Abstract
The main aim of the Leucémies de l’Enfant et l’Adolescent (LEA) project (Childhood and Adolescent Leukaemia) is to study the determinants (medical, socioeconomic, behavioural and environmental) of medium- and long-term outcomes of patients treated for childhood acute leukaemia (AL). The LEA study began in 2004 and is based on a French multicentric prospective cohort. Included are children treated for AL since January 1980 (incident and prevalent cases), surviving at month 24 for myeloblastic AL and lymphoblastic AL grafted in first complete remission or at month 48 for lymphoblastic AL not grafted in first complete remission. Information is collected during specific medical visits and notably includes the following data: socioeconomic data, AL history, physical late effects (such as fertility, cardiac function and metabolic syndrome) and quality of life. Data are collected every 2 years until the patient is 20 years old and has had a 10-year follow-up duration from diagnosis or last relapse. Thereafter, assessments are planned every 4 years. In active centres in 2013, eligible patients number more than 3000. The cohort has already included 2385 survivors, with rate of exhaustiveness of almost 80%. Data access can be requested from principal coordinators and must be approved by the steering committee.
Key Messages.
The role of modern therapy in the long-term complications of childhood acute leukaemia has been partly described. The structure and organization of the LEA cohort enable an exploration of the impact of the newer antileukaemic treatment modalities, especially haematopoietic stem cell transplantation.
The first results in the young adults surviving childhood leukaemia showed a prevalence of metabolic syndrome (MS) approximately twice as high as in the young adult general French population. Haematopoietic stem cell transplantation with total body irradiation was found to be a major risk for developing MS.
In the LEA cohort, the risk of having at least one sequela was five times higher for the transplantation survivors, compared with patients who underwent conventional therapy, resulting in a decreased physical well-being in adulthood. However, regardless of the treatment received, childhood leukaemia survivors reported that the long-term impact on psychological well-being is more important than it is on physical quality of life dimensions.
Why was the Cohort Set Up?
The need for an extended follow-up of health status after childhood leukaemia
Survival rates of childhood and adolescent acute leukaemia (AL) have improved remarkably over the past decades.1–3 These improvements lead to the need and responsibility to consider the long-term morbidity and mortality associated with the treatments responsible for the survival increase.
To varying degrees, long-term survivors are at risk of developing a spectrum of adverse outcomes, including impaired growth and development, obesity, decreased fertility, organ dysfunction, impaired cognitive function, second cancer and early death.4–8 The occurrence of late adverse events may alter patient quality of life (QoL) and social outcome.9–19 However, the data collected concerned large groups of cancer survivors without focusing on one single type of cancer and included survivors who were treated with older protocols that frequently included cranial irradiation.6,9,18,20–22 The role of recent antileukaemic therapy in these long-term complications has been partially described.13,23–31
The crucial role of prospective cohorts in this context
Several childhood cancer survivor cohorts have been established in the USA,32 Canada,11,15,33,34 and Europe35–38, especially the British Childhood Cancer Survivor Study39–45 and the Swiss Childhood Cancer Survivor Study.46 However, the Childhood Cancer Survivor Study (CCSS), funded and supported in the USA by the National Cancer Institute, currently remains the largest cohort.
The CCSS study is a multi-institutional research initiative designed to establish a large and extensively characterized cohort of childhood cancer survivors diagnosed between 1970 and 1986.47 Although the CCSS is clearly a ‘model cohort’, several limitations can be underlined as follows:
With patients diagnosed between 1970 and 1986, the treatment-related characteristics of the cohort increasingly reflect a greater historical perspective; in the particular case of leukaemia, this can likely explain some features, such as a low number of haematopoietic stem cell transplant (HSCT) recipients and a high percentage of patients who received prophylactic central nervous system irradiation.
Health status evaluations are mainly based on patient self-reported outcomes.
The information issued from this cohort study did not define the clinical care recommendations and screening guidelines applied to the patients.
The LEA cohort
According to the literature, our current knowledge on the determinants of patient health status and QoL after childhood leukaemia remains insufficient to fully optimize individual programmes designed to improve patient outcome.
The Leucémies de l’Enfant et de l’Adolescent (LEA) project (Childhood and Adolescent Leukaemia—LEA—French Childhood Cancer Survivor Study for Leukaemia) was initiated in 2004. The general objective is to study the determinants (medical, socioeconomic, behavioural and environmental) of the medium- and long-term health and QoL of a cohort of patients treated in France for childhood AL after January 1980.
Who is in the Cohort?
Inclusion criteria and participating centres
The LEA cohort consists of a dynamic/open cohort. Any subject fulfilling the following criteria is included:
Age lower than 18 years at time of diagnosis
Diagnosis of AL
Diagnosis after January 1980
Treatment of AL initiated in one of the investigatory centres
Living in France
Survivor at month 24 after the diagnosis for acute myeloblastic leukaemia (AML) patients or acute lymphoblastic leukaemia (ALL) patients grafted in first complete remission or at month 48 after the diagnosis for ALL patients not grafted in first complete remission
Has agreed to participate in the study or has been authorized to participate by the parents or legal guardian, for any subject under 18 years of age.
