Using connectivity analyses based on functional MRI, Michely et al. investigate dopaminergic modulation of neural network dynamics involved in motor control in Parkinson’s disease. The findings provide insights into the pathophysiology underlying bradykinesia and deficits in executive function, and help to explain why dopaminergic treatments have a greater effect on the former.
Keywords: effective connectivity, dopaminergic medication, bradykinesia, higher motor control, premotor loops
Abstract
Although characteristic motor symptoms of Parkinson’s disease such as bradykinesia typically improve under dopaminergic medication, deficits in higher motor control are less responsive. We here investigated the dopaminergic modulation of network dynamics underlying basic motor performance, i.e. finger tapping, and higher motor control, i.e. internally and externally cued movement preparation and selection. Twelve patients, assessed ON and OFF medication, and 12 age-matched healthy subjects underwent functional magnetic resonance imaging. Dynamic causal modelling was used to assess effective connectivity in a motor network comprising cortical and subcortical regions. In particular, we investigated whether impairments in basic and higher motor control, and the effects induced by dopaminergic treatment are due to connectivity changes in (i) the mesial premotor loop comprising the supplementary motor area; (ii) the lateral premotor loop comprising lateral premotor cortex; and (iii) cortico-subcortical interactions. At the behavioural level, we observed a marked slowing of movement preparation and selection when patients were internally as opposed to externally cued. Preserved performance during external cueing was associated with enhanced connectivity between prefrontal cortex and lateral premotor cortex OFF medication, compatible with a context-dependent compensatory role of the lateral premotor loop in the hypodopaminergic state. Dopaminergic medication significantly improved finger tapping speed in patients, which correlated with a drug-induced coupling increase of prefrontal cortex with the supplementary motor area, i.e. the mesial premotor loop. In addition, only in the finger tapping condition, patients ON medication showed enhanced excitatory influences exerted by cortical premotor regions and the thalamus upon the putamen. In conclusion, the amelioration of bradykinesia by dopaminergic medication seems to be driven by enhanced connectivity within the mesial premotor loop and cortico-striatal interactions. In contrast, medication did not improve internal motor control deficits concurrent to missing effects at the connectivity level. This differential effect of dopaminergic medication on the network dynamics underlying motor control provides new insights into the clinical finding that in Parkinson’s disease dopaminergic drugs especially impact on bradykinesia but less on executive functions.
Introduction
Patients suffering from Parkinson’s disease often present with the cardinal motor signs of bradykinesia, rigidity, tremor, and postural instability (Jankovic, 2008). Even at early stages of the disease, patients may additionally suffer from cognitive decline, especially affecting executive functions (Elgh et al., 2009; Godefroy et al., 2010). Such executive deficits comprise impaired response preparation, selection and inhibition (Siegert et al., 2002; Gauggel et al., 2004; Werheid et al., 2007; Obeso et al., 2011). Moreover, patients often show specific impairments when relying on internal control processes as opposed to preserved performance when being externally cued (Brown and Marsden, 1988; Georgiou et al., 1994; Siegert et al., 2002; Michely et al., 2012). Although dopaminergic treatment reliably ameliorates the classic motor symptoms, an effective therapy of dysexecutive symptoms to date remains elusive (Kehagia et al., 2010; Fasano et al., 2012; Narayanan et al., 2013). Previous studies investigating the effect of dopaminergic medication on executive functions in Parkinson’s disease revealed inconsistent effects. Depending on the respective task, both improvement and worsening of executive functions have been reported (for reviews see Cools, 2006; Dirnberger and Jahanshahi, 2013). Thus, dopamine seems to differentially impact upon motor control loops in the parkinsonian brain (Redgrave et al., 2010; Jenkinson and Brown, 2011).
Neuroimaging has contributed significantly to our understanding of the pathophysiology underlying Parkinson’s disease symptoms, especially with respect to motor manifestations of the disease (Rowe and Siebner, 2012). Studies consistently revealed hypoactivation/connectivity of mesial premotor regions such as the supplementary motor area (SMA) (Haslinger et al., 2001; Buhmann et al., 2003; Rowe et al., 2010; Wu et al., 2010; Esposito et al., 2013) and hyperactivation/connectivity of lateral premotor regions such as the lateral premotor cortex (PMC) (Samuel et al., 1997; Sabatini et al., 2000; Haslinger et al., 2001; Rowe et al., 2010; Wu et al., 2010). Consequently, this shift from mesial to lateral premotor loops has been suggested to constitute a core pathological or compensatory mechanism related to the impairment of internally generated as compared to externally cued movements in patients (Ceballos-Baumann, 2003; Grafton, 2004; Rowe et al., 2010). Another finding consistently reported is disturbed effective connectivity between prefrontal cortex, premotor areas, and putamen (Rowe et al., 2002, 2010; Wu et al., 2010, 2011). Despite these studies, our insights into the differential impairment of motor control mechanisms and the underlying network pathologies remain incomplete. Furthermore, whether the observed differential effects of dopaminergic drugs on basic motor function and higher motor control result from differential modulation of the different premotor loops and/or interactions with subcortical areas remains unclear (Mattay et al., 2002; Rowe et al., 2008; Jahanshahi et al., 2010).
We have previously shown that deficits in internal motor control affecting response preparation and selection respond less well to dopaminergic medication than bradykinesia (Michely et al., 2012). Based on these findings, we designed a functional MRI motor task that enabled us to contrast basic motor function (finger tapping) with higher motor control (internal response preparation and selection) (Michely et al., 2012; Hoffstaedter et al., 2013). The aim of the present study was to identify altered network interactions in patients suffering from Parkinson’s disease underlying impaired basic motor function and higher motor control by analysing effective connectivity with Dynamic Causal Modelling (DCM; Friston et al., 2003). In particular, we intended to investigate whether (i) disease-associated impairment of internal action control is due to hypoconnectivity of the mesial premotor loop and preserved externally cued performance results from compensatory hyperconnectivity of the lateral premotor loop. Moreover, we hypothesized that (ii) dopaminergic medication reinstates or strengthens connectivity of the mesial premotor loop. Finally, we were interested in testing whether (iii) differential effects of dopamine on basic motor symptoms (bradykinesia) and deficits of higher motor control are associated with a medication-induced shift from the lateral to the mesial premotor loop and/or additional effects on network dynamics.
Materials and methods
Subjects
Twelve male right-handed patients diagnosed with idiopathic Parkinson’s disease according to the British Parkinson’s Disease Society Brain Bank criteria (Hughes et al., 1992) were recruited from the Cologne University Hospital (mean age 64.5 years). Bradykinesia was the predominant symptom in the patient group, no patient was classified as tremor-dominant. All patients were tested twice, on two separate days: once with their regular dopaminergic medication (‘ON’, mean levodopa equivalent daily dose 566.3 mg; Tomlinson et al., 2010) and once after at least a 12-h overnight withdrawal from medication (‘OFF’). Longer withdrawal periods (up to 36 h) were used for long-acting dopamine agonists. The sequence of ON and OFF sessions was pseudorandomized and counterbalanced across patients. A comprehensive description of the patients’ demographic data including detailed dopaminergic medication is provided in Table 1. Twelve right-handed healthy male subjects with no history of neurological or psychiatric disease served as age-matched reference for both behavioural and neuroimaging data (mean age 62.1 years, P = 0.398). We refer to this group as ‘healthy controls’. According to the Edinburgh handedness inventory (Oldfield, 1971), all participants were strongly right-handed, with no significant difference between groups (P = 0.590). The study was approved by the local ethics committee, and carried out in accordance with the Declaration of Helsinki. All subjects gave informed written consent before entering the study.
Table 1.
Demographic and clinical parameters of patients with Parkinson’s disease
| Patient | Age | DD | H + Y | UPDRS IIION | UPDRS IIIOFF | PANDA | BDI-II | Medication | LEDD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | 4 | 2 | 17 | 21 | 30 | 6 | Pra, Ras, Ama | 715 |
| 2 | 73 | 4 | 2 | 6 | 8 | 28 | 8 | LD, Pra | 782 |
| 3 | 65 | 1 | 2 | 4 | 5 | 30 | 1 | Pra, Ras | 152 |
| 4 | 66 | 6 | 2 | 21 | 34 | 29 | 8 | LD, Ent, Rot, Ama | 1092 |
| 5 | 72 | 5 | 2.5 | 21 | 29 | 25 | 15 | LD, Pra, Ama | 610 |
| 6 | 62 | 5 | 2 | 6 | 12 | 29 | 8 | Rop, Ras | 340 |
| 7 | 72 | 4 | 2 | 8 | 11 | 26 | 4 | Pra, Ras | 310 |
| 8 | 70 | 10 | 3 | 14 | 24 | 30 | 18 | Pra, Ras | 310 |
| 9 | 53 | 9 | 2.5 | 8 | 34 | 24 | 14 | LD, Pra, Ama | 1312 |
| 10 | 50 | 7 | 2 | 27 | 31 | 26 | 10 | Rot, Pra, Ras | 212 |
| 11 | 66 | 2 | 3 | 20 | 28 | 21 | 1 | LD, Ras, Ama | 700 |
| 12 | 66 | 9 | 2.5 | 19 | 31 | 21 | 2 | Pra, Sel | 260 |
For all patients age, disease duration (DD; in years), Hoehn and Yahr stages in the OFF state (H + Y), scores of UPDRS III under dopaminergic medication (UPDRS IIION), UPDRS after medication withdrawal (UPDRS IIIOFF), PANDA, Beck’s Depression Inventory II (BDI-II), medication (Ama = Amantadine; Ent = Entacapone; LD = Levodopa; Pra = Pramipexole; Pir = Piribedil; Ras = Rasagiline; RoP = Ropinirole; Rot = Rotigotine; Sel = Selegiline); and levodopa equivalent daily dose (LEDD; in mg) at the time of examination are listed.
