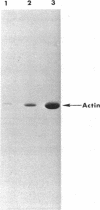
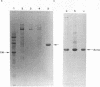
Abstract
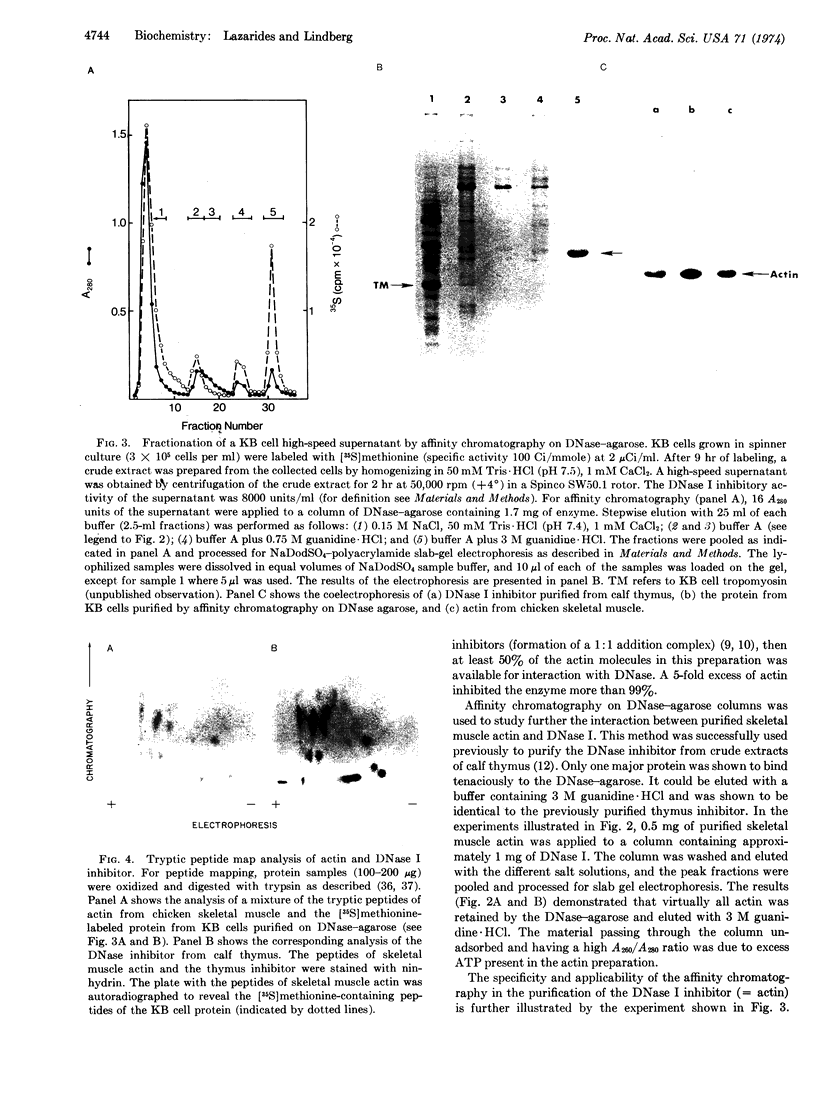
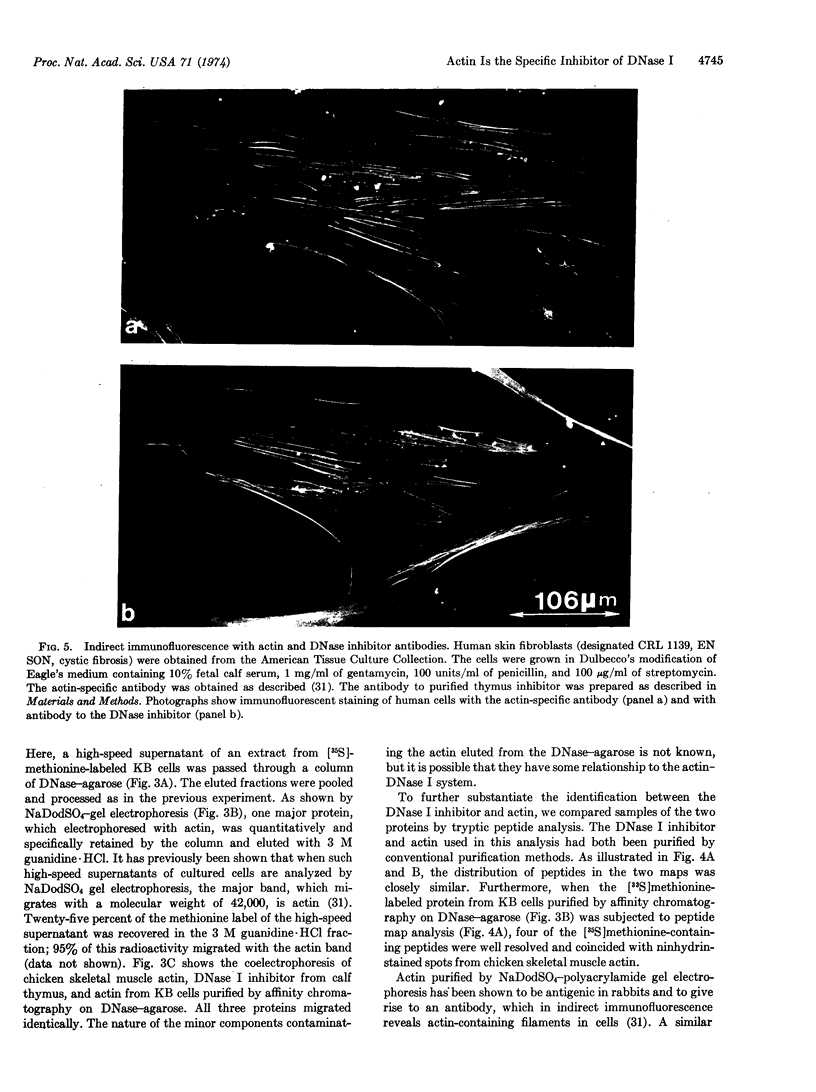
Various tissues and cells in culture contain a specific inhibitor of DNase I (EC 3.1.4.5). In this paper evidence is presented that this inhibitor is actin, one of the major structural proteins of muscle and nonmuscle cells. (a) The inhibitor is a major cellular component constituting 5-10% of the soluble protein. (b) It migrates with actin on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, having a characteristic molecular weight of 42,000. (c) It has an amino-acid composition closely similar to that of actin. (d) The peptide maps of the two proteins are nearly identical. (e) Skeletal muscle actin inhibits the enzymatic activity of DNase I. (f) DNase I-agarose affinity chromatography quantitatively retains purified skeletal muscle actin, and actin, specifically, from high-speed supernatants of whole cell extracts. (g) An antibody to purified inhibitor protein from calf thymus, used in indirect immunofluorescence on cells grown in culture, stains a two-dimensional network of fibers similar to that seen with an actin-specific antibody.
The observation that actin can be isolated by DNase-agarose affinity chromatography provides a useful tool for the biochemical study of actin under different physiological conditions.
Keywords: affinity chromatography, immunofluorescence, sodium dodecyl sulfate-gel electrophoresis
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Berger G., May P. Purification et propriétés d'un inhibiteur de la désoxyribonucléase pancreatique, extrait du sérum de rat. Biochim Biophys Acta. 1967 May 16;139(1):148–161. doi: 10.1016/0005-2744(67)90121-0. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Hoveke T. P., Rafelson M. E., Jr Human platelet actin. Isolation and properties. J Biol Chem. 1973 Jun 10;248(11):4083–4091. [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Cytoplasmic fibrils in living cultured cells. A light and electron microscope study. Protoplasma. 1967;64(4):349–380. doi: 10.1007/BF01666538. [DOI] [PubMed] [Google Scholar]