The study began in 2004 by an exhaustive recruitment in university hospitals of Marseilles and Nancy. Then, several teams joined the project throughout the following years. By 31 December 2011, the dedicated services of paediatric haematology and oncology of 10 French centres were involved: Marseilles, Nancy, Nice, Clermont-Ferrand, Grenoble, Lyon, Paris-Saint Louis, Paris-Trousseau, Paris-Robert Debré and Saint-Etienne. The potential file (population corresponding to the selection criteria) is nearly three-quarters of French childhood AL survivors since 1980 and the rate of participation in the centres is around 80%.
Incident and prevalent cases
The LEA study is based on the constitution of a multicentre historical and prospective cohort. This cohort includes both incident cases (diagnosed after the start date of the participation of the centre in the LEA programme) and prevalent cases (diagnosed between 1 January 1980 and the start date of the participation of the centre in the LEA programme).
For incident cases, the information is collected in a prospective way following a fixed schedule, starting from the initial date of diagnosis. For prevalent cases, the data collected are similar to those collected for incident cases and only the data collection differs, because it is retrospective for the events concerning the period preceding the participation of the centre in the LEA programme.
The exhaustiveness of the population included in the study is investigated taking into consideration the information available from existing data sources relating to these patients:
the national registry of childhood haematological malignancies recording the patients diagnosed since 1990
the lists of patients included in the main protocols of clinical trials implemented for childhood leukaemia since 1980: EORTC, FRALLE, and ELAM
medical information from the hospital database. Apart from the exhaustiveness control, the record linkage enables the confirmation of the representativeness of the cohort designed at a national level.
Structuring the cohort follow-up
The periodicity of the prospective follow-up evaluations is organized according to the chronological history of the pathology and the usual patterns of treatment and follow-up implemented in leukaemia patients.
The follow-up comprises three main phases of assessment.
The initial evaluation is performed, depending on the type of leukaemia, at the following times: (a) in the month 24 (M24) following the diagnosis for AML or ALL patients grafted in first complete remission or (b) in M48 following the diagnosis for ALL patients not grafted in first complete remission. Overall, this schedule corresponds to a 1-year period after the end of treatment for patients who did not experience relapse.
Then, a biannual evaluation is scheduled starting from M24 or M48 until the patient reaches at least 20 years of age and the time interval since the diagnosis (or last relapse) is at least 10 years.
Beyond 20 years of age and after at least 10 years of follow-up (since the last event), the evaluations are planned every 4 years until the patient has reached 50 years of age. The domains explored during each evaluation remain similar to those of the preceding evaluations.
Specific cases
Relapses
When a relapse occurs, the following evaluations are programmed by taking as a reference the date of diagnosis of relapse instead of the date of initial diagnosis, with an evaluation every 2 years until the patient reaches at least 20 years of age and a time period of at least 10 years elapsed since relapse. Beyond this time, the evaluation occurs every 4 years.
Death
Any death is notified to the coordinator team by the medical team caring for the patient, specifying the cause of death.
Lost to follow-up
The following patients are regarded as lost for a given time of evaluation: (i) the patients for whom no current address could be obtained, (ii) the patients who did not answer despite three consecutive reminders and (iii) the patients who moved out of France. However, new attempts will be made to obtain their replies at subsequent evaluation times.
The patients from whom no reply could be obtained at three consecutive times of evaluation are considered lost to the follow-up programme, except for any spontaneous demonstration of their willingness to participate in the study at a later time.
Population description
Due to the inclusion of prevalent and incident cases and the regular input of new participating centres, the cohort’s sample size is steadily increasing. As of 31 December 2011, 1941 childhood leukaemia survivors fulfilled the inclusion criteria in the participating centres during that period. Among them, 1545 patients agreed to participate in the study (response rate: 79.6%). The respondent group and the non-respondent group do not differ with respect to sex, type of leukaemia, history of relapse, treatment by transplantation or not, but the respondent group is older by an average of 1 year at diagnosis (P = 0.03) (Table 1). Of the participants, 43.8% have been re-evaluated and are divided as follows: 436 survivors (28.2%) have been evaluated twice, 193 survivors (12.5%) three times and 48 survivors (3.1%) four times. In total, 2511 evaluations have been conducted in the cohort. Table 2 summarizes the distribution of survivors’ ages at the time of the last evaluation in relation to time from diagnosis. At this time of cohort monitoring, among all the patients who agreed to participate at least once, 193 patients are lost for a given evaluation, only 4 are definitely lost to the follow-up programme and 11 have died.
