Abstract
Since 1986, antiretroviral therapy (ART) has been available free of charge to individuals living with HIV in British Columbia (BC), Canada, through the BC Centre of Excellence in HIV/AIDS (BC-CfE) Drug Treatment Program (DTP). The Highly Active Antiretroviral Therapy (HAART) Observational Medical Evaluation and Research (HOMER) cohort was established in 1996 to maintain a prospective record of clinical measurements and medication profiles of a subset of DTP participants initiating HAART in BC. This unique cohort provides a comprehensive data source to investigate mortality, prognostic factors and treatment response among people living with HIV in BC from the inception of HAART. Currently over 5000 individuals are enrolled in the HOMER cohort. Data captured include socio-demographic characteristics (e.g. sex, age, ethnicity, health authority), clinical variables (e.g. CD4 cell count, plasma HIV viral load, AIDS-defining illness, hepatitis C co-infection, mortality) and treatment variables (e.g. HAART regimens, date of treatment initiation, treatment interruptions, adherence data, resistance testing). Research findings from the HOMER cohort have featured in numerous high-impact peer-reviewed journals. The HOMER cohort collaborates with other HIV cohorts on both national and international scales to answer complex HIV-specific research questions, and welcomes input from external investigators regarding potential research proposals or future collaborations. For further information please contact the principal investigator, Dr Robert Hogg (robert_hogg@sfu.ca).
Keywords: HIV, highly active antiretroviral therapy, cohort studies, database, Canada
Key Messages.
Improved treatment outcomes and temporal reductions in the incidence of antiretroviral drug resistance within the HOMER cohort demonstrate the increased effectiveness of modern HAART regimens in BC.
Analyses within the HOMER cohort have shown that expanding HAART coverage within BC has reduced HIV transmission on a population level, emphasizing ‘Treatment as Prevention' as a key element of the comprehensive HIV combination prevention framework.
Contemporary research within the HOMER cohort evaluates viral and human genetic predictors of treatment outcomes, with a view to expanding human pharmacogenetic testing to guide HIV therapy.
The HIV/AIDS Drug Treatment Program (DTP)
Since 1986, antiretroviral therapy (ART) has been available free of charge to medically eligible HIV-positive individuals living in British Columbia (BC), Canada. Since 1992, the BC Centre for Excellence in HIV/AIDS (BC-CfE)1 Drug Treatment Program (DTP)2 has administered the centralized distribution of ART on behalf of the BC Ministry of Health's PharmaCare programme. Medical eligibility for ART initiation through the DTP is determined by the BC-CfE Therapeutic Guidelines.3,4 New ART prescriptions are reviewed by a BC-CfE affiliated physician, to ensure the requested medication regimen is compatible with provincial guidelines developed by a committee of physicians, virologists, pharmacists, economists and health service researchers.5 Guidelines have evolved over the past 20 years to reflect advances in research, clinical experience and availability of antiretroviral drugs. Current guidelines preferentially recommend as initial therapy the use of: two nucleoside reverse transcriptase inhibitors (NRTI), either tenofovir or alternatively abacavir, plus emtricitibine or lamuvidine, plus a third agent, either the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz or the ritonavir-boosted protease inhibitor (PI) atazanavir.4
In recent years, therapeutic guidelines have also encompassed the increasingly recognized role of ‘Treatment as Prevention’, an approach pioneered at the BC-CfE.6 Existing guidelines recommend that HAART should be offered to all HIV-positive individuals regardless of CD4 cell count, except in the case of elite controllers and long-term non-progressors.4 This treatment approach is consistent with the recommendations of the International Antiviral Society-USA7 and the Department of Health and Human Services.8
All DTP participants are entered into the BC-CfE Registry, a database of socio-demographic, clinical and laboratory variables. Individuals are entered into the BC-CfE Registry at the time of their first contact with the BC-CfE, either their first HIV viral load test or their first ART prescription in BC. The BC-CfE Registry is a secure, electronic database, which includes participant demographic and clinical information collected from HAART prescriptions, laboratory records and research ethics board-approved linkages with other health databases. At the time of DTP enrolment, participants are provided with an information sheet which describes data collection and usage and explains the measures taken to ensure confidentiality and protect privacy.9 Following an initial period of monthly monitoring and medication dispensing, HAART prescriptions are typically refilled every 2–3 months and HIV viral load and CD4 count are monitored in a schedule determined by the Primary Care Guidelines.10
For each participant, clinical staging forms, based on the US Centers for Disease Control clinical staging system,11 are mailed to the prescribing physicians annually to update clinical information and capture incident opportunistic infections. Clinical and socio-demographic information are then manually entered into the BC-CfE Registry. In BC, all HIV plasma viral load tests and most CD4 cell count tests are performed by the St Paul’s Hospital laboratories in Vancouver. These test results are then transferred electronically via linkage to the BC-CfE Registry. CD4 cell counts and hepatitis B and C tests performed at other centres in BC are captured from results written on ART prescriptions, and are manually entered into the BC-CfE Registry. Hospital and community ART-dispensing data are updated daily in the BC-CfE Registry through manual entry of pharmacy medication dispensing records.
Why was the HOMER cohort established?
The HAART Observational Medical Evaluation and Research (HOMER) cohort was established in 1996 to maintain a prospective record of clinical, laboratory and medication profiles of a subset of DTP participants, characterized by more restrictive inclusion criteria. This unique cohort provides a comprehensive data source to investigate mortality, prognostic factors and treatment responses among people living with HIV in BC from the inception of HAART.
