Abstract
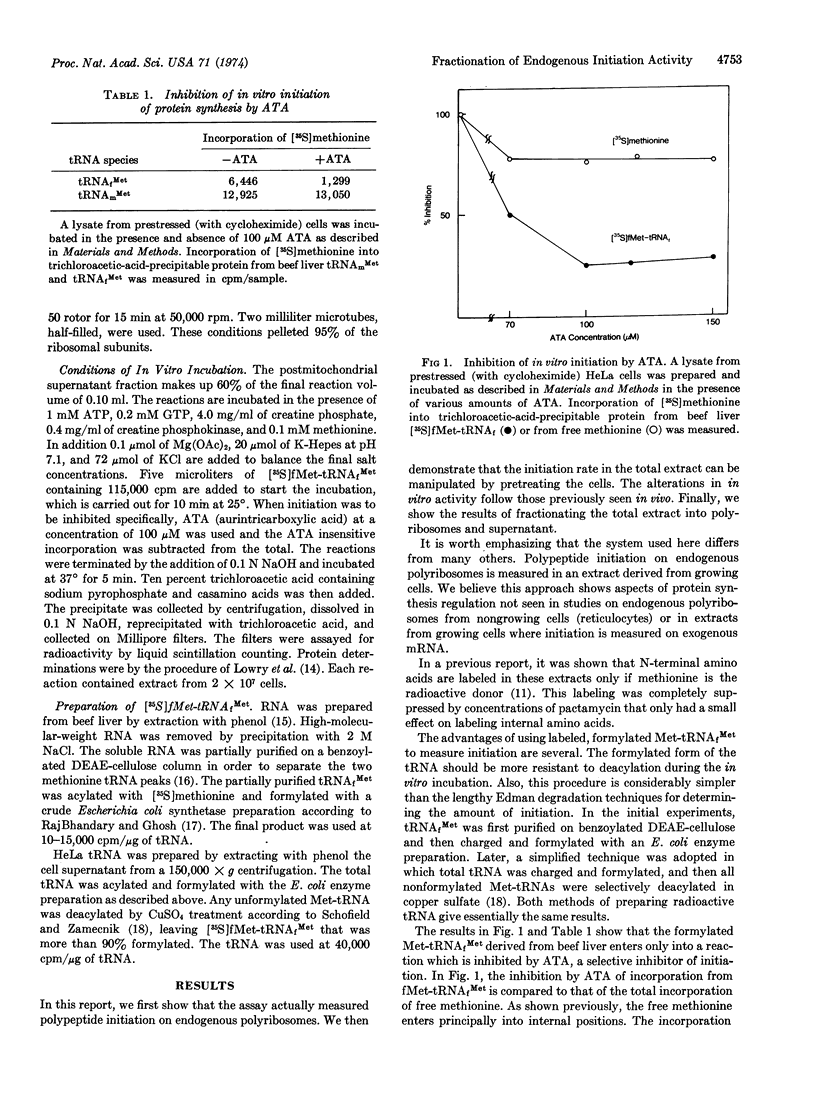
The in vitro initiation of polypeptides on endogenous polyribosomes has been studied in extracts from HeLa cells. Regulation of the rate of initiation of polypeptides can be examined. In these experiments an assay using [35S]fMet-tRNAfMet has been developed, and the system further characterized.