Clinical scores
All subjects underwent (i) Parkinson Neuropsychometric Dementia Assessment (PANDA) (Kalbe et al., 2008); and (ii) Beck’s Depression Inventory II (Beck et al., 1996). The PANDA is an assessment of cognitive features typically affected in patients with Parkinson’s disease, i.e. executive functions, working memory, attention, and visuospatial functions. (Subtle) cognitive impairment is defined for scores < 18 points (Kalbe et al., 2008). There was no significant difference in PANDA scores between groups (Patients: mean 26.6; Controls: mean 25.6, P = 0.630). In contrast, Beck’s Depression Inventory II scores were significantly higher in patients (Patients: mean 8.0; Controls: mean 2.6, P = 0.008). Importantly, some of the items that are part of the Beck’s Depression Inventory II assessment may reflect symptoms of the disease rather than symptoms of depression (Marsh et al., 2006). Therefore, when excluding items that are related to Parkinson’s disease symptomatology and treatment side effects (i.e. lack of energy, agitation, sleep changes, irritability appetite changes, concentration problems, and fatigue), no significant difference between patients and controls was observed (Patients: mean 2.8; Controls: mean 1.2, P = 0.242).
At each session, both motor function and disease severity of patients were rated using the Unified Parkinson’s Disease Rating Scale motor examination (UPDRS, part III; Fahn and Elton, 1987) and the Hoehn and Yahr (1967) rating scale. Subtests were videotaped and subsequently rated by a Movement Disorder Society certified rater (M.T.B.) blinded for state of medication (ON, OFF). Dopaminergic medication induced a significant improvement of the UPDRS motor score in patients (OFF: mean 21.4; ON mean 14.3, P = 0.002), indicating that patients showed a strong response to their regular medication.
Functional MRI paradigm
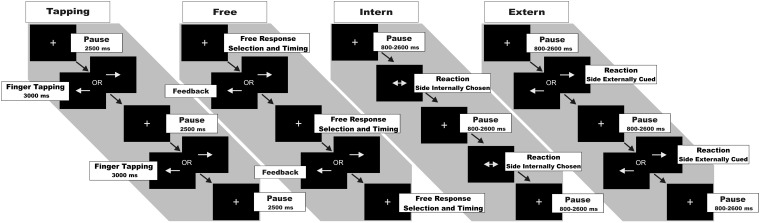
We used a computerized motor task to probe basic motor functions as well as executive functions engaged in movement preparation and selection (Fig. 1). In all conditions, subjects responded via button presses on a MRI compatible response device using the right or left index finger. Visual stimuli were generated using the ‘Presentation’ software package (Version 10.3, Neurobehavioral Systems Inc.). The task comprised four conditions [slightly modified version of the task used in Michely et al. (2012)]. Each condition was presented in blocks of 20 s duration separated by resting baselines of 16 s during which subjects watched a blank screen. Each block was introduced by a one-word instruction presented for 2.5 s, informing the subject about which of the four conditions followed next.
Condition ‘Tapping’: Finger tapping at maximum speed. In the Tapping condition, subjects were asked to perform vertical tapping movements at maximum speed using the right or left index finger. A white arrow presented in the centre of a black screen pointed to the left or right and thereby indicated which finger to use. This cue was presented throughout the entire tapping period. As in earlier studies, we used short finger tapping periods of 3 s followed by a 2.5-s break instead of continuous tapping throughout the entire 20 s block to prevent fatigue (Wang et al., 2011). In each block, four tapping periods had to be performed with fingers balanced, i.e. two right, two left.
Condition ‘Free’: Self-timed movement selection. In the Free condition, subjects were instructed to press either the left or right button at any self-chosen time. Hence, subjects were free in terms of both movement lateralization and timing. Every response was followed by an immediate visual feedback consisting of an arrow pointing to the side of the button-press (duration: 400 ms). By providing a feedback arrow we kept the conditions more comparable in terms of visual input and display delays. Moreover, during feedback, no further response was allowed to prevent repetitive finger tapping. As subjects were not allowed to press any button while the feedback arrow was presented, response times in the ‘Free’ condition reflect the interval between the end of the presentation of the feedback arrow and the next self-initiated button press. Subjects were instructed to roughly balance between left and right button presses, and to avoid extensive periods of rest between button presses.
Condition ‘Intern’: Reaction to a non-informative cue. Subjects were asked to respond to a double-headed arrow, i.e. non-informative cue (displayed for 400 ms) with a button press of either their left or right index finger. As subjects were prompted to press the right or left button as fast as possible, they were restricted with regard to the time point of movement execution, but free in terms of movement lateralization. Twelve to 14 stimuli were presented per block with varying stimulus onset asynchrony (ranging from 800 to 2600 ms), thereby minimizing anticipation of the cue. As in the Free condition, subjects were instructed to roughly balance between left- and right-sided responses.
Condition ‘Extern’: Reaction to an informative cue. In the Extern condition, subjects were instructed to respond as fast as possible to a single-headed arrow (displayed for 400 ms), pointing either to the left or right side. Hence, movements were purely reactive, and thus restricted with regard to both time point and movement lateralization. As in the Intern condition, 12–14 cues were presented per block.
Figure 1.
Functional MRI paradigm. Each block of trials started with the presentation of a fixation cross. Tapping condition: On appearance of a white arrow pointing to the left or right side, subjects were instructed to start tapping movements with maximum speed with the respective index finger as long as the respective arrow was shown (periods of 3 s), tapping periods were followed by a 2.5-s break again indicated by the fixation cross. In each block, four tapping periods were recorded. Free condition: On appearance of the fixation cross, subjects were instructed to press the left or right button with the respective index finger at any self-chosen time. Every response was followed by a visual feedback pointing to the side of the button-press. Thereafter, the fixation cross reappeared until the next response was given by the subject. Thus, subjects were free in terms of both movement lateralization and timing. Intern condition: Subjects were instructed to react as fast as possible and press the left or right button upon appearance of a double-headed arrow pointing to both sides. Hence, subjects were restricted to the time point of movement initiation but free in terms of movement lateralization. The fixation cross appeared for the time between stimuli. Extern condition: Subjects were instructed to react as fast as possible and press the left button upon appearance of an arrow pointing to the left or the right button upon appearance of an arrow pointing to the right. Thus, subjects were restricted to both time point and movement lateralization.
The Tapping condition tested for basic motor function (i.e. maximum tapping speed), whereas the other three conditions probed different aspects of higher motor control. In contrast to the Free condition where subjects were not reacting to any external cue, the Extern and Intern conditions constituted externally and internally triggered choice reaction time tasks (Jahanshahi et al., 1992). Before scanning, subjects were trained outside and inside the scanner to warrant stable task performance. A single functional MRI run lasted 21 min including eight repetitions of each condition. The four conditions were presented consecutively in blocks; however, within these blocks the order was pseudorandomized yet equal for all subjects to account for ordering effects and to maintain comparability.
Statistical analysis of behavioural data
In the reaction time conditions, i.e. Intern and Extern, we first eliminated outliers that were unlikely to represent physiologically interpretable reactions to the visual stimuli: reaction times >2000 ms and <150 ms were regarded as random or anticipatory responses (Hultsch et al., 2002). Furthermore, for each subject, all reaction times exceeding the individual mean reaction time by >3 standard deviations (SDs) were eliminated from further analysis.
Together, these steps removed 1.2% of the data in both the patients and the control group. We also excluded erroneous trials when subjects pressed more than one, or the wrong, or no button. There was no significant difference between groups and sessions regarding erroneous trials (all P > 0.1; controls: 6.4%, OFF: 5.9%, ON: 7.2%).
Statistical analyses of the behavioural data were conducted using SPSS (version 21, IBM). ANOVAs were used to test for main effects of the factors Group (levels: Patients, Controls) or Medication (levels: ON, OFF) and Condition, the interaction between the two factors when comparing Extern and Intern, respectively. Moreover, we assessed trial × trial reaction time effects regarding repetition (side of response identical to previous trial) or switching (side different from previous trial). We also tested whether subjects generated non-random response sequences in the two conditions involving self-chosen responses, i.e. Free and Intern, and how response patterns were related to disease and medication states (Supplementary material).
