Abstract
We established Project Viva to examine prenatal diet and other factors in relation to maternal and child health. We recruited pregnant women at their initial prenatal visit in eastern Massachusetts between 1999 and 2002. Exclusion criteria included multiple gestation, inability to answer questions in English, gestational age ≥22 weeks at recruitment and plans to move away before delivery. We completed in-person visits with mothers during pregnancy in the late first (median 9.9 weeks of gestation) and second (median 27.9 weeks) trimesters. We saw mothers and children in the hospital during the delivery admission and during infancy (median age 6.3 months), early childhood (median 3.2 years) and mid-childhood (median 7.7 years). We collected information from mothers via interviews and questionnaires, performed anthropometric and neurodevelopmental assessments and collected biosamples. We have collected additional information from medical records and from mailed questionnaires sent annually to mothers between in-person visits and to children beginning at age 9 years. From 2341 eligible women, there were 2128 live births; 1279 mother-child pairs provided data at the mid-childhood visit. Primary study outcomes include pregnancy outcomes, maternal mental and cardiometabolic health and child neurodevelopment, asthma/atopy and obesity/cardiometabolic health. Investigators interested in learning more about how to obtain Project Viva data can contact Project_Viva@hphc.org.
Keywords: Pregnancy, cohort, obesity, nutrition, childhood
Key Messages.
Both greater physical activity and healthful diet during pregnancy predict optimal gestational weight gain, whereas lower risk for gestational diabetes mellitus is associated with greater physical activity but not with diet during pregnancy.
Childhood obesity is associated with modifiable prenatal factors (including maternal smoking, excess gestational weight gain and caesarean birth), lower leptin levels in cord blood at delivery and postnatal factors (including rapid infant weight gain, sleep insufficiency and early introduction of solid foods).
Child cognition is related to early-life diet including maternal prenatal fish consumption and related fatty acid and mercury exposures, methyl donor intake and breastfeeding duration. These associations are robust to adjustment for socioeconomic factors, maternal intelligence and home environment.
Wheezing illnesses in early childhood are related to lower maternal intakes of vitamin E and vitamin D during pregnancy and higher adiposity during infancy.
Why was the cohort set up?
Our overall goal in creating Project Viva was to establish a longitudinal pre-birth cohort to examine the extent to which events during early development affect health outcomes over a lifetime. Analysis of these observational data could then inform future interventions to alter these events and thereby improve health and, in fact, we have now conducted a number of intervention studies informed by findings from Viva analyses.1–3 Although the initial grant funded Project Viva cohort follow-up until 6 months postpartum, our intention from the outset was to follow mothers and children for as long as possible.
From April 1999 through November 2002, we recruited women seen for prenatal care at eight urban and suburban practices of a multi-specialty group practice in eastern Massachusetts. We selected practices that: were centrally located, for ease of access by research staff; had patients from a broad range of race/ethnicity and socioeconomic status; saw a large volume of patients; and saw pregnant patients who planned to deliver at one of two participating hospitals. Since 1969, this group practice has used an electronic medical record (EMR) that includes coded information on scheduled appointments, vital signs, laboratory results and medication ordering, as well as free-text clinical encounter data. The EMR allowed us to identify potentially eligible women, meet them at initial and subsequent obstetric visits and efficiently obtain key clinical measures for enrolled women.
The specific aims of the first funded Viva grant (R01 HD 034568, PI Gillman) were to examine the associations of gestational nutritional factors, namely maternal prenatal intake of n-3 fatty acids, trans fats, and calcium, with fetal growth, length of gestation, incidence of pre-eclampsia, cognitive development and blood pressure in infancy. Subsequent grants supported cohort follow-up and ongoing assessments of cognitive development, asthma/atopy, growth and cardiovascular disease risks.
The National Institute of Child Health and Human Development (NICHD) has provided core funding to support Project Viva operations and science since its inception. Support for primary data collection has also come from other Institutes of the National Institutes of Health, primarily the National Heart, Lung, and Blood Institute (NHLBI), and other sources (see Funding section). Additional ‘ancillary’ grants have funded analyses of previously collected data or biosamples. Project Viva has also served as the substrate for a number of career development grants. eTable 1 (available as Supplementary data at IJE online) lists grant funding for Project Viva that has come from publicly available sources. Smaller grants from local or institutional programmes that provided essential pilot funding to support subsequent, larger proposals are not included in the eTable.
Who is in the cohort?
We identified potential participants at the time they scheduled their initial obstetric visit, which occurred at median 9.9 weeks of gestation. Research assistants (RAs) met women in the waiting room, introduced the study and determined eligibility. Exclusion criteria included multiple gestation, inability to answer questions in English, gestational age ≥22 weeks at recruitment and plans to move away from the study area before delivery. After the clinical visit was completed, the RA met privately with interested women to describe the study in greater detail, obtained written informed consent, conducted a brief interview and provided questionnaires for participants to complete at home and return via mail.
