ influx. (a) A representative tracing of 4 experiments. Under Ca2+-free conditions (0 Ca2+ and 0.5 mM EGTA), high K+ did not evoke contraction in a tracheal ring. After the restoration of 2 mM Ca2+, a sustained contraction occurred, which was fully inhibited by CEPN. (b) In the presence of 1 mg/mL CEPN, the identical experiments as above were performed. A Ca2+ restoration-provoked contraction was not noted. This experiment was conducted in 6 tracheal rings. These results suggest that CEPN inhibits Ca2+ influx.
3.2. CEPN Blocks VDCC Currents
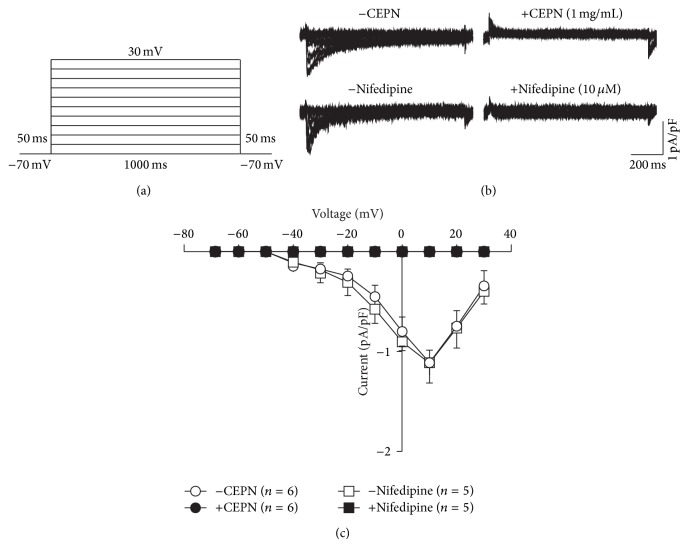
We used patch-clamp techniques to measure VDCC currents (Ba2+ as the carrier charge) [25] with voltage steps from −70 to +30 mV to further confirm the ability of CEPN to block VDCCs (Figures 3(a) and 3(b)). Currents were abrogated following applications of CEPN and nifedipine. Current-voltage (I-V) curves were constructed based on the results of 5 to 6 experiments (Figure 3(c)). These data indicate that CEPN blocked VDCCs.
Figure 3.
CEPN blocks VDCC currents. (a) The protocol used to measure VDCC currents in single ASMCs. (b) VDCC currents, recorded following depolarizations, were blocked by CEPN and nifedipine, respectively. (c) I-V relationships constructed based on the results of 5 to 6 experiments. These data indicate that CEPN blocks VDCCs.
3.3. CEPN Inhibits ACh-Induced Precontraction
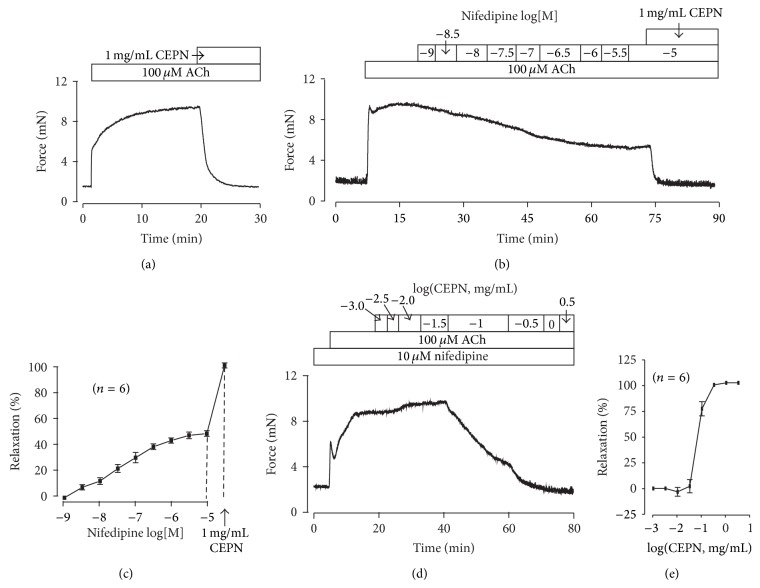
We next observed whether CEPN inhibited ACh-induced precontraction. Figure 4(a) shows that 1 mg/mL CEPN fully relaxed 100 µM ACh-induced contraction (100.7 ± 0.4%, n = 6). If CEPN was removed, the ACh-induced contraction will recover to 92.2 ± 3.4% (n = 7) within 30 min. Moreover, the precontraction was partially blocked following additions of nifedipine and the resistant component was totally inhibited by 1 mg/mL CEPN (Figure 4(b)). The maximal inhibition by nifedipine and CEPN was 52.1 ± 2.2%and 105.2 ± 2.0%, respectively (n = 6; Figures 4(b) and 4(c)). These results indicate that VDCCs and an unknown pathway mediate CEPN-induced relaxation of ACh-induced precontraction. Our data have showed that CEPN blocked VDCCs to induce relaxation of high K+-evoked precontraction. Therefore, we only focused on defining the unknown pathway. We first observed the role of the unknown pathway in the dose-response of CEPN relaxation. Figure 4(d) shows that VDCCs were blocked with 10 µM nifedipine, and ACh was added to induce a steady-state contraction. Contractions continuously declined to resting levels following cumulative additions of CEPN. The dose-response curve was constructed based on 6 experiments (Figure 4(e)). These data demonstrate that CEPN completely reduced ACh-induced precontraction through the inhibition of VDCCs and an unknown pathway.