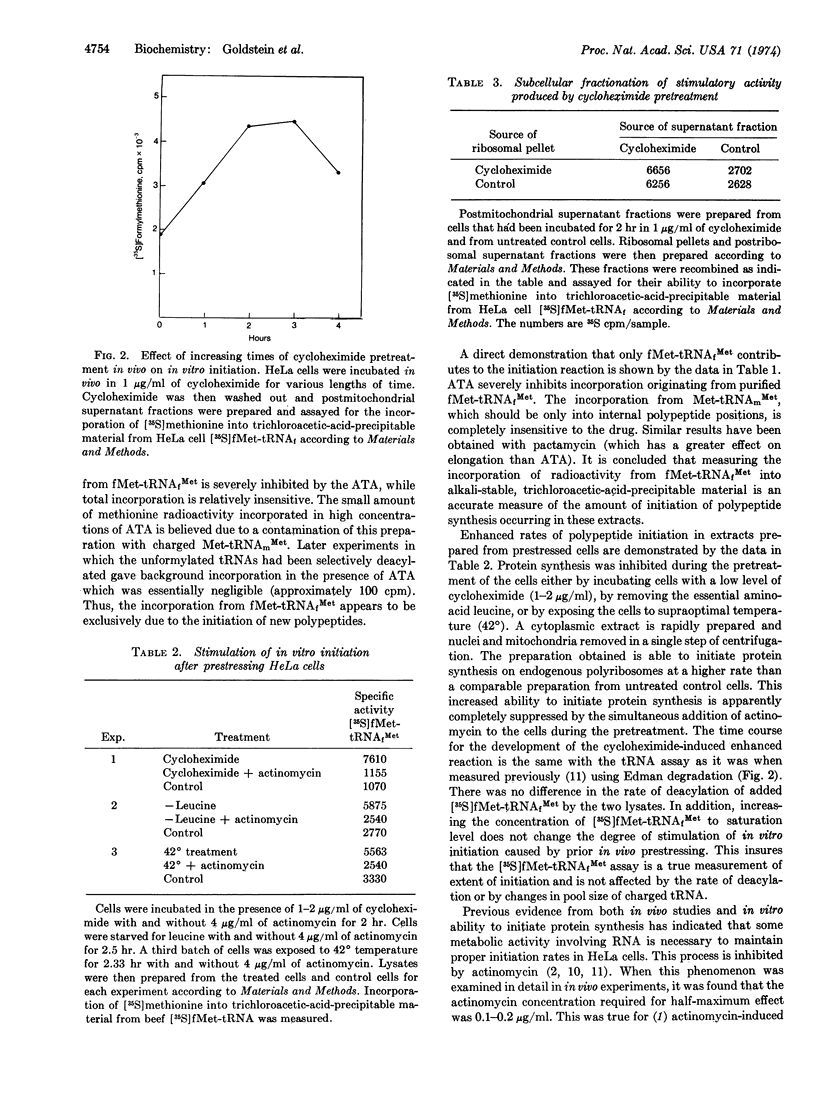
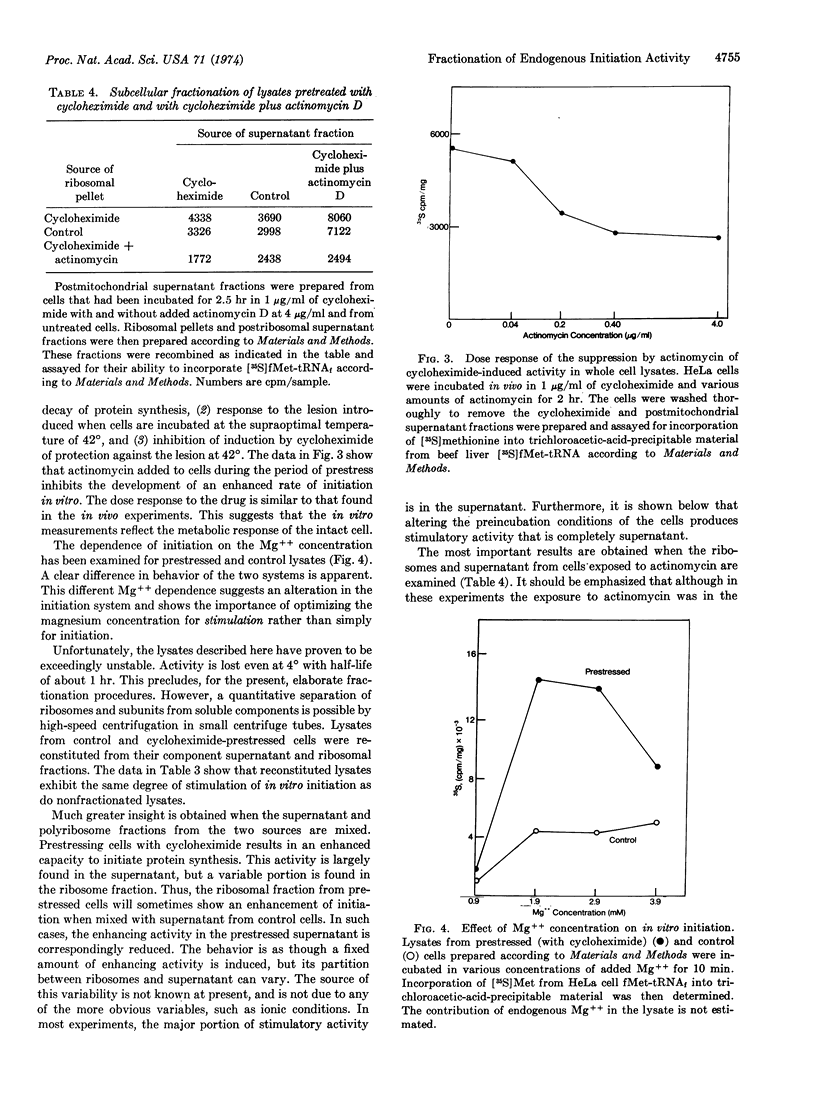
The system has been separated into a fraction containing polyribosomes with subunits and a fraction containing soluble components. The regulation of initiation has at least two distinct components.