The HOMER cohort provides longitudinal data on socio-demographic, clinical and laboratory variables for ART-naïve individuals who have initiated HAART in BC since the advent of triple combination therapy. The cohort study aims to:
evaluate clinical and virological outcomes, resistance patterns and prognostic factors associated with HAART among people living with HIV in BC
conduct surveillance of the population-level health effects of HAART
inform treatment priorities and therapeutic guidelines for HIV/AIDS management
collaborate with other HIV observational cohorts to pursue complex HIV-related research that requires large sample sizes.
Who is included in the HOMER cohort?
HOMER restricts inclusion to DTP participants who were ART-naïve in BC prior to enrolment, aged ≥19 years of age at ART initiation and whose first ART regimen included three or more antiretroviral agents initiated on/after 1 August 1996. Up until the most recent update of the cohort, eligible individuals required a baseline CD4 cell count <500 cells/mm3 or a baseline plasma viral load >5000 copies/ml. Baseline measurements are defined as the latest results available with test dates within 6 months prior to HAART initiation. In light of recent changes to the therapeutic guidelines,4 the cohort moving forward will adopt more inclusive criteria, allowing CD4 cell count to be of any value, while restricting to patients with plasma viral load measurements >200 copies/ml. This restriction will help minimize the chance of mistakenly including out-of-province ART-experienced patients into HOMER. We have explored the possibility of applying this modified inclusion criterion to the current dataset, but this failed to significantly increase the number of individuals identified. This likely reflects the lag-time between the announcement of new guidelines and community implementation.
The HOMER cohort is updated annually, with the most recent update including individuals who initiated ART between 1 August 1996 and 30 June 2011, with follow-up until 30 June 2012. The 5229 individuals enrolled in the current HOMER cohort represent approximately 63% of the 8327 patients who have ever accessed ART through the DTP up to 30 June 2011.
Characteristics of individuals in the HOMER cohort
The HOMER cohort includes individuals initiating HAART from across BC. The province is sub-divided into health authorities that separate the governing and planning of health services.12 The proportion of individuals recruited from each of the health authorities is shown in Figure 1. The majority of cohort participants reside within the catchment area of Vancouver Coastal Health Authority (58%), and live within urban localities (95%).
Figure 1.
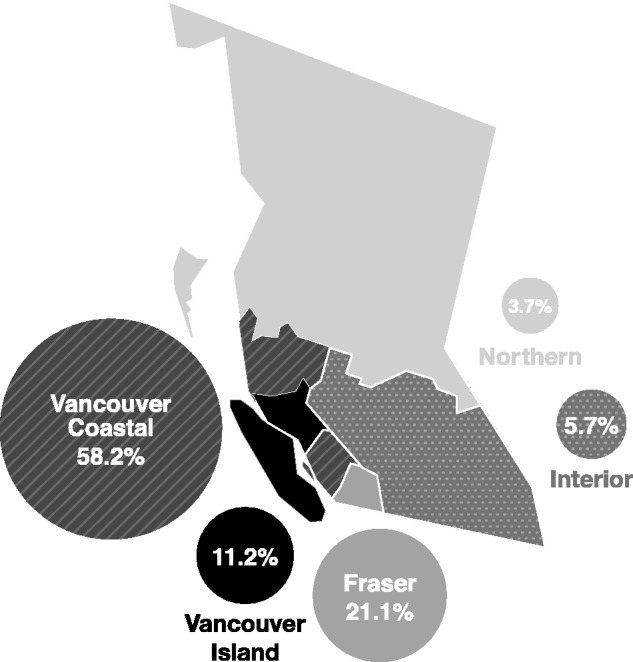
Map demonstrating the number of individuals in the HOMER cohort residing in each health authority in BC on 30 June 2012, the study end-date for the HOMER cohort at the time of writing (n = 5223). The majority of HOMER cohort participants reside within the catchment area of Vancouver Coastal Health Authority, and live in urban localities. The health authority was unknown for six participants within the cohort.
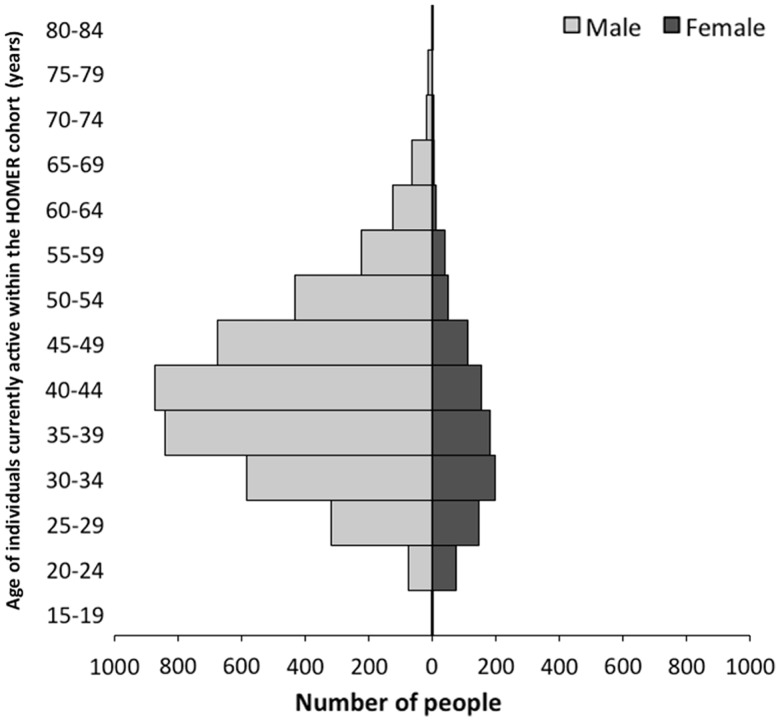
Table 2 lists socio-demographic and clinical characteristics of all HOMER cohort participants until administrative censoring on 30 June 2012. The majority (81%) of individuals in the cohort are male, and injection drug use (IDU) is the most common HIV transmission route, reported by 39% of individuals. Among individuals active in the HOMER cohort as of 30 June 2012, the median age is 48 years. Figure 2 shows the age distribution of individuals currently active within HOMER.