Functional MRI image acquisition and preprocessing
Functional magnetic resonance images were acquired using a Siemens Trio 3 T scanner (Siemens Medical Solutions) applying standard acquisition and preprocessing parameters (Supplementary material). We tested for group differences in head motion parameters from image realignment by comparing the framewise displacement and the root mean squared movement (Power et al., 2012; Satterthwaite et al., 2013). There was no difference between groups either in the framewise displacement [all P > 0.1: controls: 0.37 (SD: 0.13); OFF: 0.38 (SD: 0.13); ON: 0.34 (SD: 0.09)] or in the root mean squared movement [all P > 0.1: controls: 0.27 (SD: 0.09); OFF: 0.27 (SD: 0.09); ON: 0.24 (SD: 0.07)]. Hence, head movements did not account for differences in activity/connectivity.
Functional MRI statistical analysis
Statistical analysis was performed within the framework of the general linear model. The four experimental conditions and the instructions were separately modelled using boxcar stimulus functions convolved with a canonical haemodynamic response function. The time series in each voxel were high-pass filtered at 1/128 Hz. The six head motion parameters, as assessed by the realignment algorithm, were treated as covariates to remove movement-related variance from the image time series.
Simple main effects for each condition were computed by contrasting task activity (e.g. Tapping/Free/Intern/Extern) with the resting baselines for each subject. For group analyses, individual contrast images were entered into a second level model using a flexible factorial ANOVA to assess (i) disease-related (between-subject factor Group, levels controls/Parkinson’s disease OFF); and (ii) drug-induced (within-subject factor Medication, ON/OFF) effects on the factor Condition (i.e. Tapping/Free/Intern/Extern). Moreover, interactions between Group (or Medication) and Condition for the higher motor control conditions, i.e. Free/Intern, Free/Extern, Intern/Extern were computed.
Dynamic Causal Modelling
The focus of the present study was to investigate differences in effective connectivity between controls and patients, and the effect of dopaminergic medication on interregional interactions using DCM. In DCM, changes in neuronal states over time are modelled as in Equation 1.
| (1) |
where x is the state vector, A represents the endogenous (intrinsic) connectivity, B(j) represents the task-dependent modulations of the modelled region driven by the input function u, and C denotes the influence of direct inputs to the system. Because of the block design of the present study, the input function u covers not only the visual cues and motor responses but also the cognitive state induced by the instruction of a given condition. Endogenous connectivity (DCM-A matrix) is always present during the experiment and reflects the context-independent (i.e. constant) component of interregional coupling across the entire experimental setting. The context-dependent modulations are represented in the DCM-B matrix and reflect changes in interregional coupling evoked by a particular condition (Tapping/Free/Intern/Extern).
Regions of interest
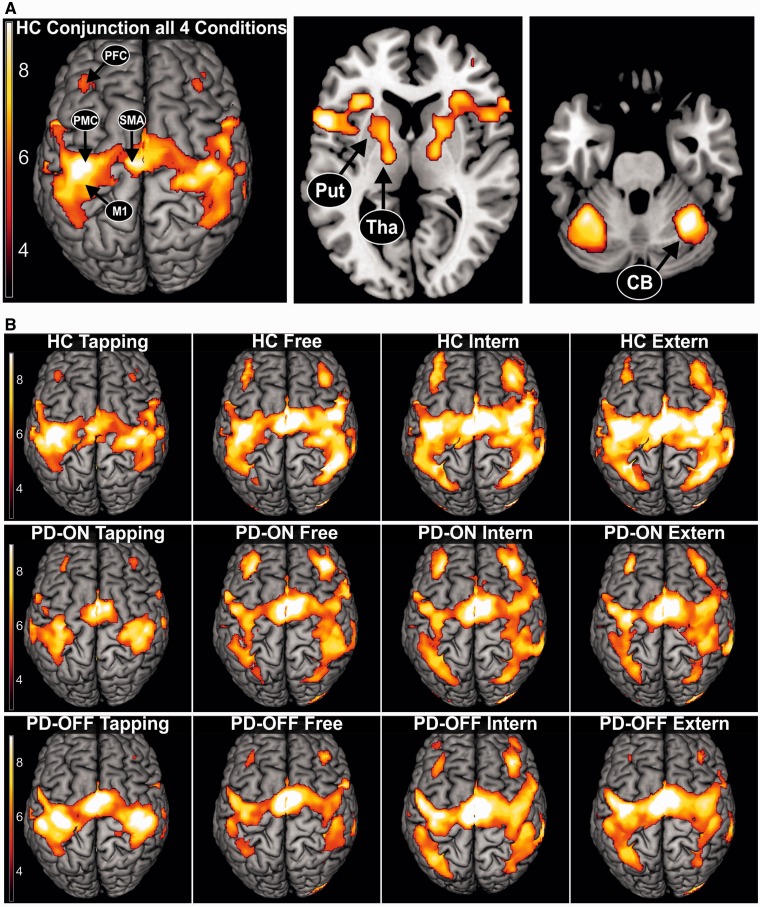
DCM as a hypothesis-driven approach relies on a priori neurobiological models comprising relevant regions and connections involved in a given task. In the current study, the selection of areas was driven by regions that have been consistently reported to show altered activity in Parkinson’s disease. Accordingly, at the cortical level, we included primary motor cortex (M1), PMC, SMA, and dorsolateral prefrontal cortex (PFC) (for reviews see Ceballos-Baumann, 2003; Grafton, 2004; Rowe and Siebner, 2012). At the subcortical level, we included putamen, thalamus and cerebellum (for reviews see Wu and Hallett, 2013; Herz et al., 2014a). All these regions constitute core areas of the motor system known to be involved in movement preparation and execution (Grefkes et al., 2008; Witt et al., 2008; Rowe et al., 2010). Figure 2 illustrates the neural network activated in each condition confirming the relevance of the regions of interest in all conditions. In DCM the number of areas that can be included in a model is limited for computational reasons (Stephan et al., 2010). Hence, we focused on the interregional coupling in the motor-dominant left hemisphere (and right cerebellum) in this group of right-handed subjects, as in earlier DCM studies in Parkinson’s disease (Rowe et al., 2010; Herz et al., 2014b). In addition, we computed the entire analysis also for the right hemisphere as an internal replication to test whether the effects found for the left hemisphere were also present in the right hemisphere.
Figure 2.
Blood oxygenation level-dependent activation pattern across all conditions. (A) Conjunction analysis of the neural network activated by all four conditions in healthy controls (HC), representing the extended cortical and subcortical motor network. Regions of interest used for DCM analysis are highlighted. P < 0.05, FWE-corrected. (B) Neural networks activated by the four different conditions (Tapping, Free, Intern, Extern). Healthy controls (HC), patients with Parkinson’s disease OFF (PD-OFF) and ON medication (PD-ON). P < 0.05, FWE corrected.
As DCMs are fitted to subject-specific blood oxygenation level-dependent time series, we extracted the time series from 4-mm radius spheres centred on seven regions of interest at subject-specific individual activation maxima based on the first-level (i.e. single subject) analyses. All regions of interest were defined by functional and anatomical criteria based on the individual activation maps superimposed on the corresponding structural T1-volume. The anatomical landmarks used for region identification are described in the Supplementary material.
The group maximum MNI coordinate was set as origin to search for the closest local maximum in the individual SPMs. As the location of maximum activation may also vary between sessions (i.e. ON/OFF), a dispersion of coordinates up to 4 mm, equivalent to the radius of the extracted sphere, was allowed between sessions to ensure within-subject consistency of anatomical areas in patients. The coordinates of all regions of interest for all groups are provided in the Supplementary material.
Connectivity models
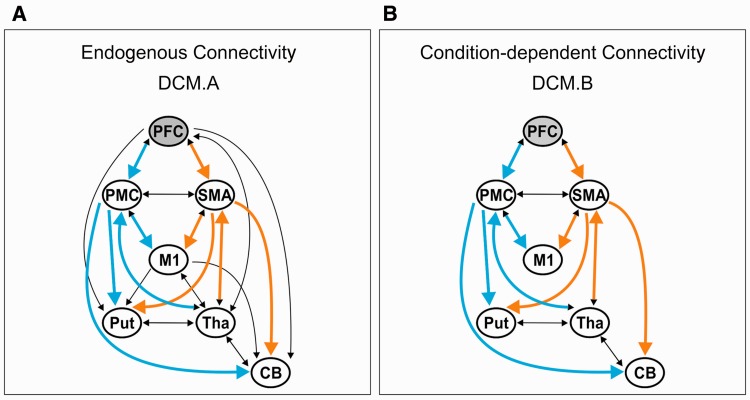
We constructed the endogenous structure of the network based on evidence obtained from invasive connectivity studies in non-human primates with respect to both cortical connectivity and subcortical loops. The condition-specific modulation of endogenous connectivity does not necessarily affect all possible connections (Friston et al., 2003; Stephan et al., 2010). Therefore, we constructed 40 neurobiologically plausible alternative models on interregional coupling (Supplementary material). These models differed in the directionality of connections (e.g. uni- and bidirectional) and cortico-subcortical connectivity between PFC, M1 and subcortical regions (Models 1–8). The same models were computed for the right hemisphere using equivalent regions.