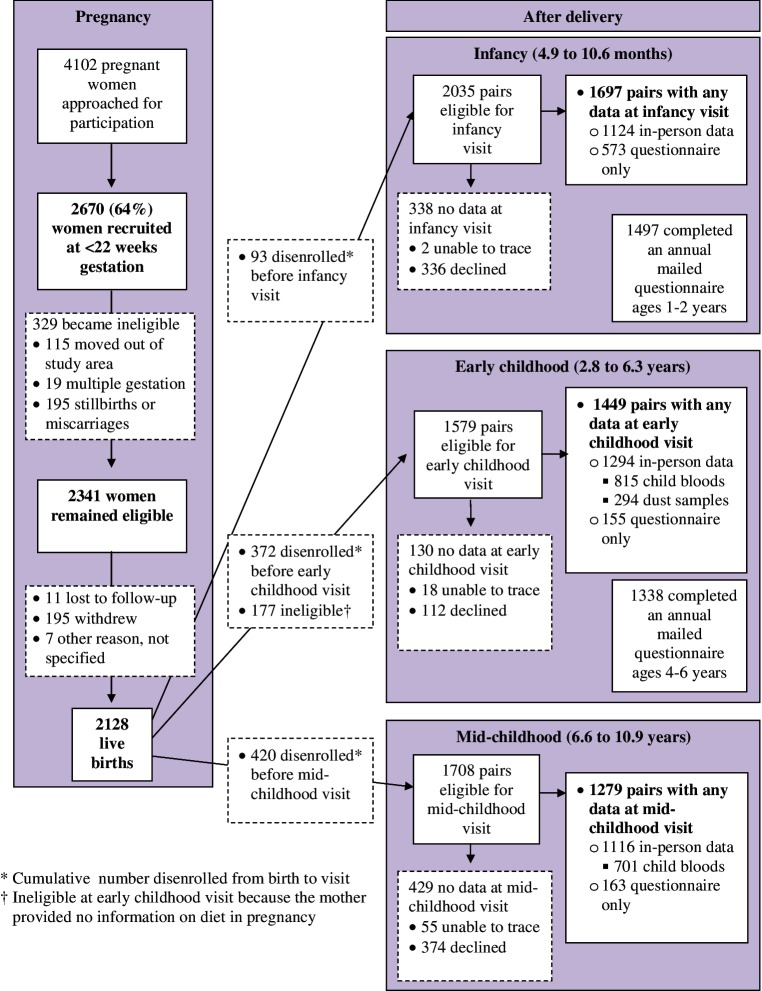
Figure 1 describes the flow of Project Viva participation. We recruited 2670 women (64% of those who were approached for participation). After recruitment we found 329 of them to be ineligible (Figure 1), leaving a total of 2341 eligible enrollees, of whom 2128 were still enrolled at the time of delivery and had live births. Table 1 describes characteristics of Viva mothers at recruitment and delivery, and of Viva children at each postnatal visit.
Figure 1.
Participant flow from recruitment through mid-childhood in the Project Viva cohort.
Table 1.
Selected characteristics of Project Viva mothers and children
| Variable (continuous) | N | Mean (SD) | Variable (categorical) | N | % |
|---|---|---|---|---|---|
| Pre- and perinatal | Maternal race/ethnicity | 2104 | |||
| Maternal age (years) | 2128 | 31.8 (5.2) | White | 66.5 | |
| Pre-pregnancy BMI (kg/m2) | 2113 | 24.9 (5.6) | Black | 16.5 | |
| Pregnancy weight gain (kg) | 2068 | 15.5 (5.7) | Asian | 5.7 | |
| 2nd trimester log CRH (pg/ml) | 1005 | 4.9 (0.6) | Hispanic | 7.3 | |
| Nutrient dataa from 2nd trimester FFQ | 1666 | Other | 3.9 | ||
| Vitamin B12 (µg/d) | 10.7 (6.3) | Maternal education | 2104 | ||
| Folate (µg/d) | 1265 (397) | <College graduate | 35.4 | ||
| Vitamin C (mg/d) | 282.0 (127) | ≥College graduate | 64.6 | ||
| Vitamin E (mg/d) | 25.8 (72.9) | Household income | 1874 | ||
| Vitamin D (IU/d) | 599.1 (187) | <$40,000/year | 15.6 | ||
| Calcium (mg/d) | 1427 (388) | $40 to $70,000/year | 23.2 | ||
| DHA (g/d) | 0.11 (0.09) | >$70,000/year | 61.2 | ||
| EPA (g/d) | 0.06 (0.08) | Maternal smoking | 2107 | ||
| Total omega-3 (g/d) | 1.17 (0.43) | Never | 68.5 | ||
| Total omega-6 (g/d) | 12.3 (3.2) | Before pregnancy | 18.9 | ||
| 2nd trimester dietary | 1666 | 1.6 (1.5) | During pregnancy | 12.6 | |
| fish intake (servings/week) | Pregnancy glucose status | 2067 | |||
| 2nd trimester plasma | 1320 | 58.5 (21.2) | GDM | 5.7 | |
| 25(OH)vitamin D (nmol/l) | IGT | 3.1 | |||
| Failed screen/normal | 8.7 | ||||
| glucose tolerance test | |||||
| Normal | 82.5 | ||||
| Birth | Gestational weight gainb | 2064 | |||
| Birthweight (kg) | 2127 | 3.46 (0.59) | Inadequate | 13.1 | |
| Birth length (cm) | 1057 | 49.8 (2.1) | Adequate | 28.9 | |
| Gestational age (wks) | 2128 | 39.4 (2.0) | Excessive | 58.0 | |
| Birthweight for gestational age z-score | 2127 | 0.17 (0.97) | Caesarean delivery | 2098 | 23.7 |
| Cord blood leptin (ng/ml) | 839 | 9.0 (6.6) | Preterm birth <37 weeks | 2128 | 7.2 |
| Cord blood | 875 | 45.6 (18.7) | Child female | 2128 | 48.5 |
| 25(OH)vitamin D (nmol/l) | Pre-eclampsia | 2083 | 3.6 | ||
| Infancy | |||||
| Change in WFL z-scorec 0 to 6 months | 692 | 0.23 (1.11) | Introduction of solids at <4 months | 1461 | 17.2 |
| WFL z-scorec at 6 months | 1158 | 0.71 (0.95) | Maternal weight retention of | 1247 | 16.4 |
| BMI (kg/m2) at 6 months | 1158 | 18.2 (1.6) | >5 kg at 12 months postpartum | ||
| Breastfeeding duration (months) | 1645 | 5.8 (4.6) | Infant eczema at 12 months | 1298 | 22.3 |
| Child at early childhood visit (median age 3.2 years) | BMI percentilec | 1258 | |||
| BMI (kg/m2) | 1258 | 16.5 (1.5) | <5th | 2.3 | |