Table 2.
Socio-demographic and clinical characteristics of HOMER cohort participants up to administrative censoring on 30 June 2012, the study end-date for the HOMER cohort at the time of writing (n = 5229)
| Variable | Number (%) or median (quartiles 1–3) |
|---|---|
| Sex | |
| Female | 985 (18.8) |
| Male | 4244 (81.2) |
| Resident in an urban localitya | 4090 (95.2) |
| Current age (years) | 48 (41–55) |
| Age at enrolment (years) | 41 (34–47) |
| Baseline CD4 cell count (cells/mm3) | 210 (110–330) |
| Most recent CD4 cell count (cells/mm3) | 450 (270–640) |
| Baseline plasma viral load (copies/ml) | 95 000 (30 700–100 010) |
| Recent viral suppression (≤50 copies/ml)b | 3673 (74.6) |
| Viral load test rate (per year follow-up) | 4.2 (3.1–5.2) |
| AIDS at baseline | 781 (14.9) |
| HIV risk factorc | |
| History of injection drug use | 2031 (38.8) |
| Heterosexual | 1207 (23.1) |
| MSM | 1310 (25.1) |
| Unknown | 1741 (33.0) |
| Hepatitis C-positive | 2193 (41.9) |
| Ethnicityc | |
| White | 1836 (35.1) |
| Aboriginal ancestry | 684 (13.1) |
| Asian | 190 (3.6) |
| Hispanic | 112 (2.1) |
| Black | 117 (2.2) |
| Unknown | 2449 (46.8) |
| Loss to follow-upd | 238 (4.6) |
| Total follow-up time (years) | 5 (3–9) |
| Crude mortality | 993 (19.0) |
| Years on ART | 4 (2–7) |
| Baseline ART resistance testing | 2839 (54.3) |
| Resistance mutations detected | 234 (8.24) |
| Treatment interruption (≥90 days) in past year of follow-up | 627 (13.0) |
| Adherence ≥95% in past year of follow-upe | 2903 (60.0) |
This table displays socio-demographic and clinical characteristics of all HOMER cohort participants up to administrative censoring, including participants who have died, moved out of BC or become lost to follow-up.
MSM, men who have sex with men; ART, antiretroviral therapy.
aDetermined by postal code data (data missing for 933 individuals).
bPlasma viral load within 12 months before last follow-up date. Viral load assays taken after April 1999 exclusively included, to reflect guideline changes and sensitivity of testing (data excluded for 305 individuals).
cIn some cases participants may identify with more than one HIV risk factor/ethnicity, therefore the total percentage is greater than 100.
dDefined as no clinical contact for ≥18 months.
eDetermined by pharmacy refill data. Individuals eligible for adherence assessment must have ≥12 months of follow-up (n = 4842).
Figure 2.
Population pyramid showing the age distribution of individuals active in the HOMER cohort on 30 June 2012, the study end-date for the HOMER cohort at the time of writing (n = 3584). This figure excludes individuals who have died, moved out of BC or become lost to follow-up. The median age of active cohort participants at the most recent study end-date was 48 years and the majority of participants identified as male sex.