- Catley B. J., Moore S., Stein W. H. The carbohydrate moiety of bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):933–936. [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FESTY B., PAOLETTI C. MISE EN 'EVIDENCE ET PROPRI'ET'ES D'UN (OU PLUSIEURS) INHIBITEUR(S) NATUREL(S) DE LA D'ESOXYRIBONUCL'EASE NEUTRE PANCR'EATIQUE. C R Hebd Seances Acad Sci. 1963 Dec 4;257:3682–3685. [PubMed] [Google Scholar]
- GUPTA S., HERRIOTT R. M. Nucleases and their inhibitors in the cellular components of human blood. Arch Biochem Biophys. 1963 Apr;101:88–95. doi: 10.1016/0003-9861(63)90538-1. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. The preparation and characterization of an active derivative of bovine pancreatic deoxyribonuclease A formed by selective cleavage with alpha-chymotrypsin. J Biol Chem. 1973 Mar 10;248(5):1712–1718. [PubMed] [Google Scholar]
- Kato I., Anfinsen C. B. Purification of synthetic ribonuclease S-peptide derivatives by specific complex formation on columns of ribonuclease S-protein bound to agarose. J Biol Chem. 1969 Nov 10;244(21):5849–5855. [PubMed] [Google Scholar]
- Kunitz M. Isolation of Crystalline Desoxyribonuclease From Beef Pancreas. Science. 1948 Jul 2;108(2792):19–20. doi: 10.1126/science.108.2792.19-a. [DOI] [PubMed] [Google Scholar]
- LINBERG U. PURIFICATION OF AN INHIBITOR OF PANCREATIC DEOXYRIBONUCLEASE FROM CALF SPLEEN. Biochim Biophys Acta. 1964 Feb 10;82:237–248. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T. H., Salnikow J., Moore S., Stein W. H. Bovine pancreatic deoxyribonuclease A. Isolation of cyanogen bromide peptides; complete covalent structure of the polypeptide chain. J Biol Chem. 1973 Feb 25;248(4):1489–1495. [PubMed] [Google Scholar]
- Lindberg M. U. Crystallization from calf spleen of two inhibitors of deoxyribonuclease I. J Biol Chem. 1966 Mar 10;241(5):1246–1249. [PubMed] [Google Scholar]
- Lindberg M. U., Skoog L. Purification from calf thymus of an inhibitor of deoxyribonuclease I. Eur J Biochem. 1970 Apr;13(2):326–335. doi: 10.1111/j.1432-1033.1970.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Eriksson S. Purification from crude extract by affinity chromatography of the inhibitor of deoxyribonucleae I. Eur J Biochem. 1971 Feb;18(4):474–479. doi: 10.1111/j.1432-1033.1971.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Lindberg U. Purification from calf spleen of two inhibitors of deoxyribonuclease. I. Physical and chemical characterization of the inhibitor II. Biochemistry. 1967 Jan;6(1):323–335. doi: 10.1021/bi00853a049. [DOI] [PubMed] [Google Scholar]