Figure 4.
CEPN inhibits ACh-induced precontraction. (a) ACh induced a contraction in a tracheal ring that was inhibited completely by CEPN. This experiment was repeated in 6 rings. (b) Nifedipine partially reduced the ACh-induced contraction in a dose-dependent manner. The resistant contraction was inhibited by CEPN. The summary results from 6 experiments are shown in (c). (d) In the presence of nifedipine, ACh induced a typical contraction, which was dose-dependently inhibited by CEPN. The summary results from 6 experiments exhibited in (e). These results show that CEPN inhibits VDCCs and another pathway to induce relaxation.
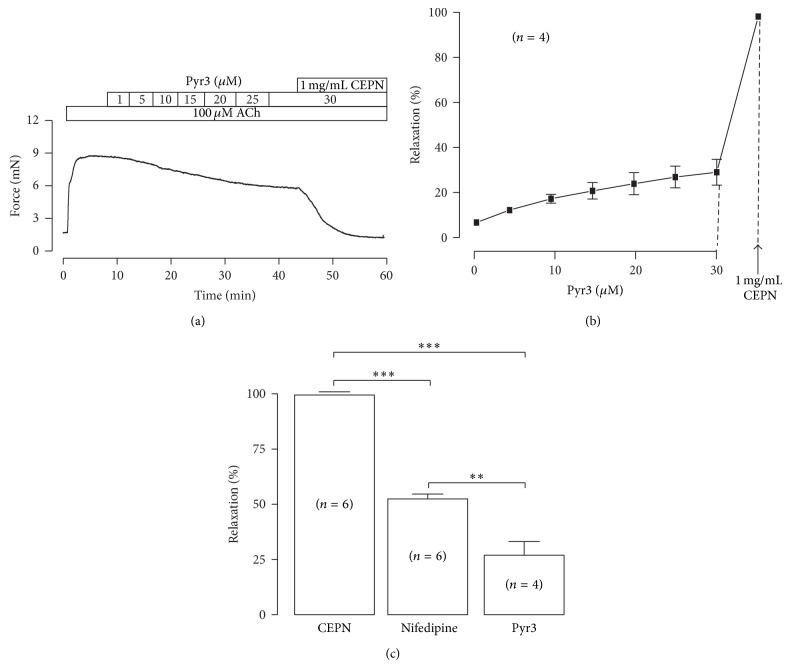
In addition, NSCCs predominantly mediate ASM contraction [29]. We used Pyr3 (an inhibitor of nonselective cation channel TRPC3 and Orai1 [30]) to examine whether TRPC3/Orai1 channels mediated ACh-induced contraction [31, 32] and found which was partially prevented by Pyr3 in a dose-dependent manner (Figures 5(a) and 5(b)). The Pyr3-induced maximal inhibition was 26.8 ± 6.2% (Figure 5(c), n = 4, 30 µM Pyr3) and the remained contraction was completely blocked by CEPN (Figures 5(a) and 5(b)). The Pyr3-induced relaxation was about half of that induced by nifedipine (52.1 ± 2.2%) as shown in Figure 5(c), suggesting that both VDCCs and NSCCs are involved in CEPN-induced relaxation.
Figure 5.