Cumulative annual enrolment, loss to follow-up and death within the HOMER cohort
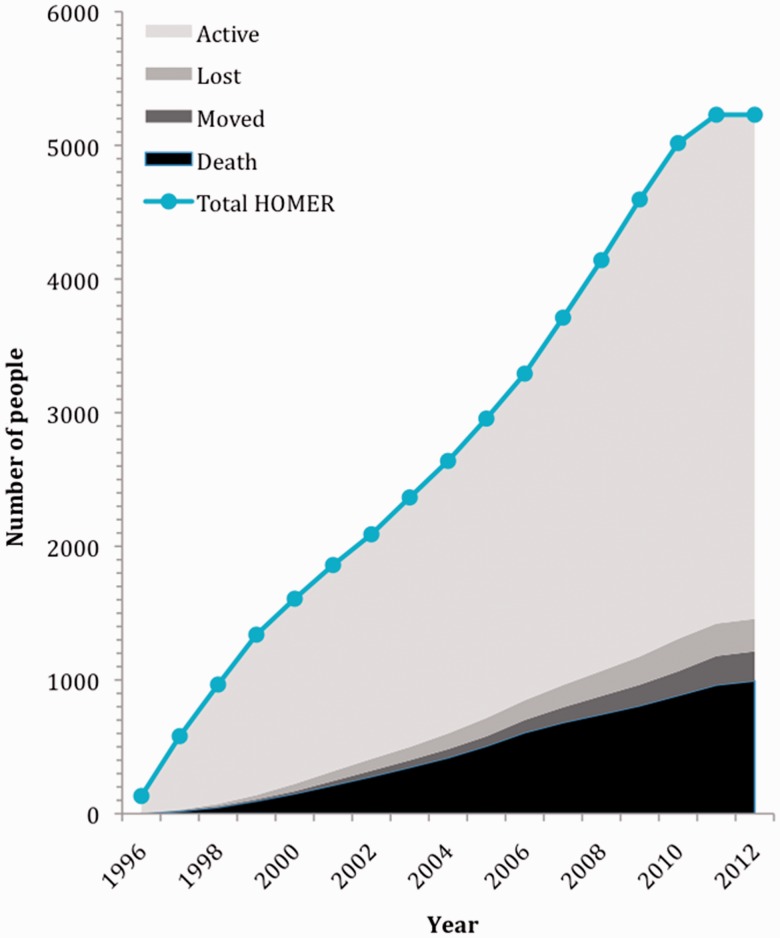
Figure 3 shows cumulative annual enrolment, loss to follow-up (LTFU) and death within the cohort since the study inception. The median number of individuals enrolled into the HOMER cohort annually is 327 individuals per year [quartiles [Q1-Q3]: 265–420]. Overall crude mortality is 19%, with a mortality rate of 3.04 per 100 person-years. The median follow-up time for individuals in the cohort is 5 years (Q1-Q3: 3–9 years). The total LTFU since cohort inception is 5%, defined as no clinical contact based on various data sources such as prescription refills, AIDS-defining illness diagnoses, CD4 cell count, viral load or other laboratory tests for ≥18 months. A recent comparison of 17 North American and European cohorts, published by the Antiretroviral Therapy Cohort Collaboration (ART-CC), demonstrated that the range of LTFU in similar HIV observational cohorts is between 2% and 18%, placing the HOMER cohort at the low end of this scale.13
Figure 3.
Cumulative enrolment, loss to follow-up and mortality in the HOMER cohort over time on 30 June 2012, the study end-date for the HOMER cohort at the time of writing. This figure demonstrates the number of participants enrolled in the HOMER cohort since the study inception in 1996. Total HOMER represents the sum of active HOMER cohort participants and individuals who had died, moved out of BC or become lost to follow-up as of 30 June 2012.
What is measured and how are the data collected?
A subset of data specific to HOMER-eligible individuals in the BC-CfE Registry is used to populate variables in the HOMER cohort. Longitudinal electronic clinical and demographic data from the BC-CfE Registry are compiled and processed to update the HOMER cohort every 12 months. Data routinely collected are shown in Table 1. Key variables captured include socio-demographic characteristics (e.g. sex, age, ethnicity, health authority), clinical variables (e.g. CD4 cell count, plasma HIV viral load, AIDS-defining illness, hepatitis C co-infection, mortality), and treatment variables (e.g. HAART regimens, date of treatment initiation, treatment interruptions, adherence data, resistance testing). The HOMER protocol has been granted ethical approval by the University of British Columbia Research Ethics Board.
Table 1.
Data routinely collected/derived for the HOMER cohort as of 30 June 2012, the study end-date for the HOMER cohort at the time of writing
| Variables | |
|---|---|
| Demographics | Age |
| Sex | |
| Ethnicity | |
| Aboriginal ancestry | |
| Postal code and/or census tract locator | |
| BC Health Authority | |
| Date of death | |
| Cause of death | |
| Clinical/laboratory variables | Year of first HIV-positive test |
| Year of ART initiation | |
| Physician’s experience of monitoring HIV-positive patients | |
| HIV clade | |
| AIDS-defining illnessesa | |
| Absolute CD4 cell count and date | |
| Plasma viral load and date | |
| Hepatitis C infection status | |
| Hepatitis B infection status | |
| Antiretroviral therapy | ART medication (drug, form, dose) |
| Start and stop date for each ART medication | |
| ART drug resistance testing and results | |
| ART adherenceb | |
| Treatment interruptions | |
| Prescribed structured treatment interruptions |
ART: antiretroviral therapy.
aDate is recorded for the first diagnosis of each ADI.
bAdherence is estimated based on pharmacy refill records and is defined as the proportion of time an individual has supply of ART dispensed during follow-up. Threshold for adherence is set at ≥95%.
The BC-CfE Registry is linked with the BC Division of Vital Statistics to monitor patient mortality on a monthly basis, which significantly minimizes LTFU, enhancing data quality and completeness within the cohort.
Knowledge translation
Research findings from the HOMER cohort have attracted a global audience as a result of numerous original research publications featuring in high-impact peer-reviewed journals, in addition to contemporary research presentations at major international conferences. Results from the cohort have informed research priorities, policy-making and programme development on a global scale. Our work is also presented through social media,14 our website15 and print media, radio and television.
What are the major findings to date?
Clinical benefits associated with HAART and prognostic indicators
Seminal research within the HOMER cohort and the BC-CfE Registry at the outset of the HIV epidemic provided strong evidence of the clinical benefits of HAART, demonstrating decreased morbidity and mortality and a superior virological response when compared with previously recommended monotherapy and dual combination therapy.16,17 The HOMER cohort continues to chronicle the evolution of HAART as antiretroviral regimens improve in efficacy and tolerability. More recent findings within the HOMER cohort18 and the BC-CfE Registry19,20 demonstrate a significant decrease in mortality and disease progression and an increase in life expectancy among HIV-positive individuals in BC over time, associated with the increased uptake of modern forms of HAART.