Table 1.
Patient characteristics
| Characteristics | Responders | Non-responders | |
|---|---|---|---|
| n = 1545 | n = 396 | P | |
| Sex [n (%)] | |||
| Female | 732 (47.4) | 169 (42.7) | 0.10 |
| Leukaemia subtype [n (%)] | |||
| ALL | 1327 (86.0) | 332 (83.8) | 0.42 |
| HSCT [n (%)] | |||
| Yes | 368 (23.8) | 86 (22.4) | 0.59 |
| Age at diagnosis (years, mean ± SD) | 6.4 ± 4.3 | 5.9 ± 4.1 | 0.03 |
| History of relapse [n (%)] | |||
| Yes | 220 (14.2) | 54 (14.0) | 0.94 |
Data as of 31 December 2011.
ALL, acute lymphoid leukaemia; HSCT, hematopoietic stem cell transplantation; SD, standard deviation.
Bold value indicates significance at P < 0.05.
Table 2.
Age of the patients in relation to time from diagnosis
|
Years from diagnosis |
||||||
|---|---|---|---|---|---|---|
| Age at the last evaluation (years) | <5 | 5 to 9 | 10 to 14 | 15 to 19 | 20 to 24 | 25+ |
| < 8-year-old [n (%)] | 94 | 28 | ||||
| 8- to 10-year-old [n (%)] | 49 | 120 | 1 | |||
| 11- to 17-year-old [n (%)] | 72 | 227 | 194 | 13 | ||
| > 18-year-old [n (%)] | 31 | 125 | 164 | 240 | 139 | 48 |
Data from 1545 participants as of 31 December 2011.
As of September 2013, in the current state of the inclusions, 2385 patients constitute the cohort; a more detailed description on this whole sample cannot be reported currently, data entry and quality control being still in progress.
What has been Measured?
Evaluation of patient’s health status
Clinical and therapeutic data relating to the disease are provided by reviewing each patient’s medical record. The reported information includes the following: complete characterization of the subtype of leukaemia, age at time of diagnosis, complete description of disease evolution and relapse occurrence, and detailed history of treatments received with special emphasis on anthracyclin cumulative dose, alkylating agents, steroids, use of radiotherapy and haematopoietic stem cell transplantation (HSCT).
The detection of the occurrence of late effects is based on (i) an exhaustive collection of every adverse medical event reported in the patient’s medical file and (ii) a specific medical visit at the time of each evaluation, with complete physical examination and adequate laboratory examinations. Physical health status assessment is organized into 14 parts or modules: height and weight growth, puberty and endocrine gonadal function, fertility, thyroid function, thyroid tumour, heart and blood vessels, ophthalmology, second cancer, viral disease (hepatitis and human immunodeficiency virus), pulmonary function, osteopenia-osteoporosis, iron overload, metabolic syndrome and its components, and other late effects (notably, osteonecrosis, diabetes, severe neurological dysfunctions, kidney failure, hearing loss and alopecia). For each module, the extent of the laboratory testing depends on predefined patient-, disease- and therapy-related features. The details of these 14 modules are reported in the Supplementary data (available at IJE online).
The results of all clinical and laboratory examinations performed to detect physical late effects are explained to the patients, their family and their general practitioner in an attempt to provide adequate medical management.
Self-reported data relating to the patient and/or the parents
According to the patient’s age at the time of evaluation, childhood leukaemia survivors and/or their parents report some personal data, such as socioeconomic status or health-related QoL. Parents of children and adolescents answer a questionnaire for themselves and for their children. Adults and children over 8 years of age complete a questionnaire concerning themselves. The full list of measurements is presented in Box 1 and is detailed in the Supplementary data (available at IJE online).
Box 1 Self-reported data by the childhood leukaemia survivors or their parents.
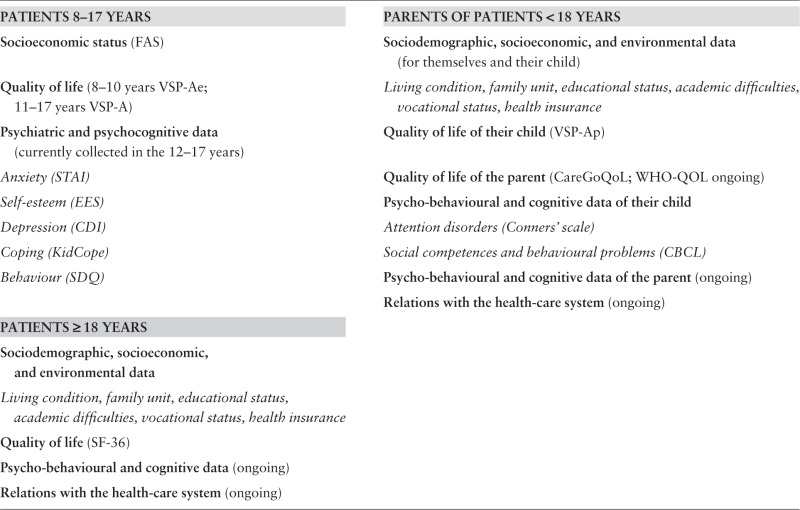 |
Further details in Supplementary data, available at IJE online
What has been Found? Key Findings and Publications
We report below the results of five studies from the cohort regarding physical sequelae little explored in the literature, and the results of one study regarding medium-term QoL of the cohort survivors.