As outlined in the introduction, we were specifically interested in changes of the mesial and lateral premotor loops. Thus, we systematically modulated connectivity of the mesial and lateral loops with respect to cortical (Models 9–24) and subcortical regions (Models 25–40). Following earlier DCM studies in Parkinson’s disease (Rowe et al., 2010; Herz et al., 2014b), we assumed that activity across conditions was driven by the PFC given the role of this area in executive control over motor output and movement preparation (Nishitani and Hari, 2000; Cieslik et al., 2013), which was necessary in all four conditions to correctly perform the requested tasks. We used Bayesian model selection to identify the model yielding the highest evidence given the data using a random effects approach (Penny et al., 2004). As we were interested in group differences, i.e. disease-related and drug-induced changes in coupling strength, we identified the most likely model for each group comparison, i.e. controls/OFF, controls/ON, and OFF/ON. To investigate the model fit of the ‘winning’ model, we computed the total mean variance explained by this model using the spm_dcm_fmri_check.m script (Friston, 2012).
Statistical analysis of connectivity data
Our hypotheses predicted specific connectivity alterations of the SMA (representing the mesial loop) and PMC (representing the lateral loop). Therefore, in our models, both areas were interconnected with the same regions at the cortical (PFC, M1) as well as at the subcortical level (putamen, cerebellum, thalamus). Hence, both loops consisted of five nodes with equivalent connections: PFC-SMA/PMC; SMA/PMC-M1; SMA/PMC-putamen; SMA/PMC-cerebellum; thalamus-SMA/PMC (Fig. 3). ANOVAs were used to test for differences between groups in the respective loops or connections, followed by post hoc t-tests in case of significant group or interaction effects. Introducing the specific factor ‘Loop’ enabled us not only to investigate differential shifts in patients versus controls or patients ON and OFF medication concerning the different premotor (SMA, PMC) loops, but also to disentangle specific connections that were driving the putative differences between these loops. For comparisons between groups (between-subject factor Group, levels controls/Parkinson’s disease-OFF), corresponding coupling parameters (within-subject factor Connection) of the mesial and lateral premotor loops (within-subject factor Loop) were entered into a mixed-design ANOVA. This procedure was conducted separately for (i) endogenous connectivity (DCM-A), and condition-specific connectivity (DCM-B) of (ii) the basic motor condition, i.e. Tapping; and (iii) the three higher motor control conditions by introducing the additional within-subject factor Condition (levels Free/Intern/Extern). For comparing treatment effects in patients, the factor Group was replaced by the within-subject factor Medication (levels ON/OFF). Follow-up comparisons on the connection level for significant Group/Medication by connection interactions were Bonferroni-corrected for the respective connections tested. A threshold of P < 0.05 was considered to be significant (adjusted alpha level = 0.005). In addition, to detect dopaminergic effects that were not restricted to our hypothesis regarding a shift between the premotor loops, we also computed t-tests comparing patients ON medication versus OFF medication (paired t-tests) and versus controls (independent t-tests) across all connections, separately for all four tasks. For this more explorative analysis, data were corrected for multiple testing using the false discovery rate (FDR) approach, which is less strict than the Bonferroni approach (Benjamini and Hochberg, 1995).
Figure 3.
DCM connectivity models highlighting mesial and lateral premotor loops. (A) Endogenous connectivity (DCM.A) matrix based on structural connectivity data derived from invasive studies in non-human primates (for anatomical references see Supplementary material). (B) Condition-dependent connectivity (DCM.B) matrix of the winning Model 4 as revealed to be the most likely model across all groups by the random-effects Bayesian model selection procedure. PFC in grey indicates the region receiving the input driving network activity. Orange arrows indicate connections of the SMA, constituting the mesial premotor loop. Blue arrows indicate equivalent connections of the PMC, constituting the lateral premotor loop. CB = cerebellum; Put = putamen; Tha = thalamus.
Results
Behavioural data
Basic motor function
Maximum finger tapping speed in the Tapping condition was significantly higher in controls than patients [F(1,22) = 9.713, P = 0.005]. Furthermore, dopaminergic medication induced a significant improvement in finger tapping speed in patients ON medication compared to OFF [F(1,11) = 6.671, P = 0.025] (Table 2). Hence, medication induced a strong effect on basic motor performance.
Table 2.
Behavioural group results obtained from the functional MRI paradigm
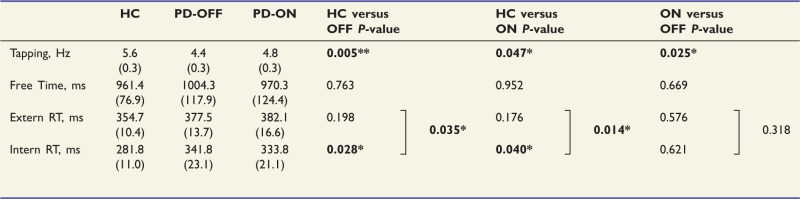 |
Healthy controls (HC), patients with Parkinson’s disease OFF (PD-OFF) and ON medication (PD-ON); Square brackets denote Group/Medication × Condition interactions relating to externally cued and internally selected reaction times. Tapping = maximum finger tapping speed; Time = intervals between button presses in the Free condition; RT = reaction time; SEM in parentheses; *P < 0.05.
Higher motor control
There was no significant difference between patients and controls in the Free condition, i.e. the two groups showed a comparable timing with regards to initiating a button press [F(1,22) = 0.093, P = 0.763]. However, when comparing internally and externally cued reactions, we found a significant main effect of Condition (Extern, Intern) [F(1,22) = 43.159, P < 0.001]. In addition, there was a significant interaction between the factors Group and Condition [F(1,22) = 5.034, P < 0.05]. Post hoc t-tests revealed that there was no difference between groups in the Extern condition. In contrast, we found a selective deficit of patients in the Intern condition: patients reacted slower when presented with a non-informative cue, i.e. when they had to choose between a left or right index finger response as fast as possible (Table 2). Dopaminergic medication did not have any significant effect on performance in any of the higher motor control conditions (all P > 0.1). Hence, despite a significant effect on bradykinesia, dopaminergic medication did not affect higher motor control functions in patients (Table 2). In summary, the behavioural findings represent a replication of the results of a previous study applying the same paradigm in a larger and independent sample of patients with Parkinson’s disease ON and OFF medication (Michely et al., 2012). This underlines the robustness of the behavioural effects across studies and samples. The differences regarding Extern and Intern between patients and controls were independent of whether a trial was a repetition or switch trial. Additionally, there was no specific effect of dopaminergic medication on repetition or switch trials (Supplementary material). There was no evidence for systematic non-random behaviour of subjects, either for patients or controls. Furthermore, there were no disease- or drug-related differences regarding non-random response patterns (Supplementary material). Moreover, to exclude systematic differences with respect to the side of responses in the conditions with self-chosen movement lateralization, we calculated the proportion of right-handed responses out of all responses in the Free and Intern condition, i.e. the response bias for the dominant hand. There were no differences across conditions between groups and sessions and a nearly perfect balance between left-right responses [all P > 0.1; Free: controls 0.52 (SD: 0.05), OFF 0.52 (SD: 0.03), ON 0.52 (SD: 0.02); Intern: controls 0.51 (SD: 0.07), OFF 0.52 (SD: 0.03); ON: 0.52 (SD: 0.04)].
Neural activation pattern
Figure 2A depicts the neural network conjointly activated by all four conditions. This ‘core network’ comprised M1, lateral PMC, SMA, dorsolateral PFC, somatosensory cortex, parietal cortex, and primary visual cortex. Furthermore, activation was found in the cerebellum, thalamus, putamen and globus pallidus. Similar patterns were evident when analysing each condition separately (Fig. 2B). Hence, the regions included in the DCM analysis were significantly activated across all conditions.
However, contrasts comparing functional MRI activity between groups did not yield significant differences (P < 0.05, family-wise error corrected at the voxel level) for any of the four conditions. Moreover, there was no interaction between the factors Group or Medication and the higher motor control conditions.
Bayesian model selection
Random-effects Bayesian model selection revealed Model 4 to be the most likely across all group comparisons, i.e. controls/OFF, controls/ON, OFF/ON (see Supplementary material for Bayesian model selection results). The computation of the entire analysis for the right hemisphere revealed the same winning model when applying the Bayesian model selection procedure across groups. Of note, the coupling parameters of this model were very similar and highly correlated between the right and left hemisphere (Supplementary material).
The total mean variance explained by Model 4 was not different between patients and controls, or between medication states [all P > 0.1; controls: 32% (SD: 15%); OFF: 24% (SD: 10%); ON: 23% (SD: 12%)].
All 40 models shared the same endogenous network structure but differed in the modulation of these endogenous connections as a function of condition. Compared to the most complex Model 1, which included condition-dependent modulation of all endogenous connections, Model 4 did not comprise modulation of connectivity between PFC or M1 and subcortical regions (putamen, thalamus, cerebellum) as a function of the experimental conditions (Fig. 3). However, all regions comprised condition-specific connectivity with both the mesial (SMA) and lateral (PMC) premotor region. This highlights the prominent role of the mesial and lateral premotor loops in all four experimental conditions across groups.