Pyr3 inhibits ACh-induced contraction. (a) Pyr3, a blocker of TRPC3 and Orai1 channels, partially inhibited ACh-induced contraction in a dose-dependent manner. The remained contraction was completely blocked by 1 mg/mL CEPN. The dose-response from 4 experiments is shown in (b). (c) Comparison of the relaxant effects of CEPN, nifedipine, and Pyr3 on ACh-induced contraction. ** P < 0.01, *** P < 0.001.
3.4. CEPN Blocks ACh-Activated Ca2+ Influx
Contraction is primarily dependent on the intracellular Ca2+ increase [33, 34]. Therefore, we investigated whether the unknown pathway involved Ca2+ influx. ACh induced a transient contraction in the presence of nifedipine (10 µM) and Ca2+-free conditions (0 mM Ca2+ and 0.5 mM EGTA) (Figure 6). Addition of 2 mM Ca2+ triggered a sustained contraction, which was blocked by 1 mg/mL CEPN. These results indicate that the steady-state contraction induced by ACh was due to a nifedipine-resistant Ca2+ influx that was inhibited by CEPN. Therefore, the nifedipine-resistant Ca2+ influx was defined as the unknown pathway.
Figure 6.
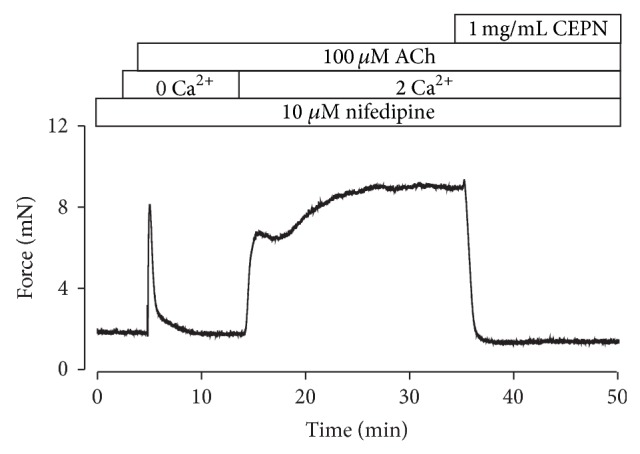
CEPN blocks ACh-evoked Ca2+ influx. Representative result of 4 experiments in the presence of nifedipine. Under Ca2+-free conditions (0 Ca2+ + 0.5 mM EGTA), ACh induced a fast transient contraction. Following the addition of 2 mM Ca2+, a strong sustained contraction occurred, which was entirely reversed by CEPN. These experiments indicate that CEPN inhibits the nifedipine-resistant Ca2+ influx activated by ACh.
3.5. CEPN Inhibits NSCCs
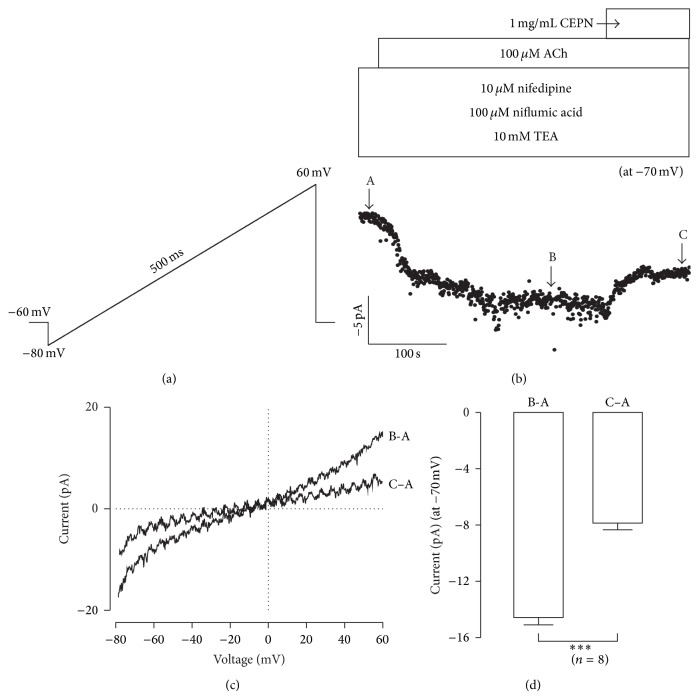
We measured ACh-activated NSCC currents and observed the effects of CEPN on these currents to further determine the nature of the Ca2+ influx because ACh activates NSCCs to increase intracellular Ca2+ [25, 35, 36]. ACh-induced NSCC currents were purely isolated in the presence of nifedipine, NA and TEA, and were recorded by a ramp (Figure 7(a)). NSCC currents were partially blocked by CEPN (Figure 7(b)). Two representative ramp current traces at time points b and c (indicated in Figure 7(b)) are shown in Figure 7(c). The leak ramp currents at time point a (indicated in Figure 7(b)) were subtracted. The mean values of current amplitudes at −70 mV were −14.5 ± 0.5 pA and −7.8 ± 0.5 pA at time points b and c, respectively (n = 8, Figure 7(d)). These data indicate that CEPN partially inhibited ACh-induced NSCCs.