Height
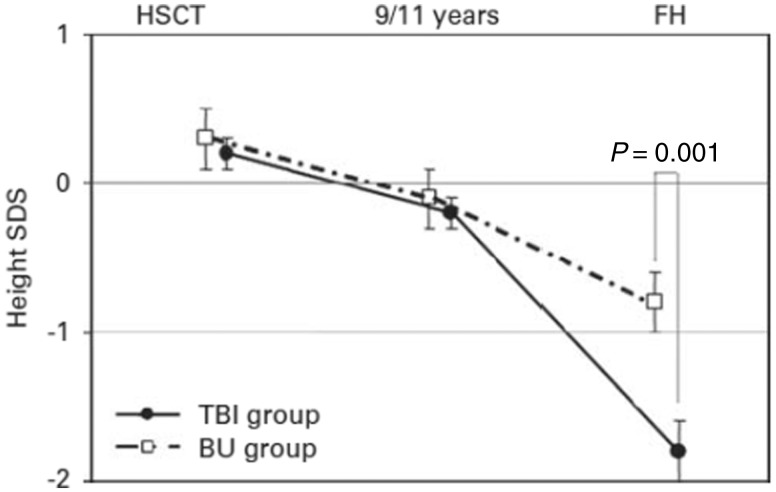
The first results concerned the growth in height of the cohort survivors. We compared the impact of a conditioning regimen with busulfan (BU) or fractionated total body irradiation (TBI) on height growth during adolescence with final height (FH) in adults transplanted for AL before adolescence (younger than 9 years for girls and 11 years for boys, and prepubertal).48 Overall, our results confirm that post-HSCT growth is severely impaired. The mean height standard deviation score was near normal at transplantation and before adolescence but reduced at FH. However, our study also reveals that preparations involving BU, although less toxic than TBI-containing regimens, also have adverse effects on growth, predominantly during adolescence (Figure 1). Another survey evaluated growth rates during the pre- and post-transplant periods up to FH in a group of children treated with HSCT, TBI and/or cranial irradiation and who received growth hormone (GH) therapy. We reported a measurable beneficial effect of GH treatment on growth up to FH.49
Figure 1.
Adults transplanted for AL before adolescence: mean height standard deviation score (± standard error) over time by conditioning regimen. The mean final SDS was lower in the TBI group compared with the BU group (P = 0.001). SDS, standard deviation score; HSCT, haematopoietic stem cell transplantation; FH, final height; TBI, total body irradiation; BU, busulfan.
Metabolic syndrome
Little is known regarding the risk factors of metabolic syndrome (MS) among childhood cancer survivors, except for an increased risk in the case of cranial radiation, and few studies have used an internationally validated MS definition. We attempted to determine the prevalence and risk factors of MS in young adults surviving childhood leukaemia.50 The overall prevalence of MS was 9.2% (95% confidence interval, 5.5–14.4), approximately twice as high as in the young adult general French population.51,52 Among all treatment modalities, HSCT with TBI was found to be a major risk factor for developing MS (adjusted odds ratio = 3.9, P = 0.03) with a dramatic impact on lipid metabolism and on hyperglycaemia.
Bone late effects
Two surveys have reported results regarding the bone sequelae. Femoral and lumbar bone mineral densities (BMDs) in young adults included in the LEA cohort53 were evaluated. Patients who had received only chemotherapy have a slight reduction in their lumbar BMD and a normal femoral BMD. Patients who received HSCT with gonadal deficiency have a reduced femoral BMD, which may increase their fracture risk later in life. We also assessed the prevalence and risk factors of osteonecrosis in childhood leukaemia survivors.54 The cumulative incidence estimation was 2.8% for all patients. A higher cumulative steroid dose, age over 10 years at diagnosis and treatment with transplantation increased the risk of osteonecrosis.