Endogenous connectivity (DCM-A)
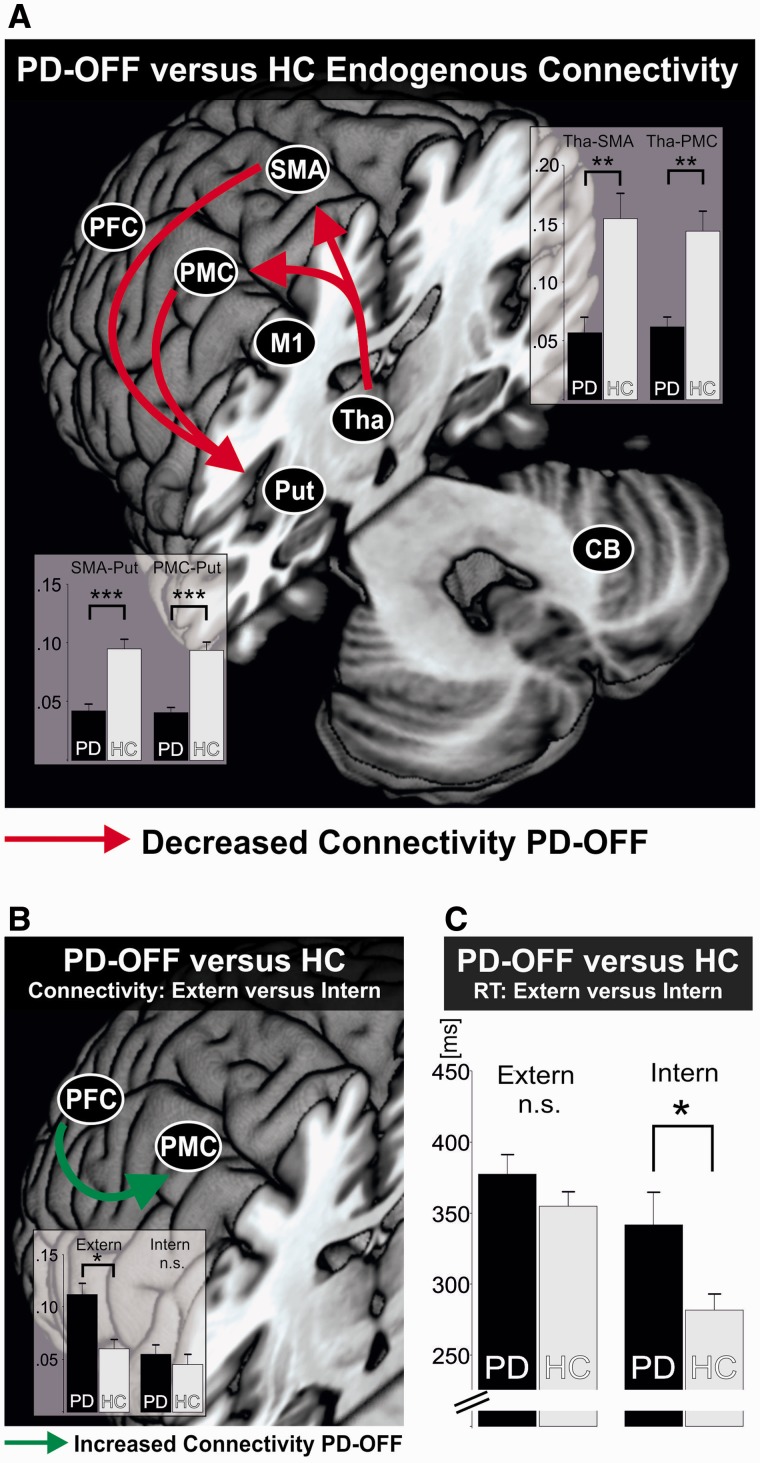
Endogenous connectivity refers to the coupling that was constant across all experimental conditions. The ANOVA for endogenous connectivity revealed a significant Group effect when comparing controls with patients OFF medication [F(1,22) = 23.246, P < 0.001]. Strongest differences were found for cortico-subcortical connections (Fig. 4A).
Figure 4.
Connectivity in patients with Parkinson’s disease in the OFF state. (A) Differences in endogenous connectivity between healthy controls (HC) and patients with Parkinson’s disease OFF medication (PD-OFF). Red arrows indicate a significantly reduced connectivity between two regions in patients as compared to controls. *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni-corrected for multiple comparisons. Bars represent coupling strength in 1/s. Error bars = SEM. (B) Selective difference in condition-specific connectivity between patients with Parkinson’s disease OFF medication (PD-OFF) and healthy controls (HC), depending on externally cued (Extern) or internally selected (Intern) movement lateralization. Green arrow indicates a significantly increased connectivity of the lateral premotor loop between PFC and PMC in patients OFF medication in the Extern condition. *P < 0.05, Bonferroni-corrected for multiple comparisons; n.s. = not significant. Bars represent coupling strength in 1/s. Error bars = SEM. (C) Selective difference in behavioural performance between patients with Parkinson’s disease OFF medication (PD-OFF) and healthy controls (HC), depending on externally cued (Extern, preserved performance in patients) or internally selected (Intern, impaired performance in patients) movement lateralization. *P < 0.05; n.s. = not significant. Bars represent reaction time in milliseconds (ms). Error bars = SEM. CB = cerebellum; Put = putamen; Tha = thalamus.
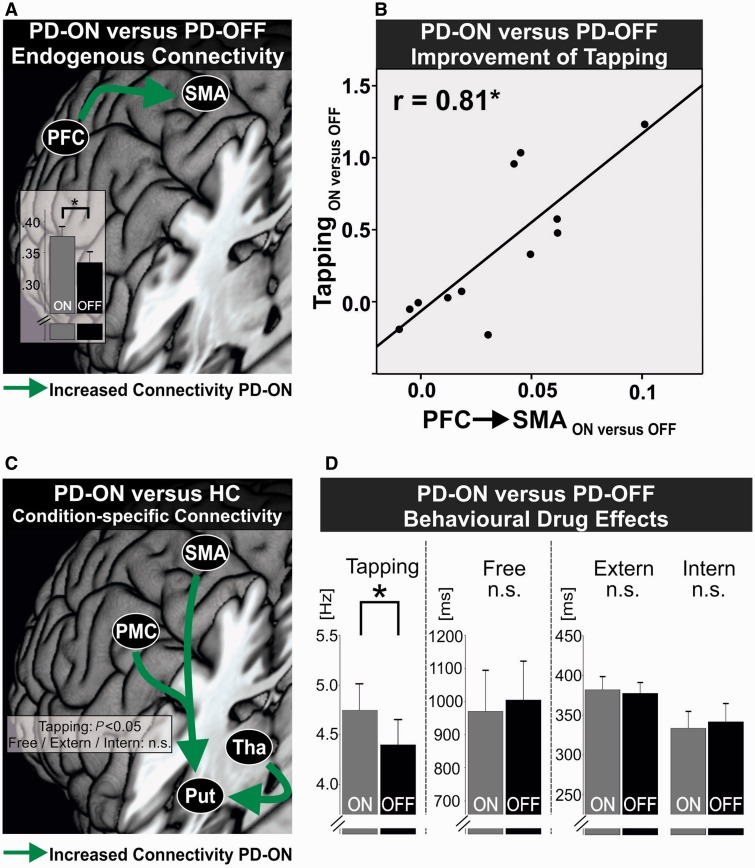
However, when examining dopaminergic effects in patients, we found a significant interaction between Medication × Loop × Connection [F(4,44) = 9.023, P <0.001]. Post hoc t-tests revealed that this interaction was primarily due to a stronger coupling in the mesial loop between PFC and SMA in the ON compared to the OFF state (P < 0.05, Bonferroni-corrected, Fig. 5A). Equivalent effects were found for endogenous connectivity of the right hemisphere.
Figure 5.
Dopaminergic effects on connectivity and behaviour in patients with Parkinson’s disease. (A) Difference in endogenous connectivity between patients with Parkinson’s disease ON (PD-ON) and OFF medication (PD-OFF). Green arrow indicates a significantly increased connectivity between PFC and SMA in patients ON medication. *P < 0.05, Bonferroni-corrected for multiple comparisons. Bars represent coupling strength in 1/s. Error bars = SEM. (B) Correlation between increase in endogenous connectivity of the mesial premotor loop between PFC and SMA (PFC→SMA) and improvement of finger tapping speed in the Tapping condition under dopaminergic medication (ON versus OFF). Pearson’s correlation, *P < 0.05, Bonferroni-corrected for multiple comparisons. (C) Difference in condition-specific connectivity between patients with Parkinson’s disease ON medication (PD-ON) and healthy controls (HC). Green arrows indicate a significantly increased connectivity targeting the putamen in patients ON medication in the Tapping condition with no such differences in the higher motor control conditions (Free/Intern/Extern). FDR-corrected for multiple comparisons, P < 0.05. (D) Significant improvement in finger tapping speed between patients with Parkinson’s disease ON (PD-ON) and OFF medication (PD-OFF). No differences in patients in the higher motor control conditions under dopaminergic medication. *P < 0.05; n.s. = not significant. Error bars = SEM.