Figure 7.
CEPN inhibits NSCC currents. (a) The ramp employed to record NSCC currents in single ASMCs. (b) Ramp current values at −70 mV were used to construct current-time traces, which exhibit ACh-induced NSCC currents that were partially blocked by CEPN. (c) The net ramp currents at time points B and C (shown in (b)). (d) At −70 mV, the mean currents at time points B and C. *** P < 0.001. These data suggest that CEPN partially inhibits NSCCs.
4. Discussion
The present study demonstrated that CEPN induced strong relaxation in precontracted mouse ASM induced by high K+ and ACh through blockade of Ca2+ influx mediated by VDCCs and NSCCs. This finding suggests that CEPN may be a potential bronchodilator.
High K+ induces membrane depolarization, which opens VDCCs that mediate extracellular Ca2+ influx and induce contractions [27, 28]. The present study showed that CEPN completely inhibited high K+-induced contractions in ASM (Figures 1(a) and 1(b)), which suggests that this inhibition was due to the blockade of VDCCs by CEPN. We designed three different experiments to further support this result and showed that the selective blocker of VDCCs nifedipine totally inhibited high K+-evoked contractions (Figure 1(c)), CEPN inhibited Ca2+ influx-induced contractions (Figure 2), and CEPN directly blocked VDCC-mediated currents (Figures 3(b) and 3(c)). These data demonstrate that CEPN blocked VDCCs, which terminated the Ca2+ influx that leads to the relaxation of high K+-induced precontracted mouse ASM.
The muscarinic receptor agonist ACh activates both VDCCs and NSCCs, which leads to Ca2+ influx and an increase in intracellular Ca2+ to trigger ASM contraction [35, 36]. This pathway was demonstrated in our recent results [25]. The present findings implied that CEPN-induced relaxation of ACh-evoked contraction (Figure 4(a)) resulted from the inhibition of both VDCCs and NSCCs by CEPN. This is because the fact that (1) CEPN completely blocked ACh-induced contraction (Figure 4(a)), (2) the selective blocker of VDCCs, nifedipine, partially inhibited ACh-induced contraction and the remaining component was blocked by CEPN (Figures 4(b), 4(c), 4(d), and 4(e)), and the latter was due to the inhibition of Ca2+ influx by CEPN (Figure 6), (3) ACh-induced NSCC currents, which mediate Ca2+ influx, were partially blocked by CEPN (Figure 7), and (4) ACh-induced contraction was partially blocked by Pyr3, a selective blocker of nonselective cation channel TRPC3 and Orai (Figure 5). Therefore, we conclude that CEPN blocks VDCC- and NSCC-mediated Ca2+ influx to result in relaxation of ACh-precontracted ASM.
5. Conclusions
CEPN induced relaxation of precontracted mouse ASM through the inactivation of VDCCs and NSCCs. This study supports the development of new drugs from CEPN to treat airway hyperresponsiveness in asthmatic and COPD patients. Further investigation will be required to identify the components that are responsible for the relaxation action.
Acknowledgments
This work was supported by the 973 research program (2011CB809100 to Qing-Hua Liu and Guangju Ji), the National Natural Science Foundation of China (31140087 and 30971514 to Qing-Hua Liu, 81400015 to Weiwei Chen, and 81170227 to Jinhua Shen), the National Key Technology Support Program (2012BAI39B01 to Jinhua Shen and Chaojun Li), and the Natural Science Foundation of Hubei Province, China (2013CFB455 to Weiwei Chen).
Conflict of Interests
The authors declare no conflict of interests.
Authors' Contribution
Li Tan, Weiwei Chen, and Ming-Yu Wei contributed equally to this work.