Impact of the physical late effects on the quality of life
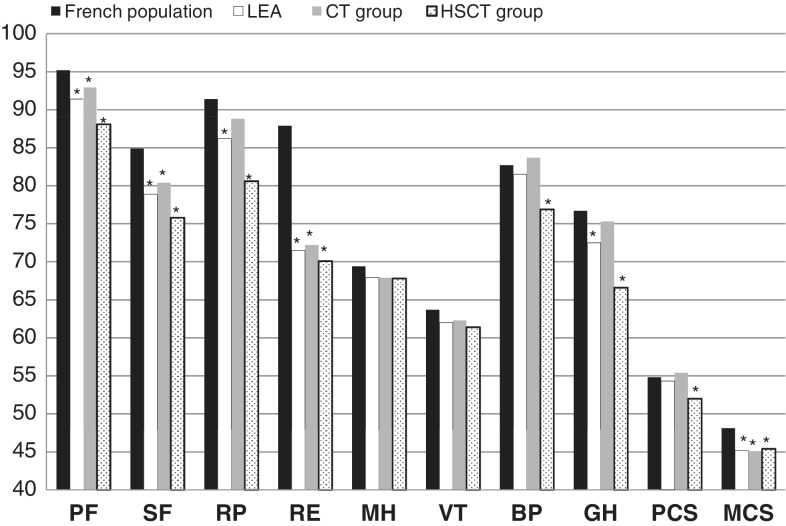
Overall, of all the survivors included as of the end of 2009, more than 70% had at least one sequela (among the 14 modules explored), with the risk being 5.0 (95% confidence interval, 3.0-8.6) times higher for transplantation survivors.55 This substantial effect of HSCT on occurrence of late effects resulted in a decreased physical well-being in adulthood. However, long after treatment was completed, childhood leukaemia survivors reported that effects on psychological well-being are more important than they are on physical QoL dimensions (Figure 2).
Figure 2.
Comparison of the assessment of quality of life of the LEA cohort adult survivors and a French population sample (SF-36 questionnaire). LEA indicates adult survivors. CT, chemotherapy; HSCT, haematopoietic stem cell transplantation; PF, physical functioning; SF, social functioning; RP, role limitations due to physical health problems; RE, role limitations due to emotional problems; MH, general mental health; VT, vitality; BP, bodily pain; GH, general health perceptions; PCS, physical composite score; MCS, mental composite score.
*French population reference group P < 0.05 paired for age and sex.
Ongoing and planned studies
Studies in progress are exploring the classical epidemiological field of late side effects, including uncommon or sparsely explored sequelae (such as thyroid tumours), and their risk factors.
We aim to extend the range of the data collected. The role of genetic susceptibility in the development of treatment-related adverse effects remains largely unknown, and the LEA cohort, according to its structure and organization, is an interesting device enabling genome-wide association studies. Molecular explorations will be planned, with which we will conduct a whole-genome scan to identify the variations associated with late side effects in survivors of childhood leukaemia.
In addition, the LEA cohort proves to be a structured and standardized monitoring system after childhood leukaemia. It enables an optimization of tertiary prevention strategies with earlier identification and treatment of serious late side effects for both the patient and society. Thus, the LEA cohort provides a very good model for studying the reduction of social inequalities facing the consequences of chronic disease. A study is ongoing to compare, inside the cohort, the most disadvantaged populations (economically, culturally, geographically) with other populations for whom access to suitable examinations is facilitated by their socioeconomic status or geographical proximity to healthcare providers.
What are the Main Strengths and Weaknesses?
A major strength of the current project is to propose assessments based on clinical and laboratory examinations to document the occurrence of late side effects in association with an evaluation of socioeconomic data and health-related QoL reported by the patients themselves or their parents. Another strength of the LEA cohort is the high response rate observed in the study, with no differences between respondent and non-respondent patients regarding demographic and clinical characteristics, with the exception of the age at diagnosis, although the observed difference was not clinically relevant. In addition, the LEA study can easily be extended to include survivors of AL diagnosed in young adulthood (18–25 years old) because the long-term health status of these patients has been little explored in the literature.
Several weaknesses have to be underscored. First, the LEA cohort is restricted to the malignant haematological diseases, particularly AL. Although an extension to include lymphoma is under consideration, the cohort does not cover the field of solid tumours. However, the LEA cohort (P Auquier and G Michel) is a part of the French national consortium HOPE- Epi (coordinated by J Clavel) and is thus linking up (1) with the national platform for observation of childhood cancers developed by the national registries (the French national registry of childhood hematopoietic malignancies, J Clavel; and the French national registry of childhood solid tumors, B Lacour), and (2) with the French Childhood Cancer Survivor Study (F deVathaire), following the patients with a diagnosis of childhood solid tumors before 2000. Second, only the survivors are included in the cohort. Nevertheless, record linkage with the national registry would allow collection of data from diagnosis to inclusion in the cohort, and currently, an extension of the protocol for an inclusion at the time of AL diagnosis is under consideration. Third, the definition of the patients definitely lost to the study can be debated. Ceasing attempts when no answer can be obtained after three consecutive evaluation points is consistent with the patient’s consent concept. However, the age range of a survivor can change from one evaluation to another: a young adult may agree to participate in the study where his parents had previously refused. Finally, the current participating centres do not cover the entire French area, even though the cohort allows a geographical coverage of three-quarters of French paediatric onco-haematology centres. Since 2012, new recruitment centres have joined the project and begun inclusions (Rennes, Montpellier, Bordeaux, Strasbourg), and recently, a new team have wished to join the LEA consortium (Toulouse). This extension may greatly improve the cohort sample size, ensuring a higher representation at the national level and making it competitive at the international level.