There is one factor in the soluble fraction which develops a stimulated response after protein synthesis has been inhibited in intact cells. This stimulation does not require new RNA synthesis during the period of cell “stress.”
A second component is associated with ribosomes. This factor is necessary for the initiation of polypeptides on endogenous polyribosomes. It disappears gradually when cells are exposed to actinomycin. The disappearance is first manifested by an inability of polyribosomes to respond to stimulated supernatants. This unstable component, which decays in the presence of actinomycin, has no apparent counterpart in systems that measure initiation on exogenous mRNA.
Keywords: endogenous activity, actinomycin inhibition, in vivo regulation, polyribosome initiation inhibition, soluble initiation stimulation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig N. The effects of inhibitors of RNA and DNA synthesis on protein synthesis and polysome levels in mouse L-cells. J Cell Physiol. 1973 Oct;82(2):133–150. doi: 10.1002/jcp.1040820202. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Goldstein E. S., Penman S. Regulation of protein synthesis in mammalian cells. V. Further studies on the effect of actinomycin D on translation control in HeLa cells. J Mol Biol. 1973 Oct 25;80(2):243–254. doi: 10.1016/0022-2836(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Inhibition of protein synthesis directed by added viral and cellular messenger RNAs in extracts of interferon-treated Ehrlich ascites tumor cells. Location and dominance of the inhibitor(s). Biochem Biophys Res Commun. 1973 Sep 18;54(2):777–783. doi: 10.1016/0006-291x(73)91491-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Leibowitz R., Penman S. Regulation of protein synthesis in HeLa cells. 3. Inhibition during poliovirus infection. J Virol. 1971 Nov;8(5):661–668. doi: 10.1128/jvi.8.5.661-668.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- McCormick W., Penman S. Regulation of protein synthesis in HeLa cells: translation at elevated temperatures. J Mol Biol. 1969 Jan;39(2):315–333. doi: 10.1016/0022-2836(69)90320-9. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- Reichman M., Penman S. Stimulation of polypeptide initiation in vitro after protein synthesis inhibition in vivo in HeLa cells. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2678–2682. doi: 10.1073/pnas.70.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P., Zamecnik P. C. Cupric ion catalysis in hydrolysis of aminoacyl-tRNA. Biochim Biophys Acta. 1968 Feb 26;155(2):410–416. doi: 10.1016/0005-2787(68)90185-8. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Becker H. Control of macromolecular synthesis in proliferating and resting Syrian hamster cells in monolayer culture. I. Ribosome function. J Cell Physiol. 1971 Feb;77(1):31–42. doi: 10.1002/jcp.1040770105. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Hansen B. S. Control of initiation of protein synthesis in human cells. Evidence for a role of uncharged transfer ribonucleic acid. J Biol Chem. 1973 Oct 25;248(20):7087–7096. [PubMed] [Google Scholar]
- Walker T. A., Pace N. R., Erikson R. L., Erikson E., Behr F. The 7S RNA common to oncornaviruses and normal cells is associated with polyribosomes. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3390–3394. doi: 10.1073/pnas.71.9.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]