We next used a linear step-wise regression analysis to test whether changes in endogenous connectivity related to changes in behavioural parameters (change of performance parameters of Tapping, Intern, and Extern [ON − OFF] were entered as independent variables and change of PFC-SMA [ON − OFF] as dependent variable). The analysis revealed that the change of PFC-SMA coupling was best explained by performance changes in the basic motor condition, i.e. Tapping [F(1,11) = 18.7; r = 0.81; P < 0.05, Bonferroni-corrected, Fig. 5B].
In summary, dopaminergic medication caused a general shift from the lateral to the mesial premotor loop in patients. This shift was implemented by a stronger excitatory influence exerted by PFC upon SMA. Moreover, patients featuring stronger drug-induced enhancement of PFC-SMA coupling showed a stronger improvement of basic motor function.
Condition-specific connectivity (DCM-B)
Basic motor function
The ANOVA for coupling parameters in the Tapping condition revealed a significant Group effect when comparing controls with patients OFF medication [F(1,22) = 7.326, P = 0.013] without a significant interaction. There was no significant difference between patients ON and OFF, i.e. no Medication effect, nor an interaction (P > 0.05).
When directly testing each and every connection in an exploratory approach independent of the different premotor loops, we found a stronger facilitating input from premotor regions (PMC, SMA) and the thalamus exerted upon the putamen in patients ON medication compared to controls (P < 0.05, FDR-corrected; Fig. 5C). This difference was not present between patients OFF medication and controls. Similar effects were found for connections targeting the putamen of the right hemisphere. Thus, during maximum motor performance, patients under dopaminergic medication showed an enhanced excitatory drive of cortical premotor regions and the thalamus influencing activity in the putamen over and above the levels observed in controls. Notably, this effect was independent from a shift between the mesial or lateral premotor loops, and highlights that cortico-striatal connectivity from premotor regions was generally enhanced during finger tapping with maximum speed.
Higher motor control
The mixed-ANOVA for the higher motor control conditions revealed a significant interaction for Group × Condition × Loop × Connection between patients OFF medication and controls [F(8,176) = 3.412, P < 0.001]. Post hoc t-tests revealed that this interaction was driven by a significantly stronger excitatory input exerted in the lateral loop by PFC upon PMC in patients OFF medication compared to controls in the Extern condition (P < 0.05, Bonferroni-corrected, Fig. 4B). Again, a corresponding effect during external cueing was also observed for the PFC-PMC connection of the right hemisphere. Interestingly, PFC-PMC coupling OFF medication was also significantly higher in the Extern as compared to both the Free and Intern conditions, i.e. the two voluntary action selection conditions (P < 0.05, Bonferroni-corrected). There was no such difference in the corresponding connection of the mesial loop, i.e. PFC-SMA, either between patients and controls, or in the patients group between Extern and the two voluntary conditions (P > 0.1). Here no significant Medication × Condition interaction was observed when directly comparing patients ON and OFF medication (P > 0.05). Rather, when selectively comparing the PFC-PMC connection in the Extern condition between the OFF and ON state in patients, we found that the hyperconnectivity was significantly reduced under dopaminergic treatment (P < 0.05). The other conditions (Intern, Free) did not differ between groups in terms of their specific connectivity pattern induced by the respective tasks. Moreover, changes in connectivity in the higher motor control conditions did not significantly correlate with behavioural performance (P > 0.1).
In summary, patients OFF medication featured pronounced connectivity of the lateral premotor loop compared to controls, induced by excitatory prefrontal influences on PMC. This shift to the lateral premotor loop in patients was exclusively present in the Extern condition after dopaminergic withdrawal.
Discussion
We used functional MRI to assess motor network dynamics and the impact of dopaminergic medication in Parkinson’s disease with regard to mesial and lateral premotor loops at the cortical level as well as cortico-subcortical interactions. We found that in patients endogenous connectivity was disturbed particularly between cortical premotor areas and subcortical regions. As endogenous connectivity in DCM reflects neural coupling constant across conditions, this accounts for the general affection of motor abilities encountered in Parkinson’s disease, resulting in bradykinesia and impairment in motor control. In contrast, patients’ performance was preserved during external cueing, which was associated with enhanced connectivity of the lateral premotor loop (PFC-PMC) after dopaminergic withdrawal. Dopaminergic medication increased connectivity of the mesial premotor loop (PFC-SMA), which correlated with improvement of basic motor performance, i.e. finger tapping. Concurrently, during finger tapping, patients ON medication also featured enhanced excitatory influences exerted by cortical premotor regions and the thalamus upon the putamen. Thus, dopaminergic treatment impacted on bradykinesia via multi-level network effects, i.e. via a cortical shift to the mesial premotor loop and enhanced cortico-striatal connectivity. In contrast, internal movement control of patients did not respond to medication, concurrent to missing effects at the connectivity level.
Higher motor control: use of external cues
The phenomenon that patients with Parkinson’s disease can overcome their impairment of initiating a movement by drawing upon an external sensory cue has been initially described as ‘kinesia paradoxica’ (Souques, 1921; Martin, 1967). The facilitation of movements by sensory cues has been frequently reproduced, e.g. with regard to gait, reaching movements and sit-to-stand movements (Majsak et al., 1998; Azulay et al., 1999; Mak and Hui-Chan, 2004; Nieuwboer et al., 2007). Similar effects have also been reported for more cognitively controlled tasks, e.g. reactions to visual cues requiring a self-selected or externally specified response or self-initiated versus externally triggered task preparation during task-switching (Siegert et al., 2002; Werheid et al., 2007; Michely et al., 2012). Although in the present study the two conditions Extern and Intern both constituted choice reaction tasks, patients’ performance differed in the two tasks: while patients performed as healthy subjects in the condition with externally cued lateralized movements (Extern), patients showed a significant deficit in the condition necessitating a (fast) internal choice regarding which hand to move (Intern). The lateral PMC is implicated in guiding externally triggered visuomotor actions by mapping external cues to the adequate movement (Passingham and Toni, 2001; Moisa et al., 2012). Increased activity, respective connectivity of the lateral PMC, has been illustrated before in neuroimaging studies of Parkinson’s disease applying various types of motor tasks (Samuel et al., 1997; Sabatini et al., 2000; Haslinger et al., 2001; Mallol et al., 2007; Rowe et al., 2010; Wu et al., 2010). Of note, in the present study connectivity between PFC and lateral PMC was significantly enhanced only in the Extern condition in patients OFF medication compared to controls and compared to connectivity in the conditions assessing self-chosen actions. This finding strongly suggests a compensatory role of the PFC-PMC connection in the hypodopaminergic state to maintain task performance at normal levels during external cueing. Such a compensatory mechanism is consistent with a number of studies that have demonstrated enhanced prefrontal activity in cognitive tasks when patients were OFF medication (Mattay et al., 2002; Farid et al., 2009; Cools et al., 2010).
This interpretation is also compatible with the observation that PFC-PMC hyperconnectivity was reduced ON medication without worsening of behavioural performance in the Extern condition. Therefore, our data suggest that hyperconnectivity of the lateral premotor loop, connecting PFC and lateral PMC, is not a general phenomenon of the disease, but context-specific. Thus, activation of the lateral premotor loop may constitute a dynamic, compensatory mechanism due to dopaminergic withdrawal accounting for preserved performance in externally cued tasks as frequently observed in clinical practice.
However, irrespective of the strong dopaminergic effect on basic motor performance, deficits in the condition relying on internally controlled processes (Intern) were unaffected by dopaminergic medication, both at the behavioural and the neural level. Hence, our findings add further support to the notion that executive deficits in higher order control of actions like internal movement selection and choice processes in Parkinson’s disease may be related to neurotransmitter system dysfunction beyond the dopaminergic system, such as, e.g. acetylcholine, noradrenaline or serotonin (Marsh et al., 2009; Schmitt et al., 2010; Narayanan et al., 2013; Ye et al., 2014a, b).
Basic motor performance: bradykinesia
An abnormal regional response profile of the SMA, constituting the central node of the mesial premotor loop, has been frequently considered to play a key role in motor symptoms of Parkinson’s disease (Jahanshahi et al., 1995; Haslinger et al., 2001; Escola et al., 2003; Rowe et al., 2010; Esposito et al., 2013). Moreover, increases in SMA activity levels after dopaminergic treatment correlate with the amelioration of bradykinesia (Haslinger et al., 2001; Buhmann et al., 2003). In line with that, dopaminergic medication has been shown to modulate SMA connectivity patterns with PFC (Rowe et al., 2010; Herz et al., 2014b) and sensorimotor cortex (Esposito et al., 2013). In the present study, we observed that the amount by which connectivity between PFC and SMA was enhanced by dopaminergic medication was directly related to individual improvements in finger tapping speed. Interestingly, Gonzalez-Garcia et al. (2011) found that improvements of bradykinesia following transcranial magnetic stimulation are associated with enhanced PFC-SMA connectivity in patients. The congruency of these findings based on different modulatory approaches (i.e. pharmacological and electromagnetic stimulation) suggests a pivotal role of the PFC in the improvement of bradykinesia, e.g. at the level of cognitive control of motor performance associated with a shift from lateral to mesial premotor loops (Rowe et al., 2002, 2010; Ceballos-Baumann, 2003).