| BMI z-scorec | 1258 | 0.45 (1.03) | 5 to <85 | 71.1 | |
| SS + TR (mm) | 1219 | 16.7 (4.3) | 85 to <95 | 17.2 | |
| Systolic blood pressure (mmHg) | 1193 | 92.2 (10.7) | ≥95 | 9.4 | |
| Leptin (ng/ml) | 729 | 1.95 (2.0) | Symptoms of wheeze | 1292 | 21.3 |
| Adiponectin (μg/ml) | 730 | 22.3 (5.5) | Doctor-diagnosed asthma, wheezing, or reactive airways | 1436 | 21.7 |
| Child at mid-childhood visit (median age 7.7 years) | BMI percentilec | 1110 | |||
| BMI (kg/m2) | 1110 | 17.2 (3.1) | <5th | 2.3 | |
| BMI z-scorec | 1110 | 0.39 (1.00) | 5 to <85 | 72.1 | |
| SS + TR (mm) | 1108 | 19.9 (9.8) | 85 to <95 | 13.4 | |
| Waist (cm) | 1112 | 60.0 (8.4) | ≥95 | 12.2 | |
| Waisthip ratio | 1098 | 0.88 (0.06) | Symptoms of wheeze | 1162 | 16.4 |
| Systolic blood pressure (mmHg) | 1106 | 94.6 (8.7) | Doctor-diagnosed asthma | 1242 | 22.5 |
| DXA | 876 | Current allergic rhinitis | 1159 | 33.6 | |
| Fat mass (kg) | 7.5 (3.9) | Allergic sensitization | 616 | 53.6 | |
| Fat-free mass (kg) | 21.7 (4.4) | ||||
| Body fat (%) | 24.6 (6.3) | ||||
| Verbal IQ (from KBIT-II) | 1100 | 111.8 (15.1) | |||
| Nonverbal IQ (from KBIT-II) | 1110 | 106.3 (17.0) | |||
SS + TR, subscapular + triceps skinfold thicknesses; IQ, Intelligence Quotient; KBIT, Kaufman Brief Intelligence Test; BMI, body mass index; WFL, weight for length; FFQ, Food Frequency Questionnaire; SD, standard deviation; CRH, corticotrophin-releasing hormone; DXA, dual-xray absorptiometry.
aEnergy-adjusted nutrients.
bGestational weight gain per 2009 Institute of Medicine Guidelines.
cWeight for length or BMI for age and sex, based on Centers for Disease Control and Prevention 2000 reference data.
How often have they been followed up?
RAs conducted mid-pregnancy follow-up visits with women at the time of the routinely scheduled 50-g non-fasting glucose challenge test (median 28.1 weeks of gestation). We next met mothers and children in the hospital during the birth admission (median 39.7 weeks of gestation), whereupon an RA administered a brief interview and measured the infant’s length, blood pressure and head and abdominal circumferences.
We have completed in-person visits with mothers and children during infancy (median age 6.3 months), early childhood (median 3.2 years) and mid-childhood (median 7.7 years). We are currently conducting an early teen in-person visit (∼12–13 years). At each visit, we obtained written informed consent from the mothers and, beginning in mid-childhood we got verbal consent from the child. Institutional Review Boards of participating institutions approved the study protocols. Table 2 summarizes the visit frequency and timing. We invite participants to attend in-person visits at our research clinic in Boston, but also offer home visits to those unable to attend and for early morning collection of fasting blood. We have also conducted a number of visits to participants’ homes across the USA and even as far as Europe. For each completed visit, we provide a cash incentive, reimbursement for parking or transportation cost, and small gifts for the child (e.g. books, toys, a copy of the DXA scan). Annually between visits, we mail questionnaires to mothers, as well as to children beginning at age 9 years.
Table 2.