Early data from the HOMER cohort supported the prognostic value of plasma viral load and CD4 cell count to predict treatment outcomes, disease progression and mortality.21–24 More recently, publications have explored the benefits of evaluating prognostic indicators on a programmatic level.25 The Programmatic Compliance Score (PCS) was developed and validated within the HOMER cohort in 2012. Six indicators based on the IAS-USA guidelines contributed to the final score including frequency of CD4 and viral load assessment, resistance testing prior to treatment initiation, CD4 count at ART initiation, HAART regimen commenced and virological outcomes after treatment initiation. The PCS was strongly associated with all-cause mortality and potentially represents a means of improving clinical outcomes among individuals commencing HAART.25
Treatment as prevention
Recent publications have evaluated the benefits of expanding access to HAART in BC with the aim of reducing HIV transmission on a population level, a concept known as treatment as prevention.6,26–29 The BC-CfE has used data from the HOMER cohort in combination with other datasets, to create demographic and mathematical models to evaluate the individual and public health benefits of treatment as prevention and inform future policy and guideline judgments. Findings have demonstrated that expanding coverage of HAART in BC could dramatically reduce HIV transmission on a population level.6,26,27,30 Treatment as prevention is now becoming widely accepted as a key element of the comprehensive HIV combination prevention framework and is recognized globally as an urgent implementation priority.31,32
Socio-demographic characteristics, adherence and treatment outcomes
The HOMER cohort is a unique database, allowing for evaluation of the impact of socio-demographic characteristics on health outcomes in a population where medical care and access to HAART is provided to all eligible residents free of charge. Despite the availability of a universal healthcare system, early findings from the HOMER cohort and the BC-CfE Registry observed that individuals of lower socio-economic status demonstrated increased HIV-related mortality and decreased access to HAART.33–35 Similarly, HOMER participants with a history of IDU accessing HAART demonstrated suboptimal adherence and clinical outcomes.36–39 However, novel findings within the HOMER cohort suggest that IDU status and adherence may be less important predictors of suboptimal clinical outcomes in the context of modern highly effective HAART regimens.40–42
Antiretroviral drug resistance
HIV mutations conferring antiretroviral drug resistance have been extensively evaluated since the inception of HOMER. High plasma viral load levels and suboptimal adherence have been identified as predictors of HIV antiretroviral-resistance mutations within the cohort.43,44 Studies evaluating the temporal changes in the emergence of antiretroviral drug resistance within the HOMER cohort45 and the BC-CfE Registry46,47 have shown a decline in the incidence of resistance mutations over time in BC, along with improved virological outcomes, highlighting increased efficacy of modern HAART regimens. Despite this, a recent study characterizing the epidemiology of antiretroviral multiclass resistance among HOMER cohort participants reported that 17% of the individuals evaluated demonstrated 2-class antiretroviral drug resistance, highlighting the continued importance of regular viral load and resistance monitoring among treatment-experienced individuals.48
Supplementary data collection
Supplementary data collection has been completed to enrich the HOMER dataset and answer specific research questions relating to key HIV-related health issues in BC. The Longitudinal Investigation into Supportive and Ancillary health services (LISA) study collected cross-sectional socio-demographic and behavioural data in a survey administered from 2007 to 2010, to improve the understanding of supportive service use, socio-demographic factors and quality of life among a subset of HOMER participants.51 Findings from the LISA study have confirmed the importance of expanding supportive service provision for HOMER participants demonstrating social and clinical vulnerabilities.52,53
Future research directions
The HOMER cohort continues to pursue innovative research within the field of HIV testing and treatment. One of the most novel research projects currently underway evaluates viral and human genetic predictors of response to HIV therapies. The research team aims to develop a single more sensitive test for HIV resistance, based on next-generation sequencing, and expand human pharmacogenetic testing to guide HIV therapy based on each patient’s unique DNA. This research will optimize HAART prescribing and reduce the economic and health cost of treatment failures and emerging drug resistance. Additionally, this project will establish a real-time surveillance platform to monitor HIV drug resistance among individuals living with HIV in BC, enabling rapid identification of at-risk areas with high transmission risk in need of targeted intervention.49
Another contemporary research project employs next-generation sequencing technologies to retrospectively quantify the genetic variation of HIV populations from repeated samples from a subset of HOMER cohort participants. The main objective of this research is to develop new techniques for detecting the signature of virus adaptation to the host-specific immune response. This project will use phylogenetic methods to reconstruct the evolutionary history of the virus population within each individual, to determine when certain mutations first appeared and how they proliferated over time in response to selection.50 By characterizing the adaptation of HIV within patients, these techniques may be able to provide an important resource for anti-HIV vaccine research.
Currently in the recruitment stage, the Engage Study54 aims to create a prospective cohort of HIV-positive individuals newly initiating HAART, nested within the HOMER cohort. Interviewer-administered surveys will supplement the HOMER dataset to enable evaluation of socio-demographic, economic, behavioural and structural characteristics that mediate HAART uptake, retention and clinical outcomes in the context of the increased expansion of HAART in BC.