Can I Get Hold of the Data? Where can I Find Out More?
The data collected within the LEA programme can be used for studies proposed by researchers not belonging to the project consortium, provided they are submitted to the scientific advisory board and approved by the steering committee. These studies will then be performed in partnership with the steering committee. Any enquiries or queries can be submitted to the principal coordinators (Gérard Michel, gérard.michel@ap-hm.fr; and Pascal Auquier, pascal.auquier@univ-amu.fr).
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work is supported by the French National Cancer Institute (InCA), the French National Research Agency (ANR), the Cancéropôle PACA, the Regional Council PACA and the French Institute for Public Health Research (IRESP).
Supplementary Material
Acknowledgements
We are thankful to all the survivors and their families who agreed to participate in this study. We thank the clinicians, past and present, who identified potential participants for the cohort, Dr MC Simeoni for her technical assistance, A Loundou for his contribution to the statistical analyses and T Vigne for her participation to the data entry.
The members of the LEA Group: Prof. H Chambost, Dr I Thuret, Dr C Galambrun, Dr V Barlogis, Dr C Curtillet, Dr I Herrmann, B Play, M Allouche, D Orbicini, V Villes, F Garnier, C Vercasson, Marseilles; Dr C Schmitt, Dr L Mansuy, Dr F Fouyssac, Dr I Lemelle, Dr A Salmon, Dr L Clément, Dr E Colomb, M Pernet, Nancy; Dr F Monpoux, Dr C Soler, Dr A Deville, Dr M Le Meignen-Diop, Dr F Bellmann Roqueplan, L Martinez, Nice; Dr F Isfan, Dr E Doré, Dr E Merlin, Dr F Chambon, N Rouel, E Rochette, Clermont-Ferrand; Dr A Pagnier, Dr C Armari-Alla, Dr D Adjaoud, I Schiff, Grenoble; Dr F Baleydier, Dr F Dommange-Romero, Dr C Halfon-Domenech, Dr K Kebaïli, Dr N Garnier, Dr C Renard, C Petit, Lyon; Dr A Auvrignon, Dr J Landman-Parker, Dr S Fasola, Dr C Dollfus, Dr J Donadieu, Dr A Petit, O Philippe, S Said Omar, Paris Trousseau; Dr C Berger, Dr A David, F Odier, Saint Etienne; Dr B Brethon, Dr AF Ray-Lunven, Dr T Leblanc, Paris St Louis; Dr S Azarnoush, Dr M Ouachee-Chardin, Dr R Fahd, Dr K Yacouben, Dr B Lescoeur, Dr T Bontant, Dr F Duquesne, MN Moukoko, Paris Robert Debré; Dr J Bonneau, Dr F Toutain, Dr S Taque, AM Lamour, Rennes; Dr A Cuinet, Dr S Haouy, Dr A Sirvent, Dr L Saumet, S Chanthavisouk, O Mophawe, Montpellier; Prof. Y Perel, Dr S Ansoborlo, Dr N Aladjidi, Dr C Vérité-Goulard, Dr C Jubert, Dr C De Bouyn-Icher, Dr A Notz-Carrerre, M Merched, Bordeaux; Dr S Drillon, S Ceron-Duran, Strasbourg.
Conflict of interest: None declared.