Schedule of Project Viva study visits and summary of measures at each visit
| Visit (Median age) | Type | From Viva Moms | From Viva Kids |
|---|---|---|---|
| 1st trimester (9.9 weeks of gestation) | In person | Questionnaire and interview | |
| Diet questionnaire | |||
| Blood sample | |||
| 2nd trimester (27.9 weeks of gestation) | In person | Questionnaire and interview | |
| Diet questionnaire | |||
| Blood sample | |||
| Birth (39.7 weeks of gestation) | In person | Interview | Anthropometry |
| Hair sample | Blood pressure | ||
| Cord blood sample | |||
| Infancy (6.3 months of age) | In person | Blood pressure | Anthropometry |
| Anthropometry | Blood pressure | ||
| Questionnaire and interview | Cognitive assessment | ||
| Vision | |||
| 1 and 2 years | Questionnaire | ||
| Early childhood (3.2 years of age) | In person | Questionnaire and interview | Anthropometry |
| Anthropometry | Blood pressure | ||
| Blood pressure | Cognitive assessment | ||
| Cognitive assessment | Blood sample | ||
| Blood sample | Household dust sample | ||
| 4, 5 and 6 years | Questionnaire | ||
| Mid-childhood (7.7 years of age) | In person | Questionnaire and interview | Anthropometry |
| Anthropometry | DXA scan | ||
| Cognitive assessment | Step test | ||
| Home stimulation measures | Blood pressure | ||
| Mother and teacher reports of child | Spirometry | ||
| behaviours and executive function | Cognitive assessment | ||
| Fasting blood | |||
| Hair and urine | |||
| 8 years | Questionnaire | ||
| 9, 10 and 11 years | Mail and internet | Questionnaire | Questionnaire |
| Early teen (In progress) | In person | Questionnaire and interview | Questionnaire |
| Anthropometry | Anthropometry | ||
| DXA scan | |||
| Step test | |||
| Blood pressure | |||
| Spirometry | |||
| Exhaled nitrous oxide | |||
| Nasal swab | |||
| Fasting blood | |||
| Hair and urine | |||
Women who remained in the study through mid-childhood follow-up tended to be older and more likely to be white, college graduates, non-smokers and have higher household income,4 although exact proportions differ based on the number included in each analysis. In most analyses, to account for missing data we use chained equations to multiply impute missing values.5 Including all 2128 subjects with live births,5 we generate 50 imputed datasets and combine their results in our analyses.6
What has been measured?
Diet has been a key exposure for many Project Viva analyses. During pregnancy we assessed maternal diet using food frequency questionnaires (FFQ) modified from one that had been extensively validated in non-pregnant adults, which we calibrated for use in pregnancy.7 At the infancy visit, mothers completed a brief dietary questionnaire called PrimeScreen that we validated against a full-length FFQ and plasma biomarkers.8 We assessed infant feeding via interview, and early childhood diet with full-length FFQs. Thereafter, to minimize the participant burden and because of concerns that FFQs are not well validated in childhood, we instead asked dietary questions focused on obesogenic behaviours (e.g. consumption of sugary beverages and fast food). eTable 2 (available as Supplementary data at IJE online) shows all domains we have assessed.
At in-person visits we assessed anthropometry, blood pressure and cognitive development. For each measure, content experts conducted training and periodic retraining, and we continually assessed data quality. Specific measures were appropriate to the children’s ages. For example, we assessed anthropometry at birth with length, weight and circumferences; in early childhood with the same measures plus skinfold thicknesses; and in mid-childhood with all aforementioned measures as well as foot-foot bio-impedance (Tanita scale model TBF-300A, Tanita Corporation of America, Arlington Heights, IL).9 In mid-childhood we also performed dual X-ray absorptiometry (DXA) scans (Hologic model Discovery A, Bedford, MA) for participants who attended our clinic.9 We assessed asthma and atopy by questionnaire in infancy and early childhood, and additionally via spirometry pre- and post-albuterol treatment in mid-childhood. At the ongoing early teen visit we are also measuring exhaled nitrous oxide (eNOx) and collecting nasal epithelial cells for epigenetic analysis.
Biosample collection protocols were designed to minimize participant discomfort and inconvenience while maximizing scientific yield. During pregnancy, RAs accompanied participants to their routinely scheduled prenatal blood tests, and obtained an extra tube of blood for research analysis. For those participants who delivered at one of the two study hospitals that accounted for approximately three-quarters of the births, the midwife or obstetrician obtained umbilical cord blood via syringe and needle from the umbilical vein. We obtained cord blood from about two-thirds of those births, or about one-half of the whole cohort. Collection of cord blood can be especially challenging given the varied timing of deliveries attended by different clinicians whose primary focus is on clinical care, not research. Approaches to remind the clinical staff to collect the cord blood included paper and electronic ‘flags’ on participant’s medical charts, socks with the Project Viva logo on the soles (provided for each participant to wear during delivery, thus facing the clinician) and gifts to the labour and delivery staff. At the early childhood visit, we obtained non-fasting blood because of concerns that asking young children to fast would be too burdensome, and coordinated the timing of the phlebotomy with routine age 3-yearsclinical lead and anaemia screening to avoid an extra needlestick. We collect fasting blood at the mid-childhood and the early teen visits. We have also collected hair, urine, household dust samples and nasal swabs (Table 2).