Partner cohort studies
The Seek and Treat for Optimal Prevention of HIV/AIDS (STOP HIV/AIDS) cohort55 is a partner study coordinated through the BC-CfE. More expansive in scope than the HOMER cohort, the STOP HIV/AIDS cohort includes all HIV-positive individuals resident in BC, who were diagnosed with HIV between 1 January 1996 and 31 March 2010. The HOMER cohort primarily uses the robust clinical data within the BC-CfE Registry to evaluate mortality, prognostic factors and treatment responses specifically among individuals accessing HAART in BC, with the aim of informing treatment priorities and therapeutic guidelines and evaluating the population-level health effects of HAART. Comparatively, the STOP HIV/AIDS cohort links data from the BC-CfE Registry with eight additional treatment, surveillance and administrative databases, to explore healthcare delivery indicators at a population level, including medical costs, quality of care and treatment outcomes. Ultimately, the STOP HIV/AIDS cohort seeks to critically evaluate the cascade of care and the expansion of HAART provision in BC, under the umbrella of treatment as prevention.
The HOMER study shares de-identified data with other HIV cohorts on both national and international scales to answer complex HIV-specific research questions requiring large data sets, or data from multiple geographical locations. Of note, a subset of data from the HOMER cohort has been merged with seven other cohort databases from across Canada to form the Canadian Observational Cohort (CANOC), facilitating collaborative pan-provincial research into HIV therapeutics and population and public health.56 Current international collaborations include the ART-CC57 and the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD).58
What are the main strengths and weaknesses of the HOMER cohort?
The HOMER cohort provides a unique population-level dataset to explore contemporary research questions relating to HIV/AIDS treatment. This dataset dates back to the inception of HAART, thereby providing an invaluable resource for investigating the changing prognoses and treatment outcomes of HIV-positive individuals over time in BC. As HAART is provided free of charge in BC, an economically diverse population is represented, and factors relating to financial barriers to treatment do not compromise the dataset by limiting access to HAART in certain populations. Similarly, as all participants are antiretroviral naïve, previous ART use does not confound the data.
Additional strengths include the complete data capture of deaths through linkage with vital statistics data, and the ability to monitor individuals who discontinue treatment through laboratory tests and physician reporting, resulting in a comprehensive dataset with minimal LTFU. Furthermore, we are systematically able to measure ART adherence through centralized pharmacy refill data, a feature that is unique to this cohort compared with most other HIV observational studies.
The HOMER cohort captures a large proportion of HIV-positive individuals receiving HAART in BC, as all adults accessing HAART through the universally accessible publicly funded DTP are included in the BC-CfE Registry. Although the majority of HAART-treated BC residents receive treatment through the DTP, a small proportion receive HAART via other programmes, such as through participation in clinical trials, the Federal First Nations health programme or private prescriptions. Therefore, this cohort is not entirely representative of the BC population accessing HAART.
Another potential weakness of the HOMER cohort is the incomplete capture of data indicating the mode of HIV acquisition for many cohort participants. Additionally, routine data collection relies on feedback from physicians for some variables (reporting of opportunistic infections, hepatitis C status and IDU status), therefore is often incomplete.
Where can I find out more?
The HOMER team welcomes input from external investigators regarding research proposals or opportunities for collaborations to pursue joint or comparative analyses. For further information please contact the principal investigator, Dr Robert Hogg (robert_hogg@sfu.ca).
Funding
The DTP receives funding from the provincial government of British Columbia (PharmaCare). R.S.H. has held grant funding in the past 5 years from the National Institutes of Health (NIH), CIHR, Health Canada, Merck and SSHRC. J.M. is supported by the British Columbia Ministry of Health and through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse (NIDA), at the US National Institutes of Health (NIH). J.M. has also received financial support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institutes of Health Research Office of AIDS Research, National Institute of Allergy & Infectious Diseases, the United States President’s Emergency Plan for AIDS Relief (PEPfAR), UNICEF, the University of British Columbia, Simon Fraser University, Providence Health Care and Vancouver Coastal Health Authority. J.M. has received grants from Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Acknowledgements
We thank the participants in the BC HIV/AIDS Drug Treatment Program; the physicians, nurses, social workers and volunteers who support them; and James Nakagawa and Cameron Collins for their research and technical assistance. We also thank Art Poon for his contribution to the Future Research Directions section of this manuscript. R.S.H., A.C., H.S., S.P. and B.Y. contributed to the conception and design of the study. Z.C. and B.Y. performed all statistical analyses. S.P., H.S. and A.C. drafted the manuscript. R.S.H. advised on all aspects of the study. All authors revised the manuscript critically and approved the final version submitted for publication.
Conflict of interest: None declared.