References
- 1.Bosetti C, Bertuccio P, Chatenoud L, Negri E, Levi F, La Vecchia C. Childhood cancer mortality in Europe, 1970-2007. Eur J Cancer 2010;46:384–94. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G, Zigon G, Capocaccia R, et al. Survival of European children and young adults with cancer diagnosed 1995-2002. Eur J Cancer 2009;45:992–1005. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011;29:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol 2009;27:2339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eiser C. Learning difficulties in children treated for leukemia. In: Children with cancer: the Quality of Life. Mahwah, NJ: Lawrence Erlbaum Associates, 2004. [Google Scholar]
- 6.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood 2008;111:5515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulrooney DA, Dover DC, Li S, et al. Twenty years of follow-up among survivors of childhood and young adult acute myeloid leukaemia: a report from the Childhood Cancer Survivor Study. Cancer 2008;112:2071–79. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–82. [DOI] [PubMed] [Google Scholar]
- 9.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social outcomes in the Childhood Cancer Survivor Study cohort. J Clin Oncol 2009;27:2390–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida Y, Honda M, Kamibeppu K, et al. Social outcomes and quality of life of childhood cancer survivors in Japan: a cross-sectional study on marriage, education, employment and health-related QOL (SF-36). Int J Hematol 2011;93:633–44. [DOI] [PubMed] [Google Scholar]
- 11.Maunsell E, Pogany L, Barrera M, Shaw AK, Speechley KN. Quality of life among long-term adolescent and adult survivors of childhood cancer. J Clin Oncol 2006;24:2527–35. [DOI] [PubMed] [Google Scholar]
- 12.Pogorzala M, Styczynski J, Kurylak A, Debski R, Wojtkiewicz M, Wysocki M. Health-related quality of life among paediatric survivors of primary brain tumours and acute leukaemia. Qual Life Res 2010;19:191–98. [DOI] [PubMed] [Google Scholar]
- 13.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukaemia. N Engl J Med 2003;349:640–49. [DOI] [PubMed] [Google Scholar]
- 14.Shankar S, Robison L, Jenney ME, et al. Health-related quality of life in young survivors of childhood cancer using the Minneapolis-Manchester Quality of Life-Youth Form. Pediatrics 2005;115:435–42. [DOI] [PubMed] [Google Scholar]
- 15.Speechley KN, Barrera M, Shaw AK, Morrison HI, Maunsell E. Health-related quality of life among child and adolescent survivors of childhood cancer. J Clin Oncol 2006;24:2536–43. [DOI] [PubMed] [Google Scholar]
- 16.Zebrack BJ, Landier W. The perceived impact of cancer on quality of life for post-treatment survivors of childhood cancer. Qual Life Res 2011;20:1595–608. [DOI] [PubMed] [Google Scholar]
- 17.Zebrack BJ, Zeltzer LK, Whitton J, et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin's disease, and non-Hodgkin's lymphoma: a report from the Childhood Cancer Survivor Study. Pediatrics 2002;110:42–52. [DOI] [PubMed] [Google Scholar]
- 18.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rueegg CS, Gianinazzi ME, Rischewski J, et al. Health-related quality of life in survivors of childhood cancer: the role of chronic health problems. J Cancer Surviv 2013;7:511–22. [DOI] [PubMed] [Google Scholar]
- 20.Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer 2005;104:1751–60. [DOI] [PubMed] [Google Scholar]
- 21.Langeveld NE, Ubbink MC, Last BF, Grootenhuis MA, Voute PA, De Haan RJ. Educational achievement, employment and living situation in long-term young adult survivors of childhood cancer in the Netherlands. Psychooncology 2003;12:213–25. [DOI] [PubMed] [Google Scholar]
- 22.Lund LW, Schmiegelow K, Rechnitzer C, Johansen C. A systematic review of studies on psychosocial late effects of childhood cancer: structures of society and methodological pitfalls may challenge the conclusions. Pediatr Blood Cancer 2011;56:532–43. [DOI] [PubMed] [Google Scholar]
- 23.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 2010;118:1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer 2007;49:65–73. [DOI] [PubMed] [Google Scholar]
- 25.Daly BP, Brown RT. Scholarly literature review: management of neurocognitive late effects with stimulant medication. J Pediatr Psychol 2007;32:1111–26. [DOI] [PubMed] [Google Scholar]
- 26.Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: implications for risk-based surveillance. J Clin Oncol 2009;27:2405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenney B, Diller L. Childhood cancer survivorship. In: Orkin SH, Fisher DE, Look AT, et al. (eds). Oncology of Infancy and Childhood. Philadelphia, PA: Saunders Elsevier, 2009. [Google Scholar]
- 28.Loning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Munster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood 2000;95:2770–75. [PubMed] [Google Scholar]
- 29.Massimo LM, Wiley TJ, Bonassi S, Caprino D. Longitudinal psychosocial outcomes in two cohorts of adult survivors from childhood acute leukemia treated with or without cranial radiation. Minerva Pediatr 2006;58:1–7. [PubMed] [Google Scholar]
- 30.Michel G, Socie G, Gebhard F, et al. Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukaemia in first complete remission: the impact of conditioning regimen without total-body irradiation – a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol 1997;15:2238–46. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen K, Levitt G, Bull C, Chessells J, Sullivan I. Anthracycline dose in childhood acute lymphoblastic leukaemia: issues of early survival versus late cardiotoxicity. J Clin Oncol 1997;15:61–68. [DOI] [PubMed] [Google Scholar]