- Lindberg U. Studies on the complex formation between deoxyribonuclease I and spleen inhibitor II. Biochemistry. 1967 Jan;6(1):343–347. doi: 10.1021/bi00853a051. [DOI] [PubMed] [Google Scholar]
- Orr T. S., Hall D. E., Allison A. C. Role of contractile microfilaments in the release of histamine from mast cells. Nature. 1972 Apr 14;236(5346):350–351. doi: 10.1038/236350a0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Price P. A. Characterization of Ca ++ and Mg ++ binding to bovine pancreatic deoxyribonuclease A. J Biol Chem. 1972 May 10;247(9):2895–2899. [PubMed] [Google Scholar]
- Price P. A., Liu T. Y., Stein W. H., Moore S. Properties of chromatographically purified bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):917–923. [PubMed] [Google Scholar]
- Price P. A., Moore S., Stein W. H. Alkylation of a histidine residue at the active site of bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):924–928. [PubMed] [Google Scholar]
- Price P. A., Stein W. H., Moore S. Effect of divalent cations on the reduction and re-formation of the disulfide bonds of deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):929–932. [PubMed] [Google Scholar]
- Salnikow J., Moore S., Stein W. H. Comparison of the multiple forms of bovine pancreatic deoxyribonuclease. J Biol Chem. 1970 Nov 10;245(21):5685–5690. [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. I. Fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat. 1970;109(4):431–449. [PubMed] [Google Scholar]
- Spooner B. S., Yamada K. M., Wessells N. K. Microfilaments and cell locomotion. J Cell Biol. 1971 Jun;49(3):595–613. doi: 10.1083/jcb.49.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihing R. R., Korn E. D. Acanthamoeba actin. Isolation and properties. Biochemistry. 1971 Feb 16;10(4):590–600. doi: 10.1021/bi00780a008. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- ZALITE B. R., ROTH J. S. AN ASSAY FOR AND SOME PROPERTIES OF DEOXYRIBONUCLEASE INHIBITOR IN RAT LIVER. Arch Biochem Biophys. 1964 Jul;107:16–22. doi: 10.1016/0003-9861(64)90263-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Coleman N. F. Pancreatic deoxyribonuclease. The role of dimerization in the reversible thermal inactivation at acid pH. J Biol Chem. 1971 Jan 25;246(2):309–317. [PubMed] [Google Scholar]