References
- 1.British Columbia Centre for Excellence in HIV/AIDS. About us. http://cfenet.ubc.ca/about-us (14 February 2014, date last accessed). [Google Scholar]
- 2.British Columbia Centre for Excellence in HIV/AIDS. Drug Treatment Program. http://cfenet.ubc.ca/drug-treatment-program (8 January 2014, date last accessed). [Google Scholar]
- 3.British Columbia Centre for Excellence in HIV/AIDS. Therapeutic Guidelines. http://www.cfenet.ubc.ca/therapeutic-guidelines (8 January 2014, date last accessed). [Google Scholar]
- 4.Montaner J, Guillemi S, Harris M. Therapeutic Guidelines. Antiretroviral (ARV) Treatment of Adult HIV Infection . British Columbia Centre for Excellence in HIV/AIDS; 2013. http://www.cfenet.ubc.ca/sites/default/files/uploads/Therapeutic%20Guidelines%202013-Feb-final.pdf (8 January 2014, date last accessed). [Google Scholar]
- 5.British Columbia Centre for Excellence in HIV/AIDS. Therapeutic Guidelines Committee. http://cfenet.ubc.ca/therapeutic-guidelines/committee (8 January 2014, date last accessed). [Google Scholar]
- 6.British Columbia Centre for Excellence in HIV/AIDS. Treatment as Prevention. http://cfenet.ubc.ca/tasp (8 January 2014, date last accessed). [Google Scholar]
- 7.Thompson M, Adberg J, Hoy J, et al. Antiretroviral treatment of adult HIV Infection 2012 Recommendations of the International Antiviral Society–USA Panel. JAMA 2012;308:387–402. [DOI] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2012. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf (8 January 2014, date last accessed). [Google Scholar]
- 9.British Columbia Centre for Excellence in HIV/AIDS. Patient Information Sheet for Drug Treatment Program Participants. http://cfenet.ubc.ca/sites/default/files/uploads/Patient%20Information%20Sheet_0.pdf (8 January 2014, date last accessed). [Google Scholar]
- 10.British Columbia Centre for Excellence in HIV/AIDS. Primary Care Guidelines for the Management of HIV/AIDS in British Columbia . 2011. http://cfenet.ubc.ca/sites/default/files/uploads/HIV_PRIMARY_CARE_GUIDELINES_2011.pdf (8 January 2014, date last accessed). [Google Scholar]
- 11.Schneider E, Whitmore S, Glynn M, Dominguez K, Mitsch A, McKenna M. Revised Surveillance Case Definitions for HIV Infection Among Adults, Adolescents, and Children Aged <18 Months and for HIV Infection and AIDS Among Children Aged 18 Months to <13 Years, United States, 2008. US Centre for Disease Control; http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a1.htm?s_cid=rr5710a1_epdf (8 January 2014, date last accessed). [PubMed] [Google Scholar]
- 12.British Columbia Health Authorities. British Columbia Ministry of Health . 2011. www.health.gov.bc.ca/socsec/ (8 January 2014, date last accessed). [Google Scholar]
- 13.May M, Hogg R, Justice A, et al. Heterogeneity in outcomes of treated HIV-positive patients in Europe and North America: relation with patient and cohort characteristics. Int J Epidemiol 2012;41:1807–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.British Columbia Centre for Excellence in HIV/AIDS . BC-CfE in HIV/AIDS @ bccfe. https://twitter.com/bccfe (8 January 2014, date last accessed). [Google Scholar]
- 15.British Columbia Centre for Excellence in HIV/AIDS. HOMER . http://cfenet.ubc.ca/research/homer (8 January 2014, date last accessed). [Google Scholar]
- 16.Hogg R, Yip B, Kully C, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ 1999;160:659–65. [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg R, Rhone S, Yip B, et al. Antiviral effect of double and triple drug combinations amongst HIV-infected adults: lessons from the implementation of viral load-driven antiretroviral therapy . AIDS 1998;12:279–84. [DOI] [PubMed] [Google Scholar]
- 18.Hogg R, Chan K, Cescon A, et al. Considerable gaps in life expectancy among HIV-positive individuals initiating HAART in British Columbia, Canada. Poster Exhibition. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, 30 June–3 July 2013, Kuala Lumpur, Malaysia. http://pag.ias2013.org/Abstracts.aspx?AID=1152 (14 February 2014, date last accessed). [Google Scholar]
- 19.Lima V, Hogg R, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS 2007;21:685–92. [DOI] [PubMed] [Google Scholar]
- 20.Nosyk B, Min J, Lima VD, Yip B, Hogg RS, Montaner JS. HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011 . J Aquir Immune Defic Syndr 2013;63:653–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood E, Yip B, Hogg R, et al. Full suppression of viral load is needed to achieve an optimal CD4 cell count response among patients on triple drug antiretroviral therapy. AIDS 2000;14:1955–60. [DOI] [PubMed] [Google Scholar]
- 22.Wood E, Hogg R, Yip B, et al. Higher baseline levels of plasma immunodeficiency virus type 1 RNA are associated with increased mortality after initiation of triple-drug antiretroviral therapy. J Infect Dis 2003;188:1421–25. [DOI] [PubMed] [Google Scholar]
- 23.Hogg R, Yip B, Chan K, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy . JAMA 2001;286:2568–77. [DOI] [PubMed] [Google Scholar]
- 24.Moore D, Hogg R, Chan K, Tyndall M, Yip B, Montaner J. Disease progression in patients with virological suppression in response to HAART is associated with the degree of immunological response. AIDS 2006;20:371–77. [DOI] [PubMed] [Google Scholar]
- 25.Lima V, Le A, Nosyk B, et al. Development and validation of a composite programmatic assessment tool for HIV therapy. PLoS One 2012;7:e47859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima V, Hogg R, Montaner J. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA Guidelines in British Columbia Canada. PLoS One 2010;6:e10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima V, Johnston K, Hogg R, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic . J Aquir Immune Defic Syndr 2008;198:59–67. [DOI] [PubMed] [Google Scholar]
- 28.Montaner J. Treatment as prevention – a double hat-trick. Lancet 2011;378:208–09 [DOI] [PubMed] [Google Scholar]