- 32.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol 2002;38:229–39. [DOI] [PubMed] [Google Scholar]
- 33.Carswell K, Chen Y, Nair RC, et al. Smoking and binge drinking among Canadian survivors of childhood and adolescent cancers: a comparative, population-based study. Pediatr Blood Cancer 2008;51:280–87. [DOI] [PubMed] [Google Scholar]
- 34.Shaw AK, Pogany L, Speechley KN, Maunsell E, Barrera M, Mery LS. Use of health care services by survivors of childhood and adolescent cancer in Canada. Cancer 2006;106:1829–37. [DOI] [PubMed] [Google Scholar]
- 35.Dama E, Maule MM, Mosso ML, et al. Life after childhood cancer: marriage and offspring in adult long-term survivors – a population-based study in the Piedmont region, Italy. Eur J Cancer Prev 2009;18:425–30. [DOI] [PubMed] [Google Scholar]
- 36.Debling D, Spix C, Blettner M, Michaelis J, Kaatsch P. The cohort of long-term survivors at the German childhood cancer registry. Klin Padiatr 2008;220:371–77. [DOI] [PubMed] [Google Scholar]
- 37.Harila-Saari AH, Lahteenmaki PM, Pukkala E, Kyyronen P, Lanning M, Sankila R. Scholastic achievements of childhood leukaemia patients: a nationwide, register-based study. J Clin Oncol 2007;25:3518–24. [DOI] [PubMed] [Google Scholar]
- 38.Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol 2001;19:191–96. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins MM, Lancashire ER, Winter DL, et al. The British Childhood Cancer Survivor Study: Objectives, methods, population structure, response rates and initial descriptive information. Pediatr Blood Cancer 2008;50:1018–25. [DOI] [PubMed] [Google Scholar]
- 40.Reulen RC, Taylor AJ, Winter DL, et al. Long-term population-based risks of breast cancer after childhood cancer. Int J Cancer 2008;123:2156–63. [DOI] [PubMed] [Google Scholar]
- 41.Reulen RC, Winter DL, Lancashire ER, et al. Health-status of adult survivors of childhood cancer: a large-scale population-based study from the British Childhood Cancer Survivor Study. Int J Cancer 2007;121:633–40. [DOI] [PubMed] [Google Scholar]
- 42.Reulen RC, Zeegers MP, Lancashire ER, Winter DL, Hawkins MM. Offspring sex ratio and gonadal irradiation in the British Childhood Cancer Survivor Study. Br J Cancer 2007;96:1439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reulen RC, Zeegers MP, Wallace WH, et al. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev 2009;18:2239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor AJ, Croft AP, Palace AM, et al. Risk of thyroid cancer in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. Int J Cancer 2009;125:2400–05. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AJ, Frobisher C, Ellison DW, et al. Survival after second primary neoplasms of the brain or spinal cord in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. J Clin Oncol 2009; 27:5781–87. [DOI] [PubMed] [Google Scholar]
- 46.Kuehni CE, Strippoli MP, Rueegg CS, et al. Educational achievement in Swiss childhood cancer survivors compared with the general population. Cancer 2011;118:1439–49. [DOI] [PubMed] [Google Scholar]
- 47.Pediatric Cancer Survivorship: The Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2307–415. [DOI] [PubMed] [Google Scholar]
- 48.Bernard F, Bordigoni P, Simeoni MC, et al. Height growth during adolescence and final height after haematopoietic SCT for childhood acute leukaemia: the impact of a conditioning regimen with BU or TBI. Bone Marrow Transplant 2009;43:637–42. [DOI] [PubMed] [Google Scholar]
- 49.Isfan F, Kanold J, Merlin E, et al. Growth hormone treatment impact on growth rate and final height of patients who received HSCT with TBI or/and cranial irradiation in childhood: a report from the French Leukaemia Long-Term Follow-Up Study (LEA). Bone Marrow Transplant 2011;47:684–93. [DOI] [PubMed] [Google Scholar]
- 50.Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood 2011;117:4442–48. [DOI] [PubMed] [Google Scholar]
- 51.Pannier B, Thomas F, Eschwege E, et al. Cardiovascular risk markers associated with the metabolic syndrome in a large French population: the ‘SYMFONIE’ study. Diabetes Metab 2006;32:467–74. [DOI] [PubMed] [Google Scholar]
- 52.Balkau B, Vernay M, Mhamdi L, et al. The incidence and persistence of the NCEP (National Cholesterol Education Programme) metabolic syndrome. The French D.E.S.I.R. study. Diabetes Metab 2003;29:526–32. [DOI] [PubMed] [Google Scholar]
- 53.Le Meignen M, Auquier P, Barlogis V, et al. Bone mineral density in adult survivors of childhood acute leukemia: impact of hematopoietic stem cell transplantation and other treatment modalities. Blood 2011;118:1481–89. [DOI] [PubMed] [Google Scholar]
- 54.Girard P, Auquier P, Barlogis V, et al. Symptomatic osteonecrosis in childhood leukaemia survivors: prevalence, risk factors and impact on quality of life in adulthood. Haematologica 2013;98:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berbis J, Michel G, Chastagner P, et al. A French cohort of childhood leukemia survivors: impact of hematopoietic stem cell transplantation on health status and quality of life. Biol Blood Marrow Transplant 2013;19:1065–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.