What has it found? Key findings and publications
In this section we summarize many of our notable findings, categorized by outcome. A full list of Project Viva publications is available at: http://dacp.org/viva/project-viva-publication-list.html.
Pregnancy outcomes
Diet quality during pregnancy was predicted by maternal characteristics and remained fairly stable from the first to the second trimester, with the notable exceptions of caffeine, alcohol and vitamin and supplement use.10,11 We found that both more physical activity and a healthful diet during pregnancy were predictive of appropriate gestational weight gain, itself an important predictor of both maternal and child health outcomes.12,13
Risk for developing gestational diabetes mellitus (GDM) was not associated with diet during early pregnancy, perhaps because pre-pregnancy body mass index (BMI) overwhelmed the influence of diet during pregnancy.14 However, more physical activity both before and during pregnancy, especially vigorous activity, was associated with lower risk for developing GDM.15 Also, low maternal plasma 25(OH)D concentration predicted risk for GDM.16 Both greater gestational weight gain and exposure to air pollution during the second trimester were associated with risk for impaired glucose tolerance but not GDM.17,18
Maternal intake of elongated n-3 polyunsaturated fatty acids (PUFA) and seafood intake were inversely associated with birthweight for gestational age.19 Second-trimester 25(OH)D levels <25 nmol/l were associated with higher odds of small for gestational age (SGA) birth, raising the possibility that vitamin D status may contribute to racial disparities in SGA.20
Contrary to our hypothesis, we found no association of low n-3 PUFA or fish intake with short duration of gestation or risk of preterm birth.19 We also did not find any evidence that ‘exposure’ to the 11 September tragedy during pregnancy was associated with preterm birth and in fact, contrary to expectation, women exposed in the first trimester actually had longer gestation durations.21
Epigenetic modification such as DNA methylation is one mechanism by which early-life exposures may have long-term health impact. We found that higher early pregnancy repetitive element DNA methylation predicted lower risk of preterm birth, but in contrast preterm birth was associated with lower methylation in cord blood.22 Recently funded grants (eTable 1, available as Supplementary data at IJE online) will support whole-genome epigenetic analysis of maternal and child blood.
Peripartum maternal health
Lifetime experiences of physical and sexual abuse, higher levels of perceived racial discrimination (among Black women), financial hardship and unwanted pregnancy were associated with more depressive symptoms during and after pregnancy.23,24 Elevated mid-pregnancy circulating level of placental corticotrophin-releasing hormone, a hormone related to preterm birth, was positively associated with risk of prenatal but not postpartum depressive symptoms.24
Whereas many women retain a modest amount of the weight they gained during pregnancy, a subset experience substantial postpartum weight retention, which predicts later obesity and cardiometabolic risk. Our analyses have identified modifiable factors that are associated with substantial postpartum weight retention including higher gestational weight gain (GWG) as well as higher postpartum intake of trans fats, less sleep, less walking and more television viewing.25,26 Unfortunately many women reduced their physical activity during and after pregnancy compared with before pregnancy.27
Perinatal factors were associated with later maternal metabolic health. Women with both impaired glucose tolerance and GDM had lower high-density lipoprotein and higher triglycerides 3 years after pregnancy, compared with women in the normal glucose tolerance group.28 We found the highest values for haemoglobin A1c and high-sensitivity C-reactive protein among women who had had impaired glucose tolerance in pregnancy.28 Shorter sleep duration predicted greater inflammation at 3 years postpartum;29 however, duration of lactation was not associated with later maternal metabolic risk.30
Offspring obesity and cardiovascular risk factors
We have found that blood pressure in infancy was predicted by maternal age and calcium intake, but not maternal protein intake, among this population with generally adequate protein intake.31–33 In ongoing work, we are examining associations of prenatal and lifetime air pollution with child blood pressure.
As in other observational studies, we have found an inverse relationship between breastfeeding duration and later obesity, a relationship that did not appear to be explained by maternal ‘restriction’ of child food intake, nor by early growth.34,35 Other modifiable early-life factors that we have found to be associated with excess adiposity in early or mid-childhood include greater maternal gestational weight gain, prenatal smoking, low n-3 fatty acid consumption and caesarean delivery, as well as rapid early postnatal growth, early introduction of solid foods and insufficient sleep duration.36–42
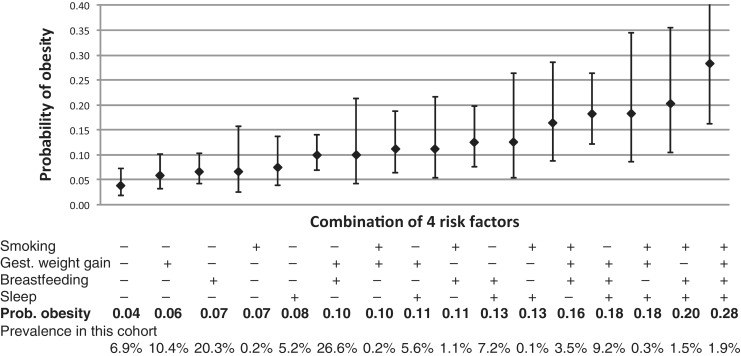
We also examined how combinations of early-life modifiable factors influence risk of later overweight. In one paper we estimated the risk of overweight in mid-childhood according to levels of maternal smoking during pregnancy, gestational weight gain, breastfeeding duration and infant sleep duration. In the prediction model, the estimated probability of overweight ranged from 0.04 among children exposed to favourable levels of all four risk factors, to 0.28 with adverse levels of all four (Figure 2).43 Additionally, we have found that racial/ethnic disparities in child adiposity and obesity were determined by factors operating in infancy and early childhood.44,45 These findings suggest that interventions to modify behaviours during pregnancy and infancy could have substantial impact on prevention of and disparities in childhood overweight.