References
- 1.An S. S., Bai T. R., Bates J. H. T., et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. European Respiratory Journal. 2007;29(5):834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert R. K., Wiggs B. R., Kuwano K., Hogg J. C., Pare P. D. Functional significance of increased airway smooth muscle in asthma and COPD. Journal of Applied Physiology. 1993;74(6):2771–2781. doi: 10.1063/1.354625. [DOI] [PubMed] [Google Scholar]
- 3.Solway J., Fredberg J. J. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. American Journal of Respiratory Cell and Molecular Biology. 1997;17(2):144–146. doi: 10.1165/ajrcmb.17.2.f137. [DOI] [PubMed] [Google Scholar]
- 4.Hershenson M. B., Brown M., Camoretti-Mercado B., Solway J. Airway smooth muscle in asthma. Annual Review of Pathology: Mechanisms of Disease. 2008;3:523–555. doi: 10.1146/annurev.pathmechdis.1.110304.100213. [DOI] [PubMed] [Google Scholar]
- 5.Postma D. S., Kerstjens H. A. M. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1998;158(5):S187–S192. doi: 10.1164/ajrccm.158.supplement_2.13tac170. [DOI] [PubMed] [Google Scholar]
- 6.West A. R., Syyong H. T., Siddiqui S., et al. Airway contractility and remodeling: links to asthma symptoms. Pulmonary Pharmacology and Therapeutics. 2013;26(1):3–12. doi: 10.1016/j.pupt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Chung K. F., Caramori G., Adcock I. M. Inhaled corticosteroids as combination therapy with β-adrenergic agonists in airways disease: present and future. European Journal of Clinical Pharmacology. 2009;65(9):853–871. doi: 10.1007/s00228-009-0682-z. [DOI] [PubMed] [Google Scholar]
- 8.Han M. K., Martinez F. J. Pharmacotherapeutic approaches to preventing acute exacerbations of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2011;8(4):356–362. doi: 10.1513/pats.201102-016rm. [DOI] [PubMed] [Google Scholar]
- 9.Tagaya E., Tamaoki J., Kawatani K., Taira M., Nagai A. Inhibition of airway smooth muscle tone by Chinese herbal medicines. European Respiratory Journal. 2000;16(6):1123–1128. doi: 10.1034/j.1399-3003.2000.16f18.x. [DOI] [PubMed] [Google Scholar]
- 10.Ge Y.-B., Dai Q., Wan D.-R., Liu Q.-H., Mei Z.-N. Relaxant effect of 1-butanol fraction from Elaeagnus pungens leaf through inhibiting L-type Ca2+ channel on guinea pig tracheal smooth muscle. Journal of Ethnopharmacology. 2013;150(1):196–201. doi: 10.1016/j.jep.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Ghayur M. N., Gilani A. H., Janssen L. J. Intestinal, airway, and cardiovascular relaxant activities of thymoquinone. Evidence-Based Complementary and Alternative Medicine. 2012;2012:13. doi: 10.1155/2012/305319.305319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai S., Wahile A., Mukherjee K., Saha B. P., Mukherjee P. K. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. Journal of Ethnopharmacology. 2006;104(3):322–327. doi: 10.1016/j.jep.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Karki R., Jung M.-A., Kim K.-J., Kim D.-W. Inhibitory effect of Nelumbo nucifera (Gaertn.) on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7. doi: 10.1155/2012/153568.153568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao C. H., Lin J. Y. Purified active lotus plumule (Nelumbo nucifera Gaertn) polysaccharides exert anti-inflammatory activity through decreasing Toll-like receptor-2 and -4 expressions using mouse primary splenocytes. Journal of Ethnopharmacology. 2013;147(1):164–173. doi: 10.1016/j.jep.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Lin J.-Y., Lai Y.-S., Liu C.-J., Wu A.-R. Effects of lotus plumule supplementation before and following systemic administration of lipopolysaccharide on the splenocyte responses of BALB/c mice. Food and Chemical Toxicology. 2007;45(3):486–493. doi: 10.1016/j.fct.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Xie Y., Zhang Y., Zhang L.-T., Zeng S.-X., Guo Z.-B., Zheng B.-D. Protective effects of alkaloid compounds from Nelumbinis Plumula on tert-butyl hydroperoxide-induced oxidative stress. Molecules. 2013;18(9):10285–10300. doi: 10.3390/molecules180910285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y., Zheng L., Liu S., Peng Z., Zhang S. Total flavonoids from Plumula Nelumbinis suppress angiotensin II-induced fractalkine production by inhibiting the ROS/NF-κB pathway in human umbilical vein endothelial cells. Experimental and Therapeutic Medicine. 2014;7(5):1187–1192. doi: 10.3892/etm.2014.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin Z., Wang H., Fu Q., et al. Simultaneous separation, identification and activity evaluation of three butyrylcholinesterase inhibitors from Plumula nelumbinis using on-line HPLC-UV coupled with ESI-IT-TOF-MS and BChE biochemical detection. Talanta. 