- 29.Montaner J, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 2006;368:531–36 [DOI] [PubMed] [Google Scholar]
- 30.Montaner J, Lima V, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study . Lancet 2010;376:532–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granich R, Crowley S, Vitoria M, et al. Highly active antiretroviral treatment as prevention of HIV transmission: Review of scientific evidence and update. Curr Opin HIV AIDS 2010;5:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Programmatic Update. Antiretroviral Treatment as Prevention (TASP) of HIV and TB. 2012. http://whqlibdoc.who.int/hq/2012/WHO_HIV_2012.12_eng.pdf (8 January 2014, date last accessed). [Google Scholar]
- 33.Wood E, Montaner J, et al. Socioeconomic status, access to triple therapy and survival from HIV-disease since 1996 . AIDS 2002;16:2065–72. [DOI] [PubMed] [Google Scholar]
- 34.Wood E, Montaner J, Tyndall M, Schechter M, O’Shaughnessy M, Hogg R. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. J Infect Dis 2003;188:1164–70. [DOI] [PubMed] [Google Scholar]
- 35.Joy R, Druyts E, Brandson E, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. J Infect Dis 2008;47:500–05 [DOI] [PubMed] [Google Scholar]
- 36.Palepu A, Tyndall M, Yip B, O’Shaughnessy M, Hogg R, Montaner J. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. J Aquir Immune Defic Syndr 2003;32:522–26. [DOI] [PubMed] [Google Scholar]
- 37.Wood E, Montaner J, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users . CMAJ 2003;169:656–61. [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connell J, Braitstein P, Hogg R, et al. Age, adherence and injection drug use predict virological suppression among men and women enrolled in a population-based antiretroviral drug treatment programme. Antivir Ther 2003;8:569–76. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd-Smith E, Brodkin E, Wood E, et al. Impact of HAART and injection drug use on life expectancy of two HIV-positive cohorts in British Columbia. AIDS 2006;20:445–50. [DOI] [PubMed] [Google Scholar]
- 40.Lima V, Nosyk B, Wood E, Kozai T, Chan K, Montaner JS. Assessing the effectiveness of antiretroviral regimens in cohort studies involving HIV-positive injection drug users. AIDS 2012;26:1491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lima V, Bangsberg D, Harrigan P, et al. Risk of viral failure declines with duration of suppression on HAART, irrespective of adherence level. J Aquir Immune Defic Syndr 2010;55:460–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood E, Hogg R, Lima V, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA 2008;300:550–54. [DOI] [PubMed] [Google Scholar]
- 43.Harrigan R, Hogg R, Dong W, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naïve cohort initiating triple antiretroviral therapy. J Infect Dis 2005;191:339–47. [DOI] [PubMed] [Google Scholar]
- 44.Tam L, Chui C, Brumme C, et al. The relationship between resistance and adherence in drug-naïve individuals initiating HAART is specific to individual drug classes. J Aquir Immune Defic Syndr 2008;49:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lima V, Gill V, Yip B, Hogg B, Montaner J, Harrigan R. Increased resilience to the development of drug resistance with modern boosted protease inhibitor-based highly active antiretroviral therapy. J Infect Dis 2008;198:51–58. [DOI] [PubMed] [Google Scholar]
- 46.Gill V, Lima V, Zhang W, et al. Improved virological outcomes in British Columbia concomitant with decreasing incidence of HIV type 1 drug resistance detection . Clin Infect Di. 2010;50:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cescon A, Kanters S, Brumme CJ, et al. Trends in plasma HIV-RNA suppression and antiretroviral resistance in British Columbia, 1997–2010. J Aquir Immune Defic Syndr 2014;65:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lima V, Harrigan PR, Senecal M, et al. Epidemiology of antiretroviral multiclass resistance. Am J Epidemiol 2010;172:460–68. [DOI] [PubMed] [Google Scholar]
- 49.Genome Canada. Viral and Human Genetic Predictors of Response to HIV Therapies.2012http://www.genomecanada.ca/medias/pdf/en/Harrigan.pdf (8 January 2014, date last accessed). [Google Scholar]
- 50.Poon A, Swenson L, Bunnik E, et al. Reconstructing the dynamics of HIV evolution within hosts from serial deep sequence data. PLoS Comput Biol 2012;8:e1002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncan K, Salters K, Forrest J, et al. Cohort Profile: Longitudinal investigations into supportive and ancillary health services. Int J Epidemiol 2013;42:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Brien N, Palmer A, Zhang W, et al. Social-structural factors associated with supportive service use among a cohort of HIV-positive individuals on antiretroviral therapy. AIDS Care 2013;25:937–47, [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Salters K, Zhang W, et al. Women’s health care utilization among harder-to-reach HIV-infected women ever on antiretroviral therapy in British Columbia. AIDS Res Treat 2012;2012:560361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.British Columbia Centre for Excellence in HIV/AIDS. The Engage Study. http://cfenet.ubc.ca/research/engage-study (8 January 20134, date last accessed). [Google Scholar]
- 55.STOP HIV/AIDS. Seek and Treat for Optimal Prevention for HIV/AIDS (http://stophivaids.ca/ (8 January 20134, date last accessed). [Google Scholar]
- 56.Palmer A, Klein M, Raboud J, et al. Cohort profile: The Canadian Observational Cohort collaboration . Int J Epidemiol 2011;40:25–32. [DOI] [PubMed] [Google Scholar]
- 57.May M, Ingle S, Costagliola D, et al. Cohort profile: Antiretroviral Therapy Cohort Collaboration (ART-CC). Int J Epidemiol 2013;57:1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gange S, Kitahata M, Saag M, et al. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol 2007;36:294. [DOI] [PMC free article] [PubMed] [Google Scholar]