Figure 2.
Predicted probability of obesity at 7 to 10 years of age for 16 combinations of four modifiable prenatal and postnatal risk factors. Data are from 1110 mother–child pairs participating in Project Viva. Estimates are adjusted for the mother’s educational level and body-mass index (BMI), household income, and the child’s race or ethnic group. Obesity was defined as a BMI above the 95th percentile for age and sex. Excessive gestational weight gain was defined according to the 2009 recommendations of the Institute of Medicine. Plus signs indicate the presence of the risk factor, and minus signs the absence of the risk factor. I bars indicate 95% confidence intervals. From Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med 2013;369:2173–75. © 2013 Massachusetts Medical Society. Reprinted with permission.43
We have also examined metabolic and hormonal factors that predict postnatal growth and childhood weight. For example, higher cord blood leptin was associated with slower gain in weight for length z score from 0 to 6 months and with lower BMI in early and mid-childhood, in line with results from animal studies suggesting that neonatal leptin levels influence developmental obesity programming.46,47 However, leptin levels in early childhood were inversely associated with greater weight gain and higher adiposity in mid childhood, more in line with associations observed in adulthood.48 Cord blood adiponectin levels were also directly associated with birth weight for gestational age, inversely associated with weight gain in the first 6 months of life and predicted an increase in central adiposity at age 3 years.49
Cognition
We have found evidence that childhood cognition is related to early-life diet. For example, cognition assessed in both infancy and early childhood was directly related to maternal prenatal fish consumption, but inversely to maternal prenatal mercury, but associations were no longer apparent in mid childhood.50–51 These findings have contributed to the active debate regarding how to balance the toxicant risks and nutrient benefits related to maternal fish consumption in pregnancy.52
Maternal intake of methyl-donor nutrients predicted child cognition in early childhood, and choline intake predicted better visual memory in mid childhood.53,54 Longer duration of any breastfeeding was associated with higher receptive vocabulary in early childhood and with greater intelligence in mid childhood, with stronger associations with exclusive breastfeeding duration.55 However other early-life factors did not predict later cognition, including variation in maternal and neonatal thyroid function within a fairly normal range of distribution, maternal prenatal depressive symptoms and early growth among term infants.56–58
Asthma and atopy
At birth, we assessed risk for atopy with umbilical cord blood immune assays. We found evidence that a predisposition to a heightened immune response is already evident at birth,59 and differences in innate immune responses according to maternal atopic history as well as by maternal factors including race/ethnicity, smoking during pregnancy, gestational weight gain and n-3 and n-6 PUFA concentrations.60–62
On annual questionnaires and at the early childhood visit, mothers reported symptoms of wheeze and doctor diagnosis of asthma. We have found that male sex, exposure to smoking, parental history of asthma and exposure to older siblings were associated with increased risk of wheezing and asthma-related outcomes in early childhood.63,64 Higher maternal prenatal intake of vitamin D and of antioxidants were associated with decreased risk for wheezing illnesses in early childhood.65,66 Fetal growth and length of gestation were not associated with wheezing, although infants with higher weight for length z scores at 6 months of age had a greater risk of recurrent wheezing in early childhood.63,64 Analyses examining predictors of lung function measures and allergic sensitization in mid-childhood are in progress.
What are the main strengths and weaknesses?
The primary strength of Project Viva is the in-person collection of exposure and outcome measures using research standard procedures conducted by trained and expert staff. Another is the wealth of covariate information we have collected, which helps to minimize confounding. For example, in analyses examining predictors of childhood cognition we can account not only for family education and sociodemographic characteristics, but also maternal IQ and home environmental stimulation.55
Weaknesses include the loss to follow-up over time. This issue plagues many longitudinal studies, but is a particular challenge during the postpartum transition, when mothers may become overwhelmed with the difficulties of caring for their new infant. This problem may have been magnified by our initial recruitment and consent procedures, which described Project Viva as a study from pregnancy through 6 months postpartum—the period of data collection in our initial grant. Now we believe that if from the outset we had told participants of our plans for long-term follow-up, we may have had less postpartum dropout. Also, because of funding cuts in the grants that supported the early childhood in-person visit, we focused the retention efforts of our reduced staff on the subset of participants who were most likely to continue their involvement in the study, based on past participation (Figure 1).
Other limitations include the extent to which findings are generalizable to other populations, since all participants resided in the greater Boston area and had health insurance, and many were college educated. A majority of our participants were White (Table 1), although the proportions of racial/ethnic minorities in Project Viva were higher than in Massachusetts as a whole, according to the 2000 United States census.67 Despite the relatively large sample size, power may not be adequate for all analyses, especially not for dichotomous outcomes or within some strata.