2013;110:180–189. doi: 10.1016/j.talanta.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Li X.-C., Tong G.-X., Zhang Y., et al. Neferine inhibits angiotensin II-stimulated proliferation in vascular smooth muscle cells through heme oxygenase-1. Acta Pharmacologica Sinica. 2010;31(6):679–686. doi: 10.1038/aps.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y. Effects of neferine on the 45Ca-influx and efflux induced by activation of α-1 adrenoceptor of vascular smooth muscle. Zhongguo Zhongyao Zazhi. 1996;21(2):117–118. [PubMed] [Google Scholar]
- 21.Chen J., Liu J. H., Jiang Z. J., et al. Effects of neferine on cytosolic free calcium concentration in corpus cavernosum smooth muscle cells of rabbits. Andrologia. 2007;39(4):141–145. doi: 10.1111/j.1439-0272.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Xiao J. H., Zhang Y. L., Ding L. L., Feng X. L., Wang J. L. Effects of isoliensinine on proliferation of porcine coronary arterial smooth muscle cells induced by phenylephrine. Yaoxue Xuebao. 2005;40(2):105–110. [PubMed] [Google Scholar]
- 23.Xiao J.-H., Zhang Y.-L., Feng X.-L., Wang J.-L., Qian J.-Q. Effects of isoliensinine on angiotensin II-induced proliferation of porcine coronary arterial smooth muscle cells. Journal of Asian Natural Products Research. 2006;8(3):209–216. doi: 10.1080/1028602042000325609. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q.-H., Zheng Y.-M., Korde A. S., et al. Protein kinase C-ε regulates local calcium signaling in airway smooth muscle cells. The American Journal of Respiratory Cell and Molecular Biology. 2009;40(6):663–671. doi: 10.1165/rcmb.2008-0323oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang T., Luo X.-J., Sai W.-B., et al. Non-selective cation channels mediate chloroquine-induced relaxation in precontracted mouse airway smooth muscle. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0101578.e101578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q.-H., Zheng Y.-M., Korde A. S., et al. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirschstein T., Rehberg M., Bajorat R., Tokay T., Porath K., Köhling R. High K+-induced contraction requires depolarization-induced Ca2+ release from internal stores in rat gut smooth muscle. Acta Pharmacologica Sinica. 2009;30(8):1123–1131. doi: 10.1038/aps.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sai W.-B., Yu M.-F., Wei M.-Y., et al. Bitter tastants induce relaxation of rat thoracic aorta precontracted with high K+ . Clinical and Experimental Pharmacology and Physiology. 2014;41(4):301–308. doi: 10.1111/1440-1681.12217. [DOI] [PubMed] [Google Scholar]
- 29.Ong H. L., Barritt G. J. Transient receptor potential and other ion channels as pharmaceutical targets in airway smooth muscle cells. Respirology. 2004;9(4):448–457. doi: 10.1111/j.1440-1843.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 30.Schleifer H., Doleschal B., Lichtenegger M., et al. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca2+ entry pathways. British Journal of Pharmacology. 2012;167(8):1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao Y., Plummer N. W., George M. D., Abramowitz J., Zhu M. X., Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson J. L., Shuttleworth T. J. How many Orai’s does it take to make a CRAC channel? Scientific Reports. 2013;3, article 1961 doi: 10.1038/srep01961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai Y., Sanderson M. J. The contribution of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2009;296(6):L947–L958. doi: 10.1152/ajplung.90288.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y., Zhang M., Sanderson M. J. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. The American Journal of Respiratory Cell and Molecular Biology. 2007;36(1):122–130. doi: 10.1165/rcmb.2006-0036oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleischmann B. K., Wang Y.-X., Kotlikoff M. I. Muscarinic activation and calcium permeation of nonselective cation currents in airway myocytes. American Journal of Physiology: Cell Physiology. 1997;272(1):C341–C349. doi: 10.1152/ajpcell.1997.272.1.C341. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y.-X., Fleischmann B. K., Kotlikoff M. I. M2 receptor activation of nonselective cation channels in smooth muscle cells: calcium and G(i)/G(o) requirements. American Journal of Physiology—Cell Physiology. 1997;273(2):C500–C508. doi: 10.1152/ajpcell.1997.273.2.C500. [DOI] [PubMed] [Google Scholar]