However, our connectivity analyses also revealed medication-induced changes of connections targeting subcortical areas, in particular the putamen. Dopamine deficiency in Parkinson’s disease is most prominent in the putamen, and its dysfunction has been frequently shown to correlate with clinical scores, especially bradykinesia (Taniwaki et al., 2003; Postuma and Dagher, 2006). For example, Vingerhoets et al. (1997) showed that measures of bradykinesia, e.g. reduced finger tapping speed, correlate with reduced striatal dopamine uptake, quantified with PET. Notably, in our study, enhanced excitatory input targeting the putamen in patients ON medication was specifically found for the condition in which task performance improved under dopaminergic medication, i.e. finger tapping. Thus, our finding that dopaminergic stimulation facilitated cortical interactions with the putamen during finger tapping extends previous assumptions of the role of the putamen in bradykinesia by suggesting that not only regional activity but especially enhanced cortical input to the putamen is a key mechanism underlying improvement of movement speed in Parkinson’s disease.
Importantly, under dopaminergic medication, excitatory drive from cortical premotor regions and the thalamus onto the putamen during finger tapping exceeded levels observed in controls. Additionally, dopaminergic treatment specifically increased endogenous PFC-SMA coupling. Notably, this connection was not reduced in the OFF state compared to controls. Taken together, the data suggest that the positive effect of dopaminergic medication upon bradykinesia does not result from reinstating physiological coupling patterns but through recruiting additional (‘unphysiological’) neural capacities relating to connectivity patterns of SMA and putamen. Consistent with this view, Herz et al. (2014c) recently found that abnormal, dopamine-induced increase of activity in the (pre-)SMA and the putamen predicted development of dyskinesia in patients with Parkinson’s disease. Moreover, several studies found significant overactivity, respectively altered cortical excitability, of the SMA in patients with levodopa-induced dyskinesia (Brooks et al., 2000; Koch, 2010; Cerasa et al., 2012). Hence, it seems crucial to further investigate whether drug-induced altered connectivity patterns in patients, initially yielding a beneficial effect on basic motor symptoms, could lead to frequently observed side effects associated with dopaminergic medication, e.g. peak dose fluctuations and levodopa-induced dyskinesia (Damier, 2009; Iravani et al., 2012).
Limitations
Patients were tested after at least 12 h withdrawal of medication, which is standard practice in Parkinson’s disease research as medication effects are minimized, yet preserving the functionality of the patients for most of the wake time (Defer et al., 1999). Although this procedure is widely applied, we cannot rule out pharmacological long-term effects on network dynamics even in the OFF state (Fahn, 2005). Moreover, patients were under their standard doses of medication in a clinically best ON state, but we cannot rule out that a dopaminergic challenge with high doses of levodopa might have induced different effects on brain network connectivity. However, the fact that we found highly significant improvements in UPDRS scores renders it very unlikely that missing effects were caused by insufficient stimulation of the dopaminergic system.
We implemented a bimanual task in a block design in order to maximize sensitivity such that we could find a group difference at both the behavioural (higher demands on the motor effectors) and neural level (higher sensitivity of block design versus event related designs). Consequently, the respective connectivity models are not specific for one hand, but rather reflect the general effect of the motor task on network connectivity. Furthermore, when computing the entire analysis for equivalent regions of the right hemisphere, similar effects were found as for the left hemisphere. This internal replication underpins the robustness of the DCM findings, suggesting that both within- and between-group analyses were mirror-symmetric in both hemispheres and that neither the use of a unilateral model nor hemispheric differences significantly impacted on our results.
Of note, our analyses did not yield strong group differences when focusing on blood oxygenation level-dependent activity. In stark contrast, analyses of the coupling estimates assessed via DCM were much more sensitive with respect to disease- and drug-related changes in network connectivity. This might be due to the region of interest approach in DCM correcting for residual interindividual variability in the precise anatomical location of areas in individual subjects. Moreover, the haemodynamic response function in DCM is computed for every region of interest separately (Friston et al., 2003) in contrast to the ‘classical’ activation analysis, which uses a canonical haemodynamic response function for all voxels. Consistent with this, a recent neuroimaging meta-analysis reported a remarkable heterogeneity of regional activation effects in Parkinson’s disease, suggesting that local activation changes in patients may be small and heterogeneous (Herz et al., 2014a). In contrast, connectivity approaches have been shown to be more sensitive especially with regard to systems level disorders like Parkinson’s disease, and both technical (transcranial magnetic stimulation, deep brain stimulation) and pharmacological treatment effects (Jahanshahi et al., 2010; Rowe, 2010; Gonzalez-Garcia et al., 2011; Esposito et al., 2013; Kahan et al., 2014).
Conclusion
Our findings highlight that facilitation of movement initiation during external cueing in Parkinson’s disease is specifically associated with hyperconnectivity of the lateral premotor loop. This hyperconnectivity constitutes a context-dependent compensatory mechanism in the hypodopaminergic state. Moreover, our study suggests that dopaminergic medication selectively improves bradykinesia as opposed to deficits in higher motor control via specific multi-level network effects, i.e. at the cortical level with a dopamine-induced shift to the mesial premotor loop and via strengthened cortico-striatal coupling targeting the putamen. The observed connectivity changes underlying the dopaminergic improvement of bradykinesia could not be observed for higher motor control. Hence, our data suggest that higher motor control deficits in patients with Parkinson’s disease are related to factors beyond mere dopaminergic depletion.
Funding
J.M., M.T.B., L.T., G.R.F. and C.G. are supported by the German Research Foundation (Clinical Research Group KFO219 ‘Basal-Ganglia-Cortex-Loops: Mechanisms of Pathological Interactions and Therapeutic Modulation’; GR 3285/5–1). S.V., G.R.F and C.G. receive additional funding from the University of Cologne Emerging Groups Initiative (CONNECT group) implemented into the Institutional Strategy of the University of Cologne and the German Excellence Initiative. S.B.E. acknowledges funding by the Helmholtz Initiative on Systems-Biology ‘The Human Brain Model’ and the NIH (R01-MH074457). G.R.F. gratefully acknowledges additional support from the Marga and Walter Boll Stiftung.
Supplementary material
Supplementary material is available at Brain online
Glossary
Abbreviations
- DCM
Dynamic Causal Modelling
- M1
primary motor cortex
- PANDA
Parkinson Neuropsychometric Dementia Assessment
- PFC
prefrontal cortex
- PMC
premotor cortex
- SMA
supplementary motor area
- UPDRS
Unified Parkinson’s Disease Rating Scale
References
- Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, Pouget J. Visual control of locomotion in Parkinson's disease. Brain. 1999;122(Pt 1):111–20. doi: 10.1093/brain/122.1.111. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory. 2nd edn. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- Brooks DJ, Piccini P, Turjanski N, Samuel M. Neuroimaging of dyskinesia. Annals of neurology. 2000;47(4 Suppl 1):S154–8; discussion S8–9. [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Internal versus external cues and the control of attention in Parkinson's disease. Brain. 1988;111(Pt 2):323–45. doi: 10.1093/brain/111.2.323. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI—cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 2003;126(Pt 2):451–61. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Ceballos-Baumann AO. Functional imaging in Parkinson's disease: activation studies with PET, fMRI and SPECT. J Neurol. 2003;250(Suppl 1):I15–23. doi: 10.1007/s00415-003-1103-1. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Pugliese P, Messina D, Morelli M, Gioia MC, Salsone M, et al. Prefrontal alterations in Parkinson's disease with levodopa-induced dyskinesia during fMRI motor task. Mov Disord. 2012;27:364–71. doi: 10.1002/mds.24017. [DOI] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, et al. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. 2013;23:2677–89. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D'Esposito M. Enhanced frontal function in Parkinson's disease. Brain. 2010;133(Pt 1):225–33. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P. Drug-induced dyskinesias. Curr Opin Neurol. 2009;22:394–9. doi: 10.1097/WCO.0b013e32832d9dc4. [DOI] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD) Mov Disord. 1999;14:572–84. doi: 10.1002/1531-8257(199907)14:4<572::aid-mds1005>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: a population-based study. Eur J Neurol. 2009;16:1278–84. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- Escola L, Michelet T, Macia F, Guehl D, Bioulac B, Burbaud P. Disruption of information processing in the supplementary motor area of the MPTP-treated monkey: a clue to the pathophysiology of akinesia? Brain. 2003;126(Pt 1):95–114. doi: 10.1093/brain/awg004. [DOI] [PubMed] [Google Scholar]
- Esposito F, Tessitore A, Giordano A, De Micco R, Paccone A, Conforti R, et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain. 2013;136(Pt 3):710–25. doi: 10.1093/brain/awt007. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent developments in Parkinson’s Disease. FlorhamPark, NJ: MacMillanHealthCare; 1987. pp. 153–63. [Google Scholar]
- Fahn S. Parkinson Study G. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. 2005;252(Suppl 4):IV37–42. doi: 10.1007/s00415-005-4008-5. [DOI] [PubMed] [Google Scholar]
- Farid K, Sibon I, Guehl D, Cuny E, Burbaud P, Allard M. Brain dopaminergic modulation associated with executive function in Parkinson's disease. Mov Disord. 2009;24:1962–9. doi: 10.1002/mds.22709. [DOI] [PubMed] [Google Scholar]
- Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson's disease with deep brain stimulation. Lancet Neurol. 2012;11:429–42. doi: 10.1016/S1474-4422(12)70049-2. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston K. SPM Helpline. 2012. Available from: https://www.jiscmail.ac.uk/cgi-bin/webadmin?A2=spm;bebd494.1203.
- Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:539–44. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Iansek R, Phillips JG, Mattingley JB, Bradshaw JA. Reduction in external cues and movement sequencing in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:368–70. doi: 10.1136/jnnp.57.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy O, Azouvi P, Robert P, Roussel M, LeGall D, Meulemans T, et al. Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol. 2010;68:855–64. doi: 10.1002/ana.22117. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia N, Armony JL, Soto J, Trejo D, Alegria MA, Drucker-Colin R. Effects of rTMS on Parkinson's disease: a longitudinal fMRI study. J Neurol. 2011;258:1268–80. doi: 10.1007/s00415-011-5923-2. [DOI] [PubMed] [Google Scholar]
- Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol. 2004;14:715–9. doi: 10.1016/j.conb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. Neuroimage. 2008;41:1382–94. doi: 10.1016/j.neuroimage.2008.03.048. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, et al. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124(Pt 3):558–70. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Lokkegaard A, Siebner HR. Functional neuroimaging of motor control in parkinson's disease: a meta-analysis. Hum Brain Mapp. 2014a;35:3227–37. doi: 10.1002/hbm.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz DM, Siebner HR, Hulme OJ, Florin E, Christensen MS, Timmermann L. Levodopa reinstates connectivity from prefrontal to premotor cortex during externally paced movement in Parkinson's disease. Neuroimage. 2014b;90:15–23. doi: 10.1016/j.neuroimage.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Herz DM, Haagensen BN, Christensen MS, Madsen KH, Rowe JB, Lokkegaard A, et al. The acute brain response to levodopa heralds dyskinesias in Parkinson disease. Ann Neurol. 2014c;75:829–36. doi: 10.1002/ana.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The “what” and “when” of self-initiated movements. Cereb Cortex. 2013;23:520–30. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57:P101–15. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Iravani MM, McCreary AC, Jenner P. Striatal plasticity in Parkinson's disease and L-dopa induced dyskinesia. Parkinsonism Rel Disord. 2012;18(Suppl 1):S123–5. doi: 10.1016/S1353-8020(11)70038-4. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Brown RG, Marsden CD. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson's disease. Brain. 1992;115(Pt 2):539–64. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118(Pt 4):913–33. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010;133(Pt 3):727–45. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–8. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, et al. Resting state functional MRI in Parkinson's disease: the impact of deep brain stimulation on ‘effective' connectivity. Brain. 2014;137(Pt 4):1130–44. doi: 10.1093/brain/awu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, Wittchen HU, et al. Screening for cognitive deficits in Parkinson's disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Rel Disord. 2008;14:93–101. doi: 10.1016/j.parkreldis.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol. 2010;9:1200–13. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- Koch G. rTMS effects on levodopa induced dyskinesias in Parkinson's disease patients: searching for effective cortical targets. Restor Neurol Neurosci. 2010;28:561–8. doi: 10.3233/RNN-2010-0556. [DOI] [PubMed] [Google Scholar]
- Majsak MJ, Kaminski T, Gentile AM, Flanagan JR. The reaching movements of patients with Parkinson's disease under self-determined maximal speed and visually cued conditions. Brain. 1998;121(Pt 4):755–66. doi: 10.1093/brain/121.4.755. [DOI] [PubMed] [Google Scholar]
- Mak MK, Hui-Chan CW. Audiovisual cues can enhance sit-to-stand in patients with Parkinson's disease. Mov Disord. 2004;19:1012–9. doi: 10.1002/mds.20196. [DOI] [PubMed] [Google Scholar]
- Mallol R, Barros-Loscertales A, Lopez M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson's disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. 2007;1147:265–71. doi: 10.1016/j.brainres.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Marsh L, Biglan K, Gerstenhaber M, Williams JR. Atomoxetine for the treatment of executive dysfunction in Parkinson's disease: a pilot open-label study. Mov Disord. 2009;24:277–82. doi: 10.1002/mds.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L, McDonald WM, Cummings J, Ravina B Depression NNWGo, Parkinson's D. Provisional diagnostic criteria for depression in Parkinson's disease: report of an NINDS/NIMH Work Group. Mov Disord. 2006;21:148–58. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]
- Martin JP. The basal ganglia and posture. London: Pitman Medical; 1967. [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. Dopaminergic modulation of cortical function in patients with Parkinson's disease. Ann Neurol. 2002;51:156–64. doi: 10.1002/ana.10078. [DOI] [PubMed] [Google Scholar]
- Michely J, Barbe MT, Hoffstaedter F, Timmermann L, Eickhoff SB, Fink GR, et al. Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson's disease. Neuropsychologia. 2012;50:2506–14. doi: 10.1016/j.neuropsychologia.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Moisa M, Siebner HR, Pohmann R, Thielscher A. Uncovering a context-specific connectional fingerprint of human dorsal premotor cortex. J Neurosci. 2012;32:7244–52. doi: 10.1523/JNEUROSCI.2757-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson's disease. Rev Neurosci. 2013;24:267–78. doi: 10.1515/revneuro-2013-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–40. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proc Natl Acad Sci USA. 2000;97:913–8. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Casabona E, Bringas ML, Alvarez M, Alvarez L, et al. Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp Brain Res. 2011;212:371–84. doi: 10.1007/s00221-011-2736-6. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Toni I. Contrasting the dorsal and ventral visual systems: guidance of movement versus decision making. Neuroimage. 2001;14(1 Pt 2):S125–31. doi: 10.1006/nimg.2001.0836. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Modelling functional integration: a comparison of structural equation and dynamic causal models. Neuroimage. 2004;23(Suppl 1):S264–74. doi: 10.1016/j.neuroimage.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci. 2010;11:760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB. Connectivity analysis is essential to understand neurological disorders. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00144. pii: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R. Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain. 2002;125(Pt 2):276–89. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, et al. Parkinson's disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain. 2008;131(Pt 8):2094–105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes LE, Barker RA, Owen AM. Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment? Neuroimage. 2010;52:1015–26. doi: 10.1016/j.neuroimage.2009.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Siebner HR. The motor system and its disorders. Neuroimage. 2012;61:464–77. doi: 10.1016/j.neuroimage.2011.12.042. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123(Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, et al. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain. 1997;120(Pt 6):963–76. doi: 10.1093/brain/120.6.963. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–56. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Farlow MR, Meng X, Tekin S, Olin JT. Efficacy of rivastigmine on executive function in patients with Parkinson's disease dementia. CNS Neurosci Ther. 2010;16:330–6. doi: 10.1111/j.1755-5949.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert RJ, Harper DN, Cameron FB, Abernethy D. Self-initiated versus externally cued reaction times in Parkinson's disease. J Clin Exp Neuropsychol. 2002;24:146–53. doi: 10.1076/jcen.24.2.146.991. [DOI] [PubMed] [Google Scholar]
- Souques AA. Older description of parkinsonian persons who can run much easier than walk. Revue Neurologique (Paris) 1921;37:559–60. [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49:3099–109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T, Okayama A, Yoshiura T, Nakamura Y, Goto Y, Kira J, et al. Reappraisal of the motor role of basal ganglia: a functional magnetic resonance image study. J Neurosci. 2003;23:3432–8. doi: 10.1523/JNEUROSCI.23-08-03432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- Vingerhoets FJ, Schulzer M, Calne DB, Snow BJ. Which clinical sign of Parkinson's disease best reflects the nigrostriatal lesion? Ann Neurol. 1997;41:58–64. doi: 10.1002/ana.410410111. [DOI] [PubMed] [Google Scholar]
- Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol. 2011;69:375–88. doi: 10.1002/ana.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werheid K, Koch I, Reichert K, Brass M. Impaired self-initiated task preparation during task switching in Parkinson's disease. Neuropsychologia. 2007;45:273–81. doi: 10.1016/j.neuropsychologia.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. Neuroimage. 2008;42:343–56. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136(Pt 3):696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. 2011;55:204–15. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Li K, Chan P. Neural correlates of bimanual anti-phase and in-phase movements in Parkinson's disease. Brain. 2010;133(Pt 8):2394–409. doi: 10.1093/brain/awq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Improving response inhibition in Parkinson's disease with atomoxetine. Biol Psychiatry. 2014a doi: 10.1016/j.biopsych.2014.01.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, et al. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson's disease. Brain. 2014b;137(Pt 4):1145–55. doi: 10.1093/brain/awu032. [DOI] [PMC free article] [PubMed] [Google Scholar]