Can I get hold of the data? Where can I find out more?
One of our explicit goals has been to make Project Viva data available to numerous investigators, while at the same time respecting the confidentiality of our participants and ensuring the integrity of the data. We ask that interested investigators, with the assistance of our data manager, analysts and/or biostatistician, draft and present a detailed analysis plan to the core group of Viva co-investigators (the ‘decision-making group’) which meets monthly. The discussion of the decision-making group ensures that the outside investigator has adequate knowledge of the data and understands how the proposed analysis relates to previous analyses. This approach also ensures that we will avoid scientific overlap between projects and optimize use of scarce resources such as biosamples. The decision-making group then approves the analysis plan or asks the investigator to make changes before approval or (rarely) rejects proposals.
Once an analysis plan is approved, our data manager and analysts create de-identified analysis datasets based on the required data elements. Each analysis dataset combines native variables with those derived from more than one native variable de novo or for previous analyses. To ensure consistency across analyses, we keep a master file of all derived variables. All Project Viva policies, forms, approved analysis plans and a data dictionary are stored on our password-protected website; we share the password with prospective investigators upon request. In most instances prospective investigators will find it useful to collaborate with one or more established Viva investigators to an extent that co-authorship is appropriate, though this is not required. Investigators interested in learning more about how to obtain Project Viva data can contact the Project Viva PI Dr Matthew Gillman at: Project_Viva@hphc.org.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Project Viva and its team of co-investigators have been funded by grants from the National Institutes of Health (R01 HD 034568, R01 HL 075504, R01 HL 64925, R01 MH 068596, R01 HD 034568, R01 HD 064925, R01 ES 016314, U54 CA155626, R37 HD 034568, R01 AI 102960, R03 DE14004, P30 ES000002, R21 DK 073739, R21 DK 082661, R01 MD 003963, RC1 HD 063590, R01 HD 034568-0951, R01 ES21447, R01 NR013945, R01 HL111108, K24 HL 068041, R01 HL 64925, K23 HD 044807, T32 DK 081505, K23 DK083817, K24 HD069408, K12 DK094721), the Centers for Disease Control and Prevention (200-95-0957), the Environmental Protection Agency (RD83479801), and the March of Dimes Foundation, with additional support from the Harvard Pilgrim Health Care Foundation.
Supplementary Material
Acknowledgements
We are indebted to the Project Viva mothers, children and families for their ongoing participation. We appreciate the assistance with manuscript preparation that we received from Denise Simon and Karen Switkowski.
Conflict of interest: None declared.
References
- 1.Oken E, Guthrie LB, Bloomingdale A, et al. A pilot randomized controlled trial to promote healthful fish consumption during pregnancy: the Food for Thought Study. Nutr J 2013;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taveras EM, Blackburn K, Gillman MW, et al. First Steps for Mommy and Me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J 2012;15:1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taveras EM, Gortmaker SL, Hohman KH, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med 2011;165:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 2013;36:3045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 6.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: Wiley-Interscience, 2004. [Google Scholar]
- 7.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol 2004;14:754–62. [DOI] [PubMed] [Google Scholar]
- 8.Rifas-Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr 2001;4:249–54. [DOI] [PubMed] [Google Scholar]
- 9.Boeke CE, Oken E, Kleinman KP, Rifas-Shiman SL, Taveras EM, Gillman MW. Correlations among adiposity measures in school-aged children. BMC Pediatr 2013;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol 2006;20:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009;109:1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am J Epidemiol 2009;170:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol 2009;201:e51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radesky JS, Oken E, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Gillman MW. Diet during early pregnancy and development of gestational diabetes. Paediatr Perinat Epidemiol 2008;22:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol 2006;108:1200–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burris HH, Rifas-Shiman SL, Kleinman K, et al. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am J Obstet Gynecol 2012;207:e181–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleisch AF, Gold DR, Rifas-Shiman SL, et al. Air pollution exposure and abnormal glucose tolerance during pregnancy: The project viva cohort. Environ Health Perspect , 2014; 122:378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herring SJ, Oken E, Rifas-Shiman SL, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol 2009;201:e61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol 2004;160:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burris HH, Rifas-Shiman SL, Camargo CA, Jr, et al. Plasma 25-hydroxyvitamin D during pregnancy and small-for-gestational age in black and white infants. Ann Epidemiol 2012;22:581–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich-Edwards JW, Kleinman KP, Strong EF, Oken E, Gillman MW. Preterm delivery in Boston before and after September 11th, 2001. Epidemiology 2005;16:323–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burris HH, Rifas-Shiman SL, Baccarelli A, et al. Associations of LINE-1 DNA methylation with preterm birth in a prospective cohort study. J Dev Orig Health Dis 2012;3:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rich-Edwards JW, Kleinman K, Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health 2006;60:221–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rich-Edwards JW, Mohllajee AP, Kleinman K, et al. Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab 2008;93:1946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunderson EP, Rifas-Shiman SL, Oken E, et al. Association of fewer hours of sleep at 6 months postpartum with substantial weight retention at 1 year postpartum. Am J Epidemiol 2008;167:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oken E, Taveras EM, Popoola FA, Rich-Edwards JW, Gillman MW. Television, walking, and diet: Associations with postpartum weight retention. Am J Prev Med 2007;32:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira MA, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project Viva. Am J Prev Med 2007;32:312–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuebe AM, Mantzoros C, Kleinman K, et al. Gestational glucose tolerance and maternal metabolic profile at 3 years postpartum. Obstet Gynecol 2011;118:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taveras EM, Rifas-Shiman SL, Rich-Edwards JW, Gunderson EP, Stuebe AM, Mantzoros CS. Association of maternal short sleep duration with adiposity and cardiometabolic status at 3 years postpartum. Obesity (Silver Spring) 2011;19:171–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuebe AM, Kleinman K, Gillman MW, Rifas-Shiman SL, Gunderson EP, Rich-Edwards J. Duration of lactation and maternal metabolism at 3 years postpartum. J Womens Health (Larchmt) 2010;19:941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh SY, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Lipshultz SE, Gillman MW. Maternal protein intake is not associated with infant blood pressure. Int J Epidemiol 2005;34:378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr 2004;144:240–45. [DOI] [PubMed] [Google Scholar]
- 33.Gillman MW, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Lipshultz SE. Maternal calcium intake and offspring blood pressure. Circulation 2004;110:1990–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rossem L, Taveras EM, Gillman MW, et al. Is the association of breastfeeding with child obesity explained by infant weight change? Int J Pediatr Obes 2011;6:e415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taveras EM, Rifas-Shiman SL, Scanlon KS, Grummer-Strawn LM, Sherry B, Gillman MW. To what extent is the protective effect of breastfeeding on future overweight explained by decreased maternal feeding restriction? Pediatrics 2006;118:2341–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med 2008;162:305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res 2005;13:2021–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 2007;196:e321–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics 2009;123:1177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr 2011;93:780–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh SY, Rifas-Shiman SL, Zera CA, et al. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child 2012;97:610–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huh SY, Rifas-Shiman SL, Taveras EM, Oken E, Gillman MW. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011;127:e544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med 2013;369:2173–75. [DOI] [PubMed]
- 44.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics 2010;125:686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taveras EM, Gillman MW, Kleinman KP, Rich-Edwards JW, Rifas-Shiman SL. Reducing racial/ethnic disparities in childhood obesity: the role of early life risk factors. JAMA Pediatr 2013:167:731–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker M, Rifas-Shiman SL, Belfort MB, et al. Gestational glucose tolerance and cord blood leptin levels predict slower weight gain in early infancy. J Pediatr 2011;158:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes 2007;14:17–22. [DOI] [PubMed] [Google Scholar]
- 48.Boeke CE, Mantzoros CS, Hughes MD, et al. Differential associations of leptin with adiposity across early childhood. Obesity (Silver Spring) 2013;21:1430–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics 2009;123:682–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect 2005;113:1376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oken E, Radesky JS, Wright RO, et al. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol 2008;167:1171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oken E, Choi AL, Karagas MR, et al. Which fish should I eat? Perspectives influencing fish consumption choices. Environ Health Perspect 2012;120:790–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol 2013;177:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villamor E, Rifas-Shiman SL, Gillman MW, Oken E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol 2012;26:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belfort M, Rifas-Shiman SL, Guthrie LB, et al. Infant feeding and childhood cognition at ages 3 and 7 years: effects of breastfeeding duration and exclusivity. JAMA Pediatrics 2013;167:836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oken E, Braverman LE, Platek D, Mitchell ML, Lee SL, Pearce EN. Neonatal thyroxine, maternal thyroid function, and child cognition. J Clin Endocrinol Metab 2009;94:497–03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belfort MB, Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Infant growth and child cognition at 3 years of age. Pediatrics 2008;122:e689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tse AC, Rich-Edwards JW, Rifas-Shiman SL, Gillman MW, Oken E. Association of maternal prenatal depressive symptoms with child cognition at age 3 years. Paediatr Perinat Epidemiol 2010;24:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaub B, Tantisira KG, Gibbons FK, et al. Fetal cord blood: aspects of heightened immune responses. J Clin Immunol 2005;25:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gold DR, Willwerth BM, Tantisira KG, et al. Associations of cord blood fatty acids with lymphocyte proliferation, IL-13, and IFN-gamma. J Allergy Clin Immunol 2006;117:931–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroeter CH, Schaub B, Gold DR, et al. Nuclear factor kappa B activation in human cord blood mononuclear cells. Pediatr Res 2004;56:212–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaub B, Campo M, He H, et al. Neonatal immune responses to TLR2 stimulation: influence of maternal atopy on Foxp3 and IL-10 expression. Respir Res 2006;7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taveras EM, Camargo CA, Jr, Rifas-Shiman SL, et al. Association of birth weight with asthma-related outcomes at age 2 years. Pediatr Pulmonol 2006;41:643–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taveras EM, Rifas-Shiman SL, Camargo CA, Jr, et al. Higher adiposity in infancy associated with recurrent wheeze in a prospective cohort of children. J Allergy Clin Immunol 2008;121:1161–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Litonjua AA, Rifas-Shiman SL, Ly NP, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr 2006;84:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.United States Census Bureau. American FactFinder. 2000. http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml (5 July 2013, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.