Abstract
Achyrocline alata, known as Jateí-ka-há, is traditionally used to treat several health problems, including inflammations and infections. This study aimed to optimize an active extract against Streptococcus mutans, the main bacteria that causes caries. The extract was developed using an accelerated solvent extraction and chemometric calculations. Factorial design and response surface methodologies were used to determine the most important variables, such as active compound selectivity. The standardized extraction recovered 99% of the four main compounds, gnaphaliin, helipyrone, obtusifolin and lepidissipyrone, which represent 44% of the extract. The optimized extract of A. alata has a MIC of 62.5 μg/mL against S. mutans and could be used in mouth care products.
Introduction
Natural products are an important source of bioactive compounds; however, many challenges exist when transforming them into phytotherapy medicines. The main difficulties involve identifying the bioactive compounds, the variations in secondary metabolites and the low reproducibility of traditional extractions [1]. The extractive method directly affects the pharmaceutical industry because it determines the quality and safety of the final product [2]. Modern techniques, such as accelerated solvent extraction (ASE) assisted by chemometric calculations are good alternate solutions to this problem and allow the extraction time, solvent quantity and yield to be optimized [3,4].
ASEs enable reproducible, exhaustive and stable extractions by using an inert nitrogen atmosphere with high temperature and pressure [5]. The parameter range allowed by the equipment yields extracts with different chemical and biological profiles. Furthermore, chemometric techniques, such as factorial experiments and a response surface method (RSM), can optimize the extractive variables and allow the monitored response variables to be understood, which yields the ideal extract for a specific purpose [6]. The monitored responses can be a chemical profile or biological activity as demonstrated by Zhou et al. [7], who used a DPPH (diphenyl-(2,4,6-trinitrophenyl) iminoazanium) antioxidant assay to establish the best production conditions of an antioxidant extracted from Clerodendrum cyrtophyllum.
Extract optimization must be based on the biologically active chemical constituents with aid of the ethnopharmacological use. A bioactivity assay could help identify the fraction/compounds responsible for an observed activity [8]; this step is required for widely used plants, such as species of the genus Achyrocline (Asteraceae), which are recommended for many diseases as inflammation and infections [9]. Achyrocline alata, a species of this genus found in Brazil, Paraguay and Argentina, contains various classes of secondary metabolites, such as flavonoids, terpenes and polyketides [10, 11]. Furthermore, the variety of traditional uses obfuscates which constituents must be present for each reported activity.
Hence, this study aimed to identify the antimicrobial metabolites in A. alata and standardize an active extract for use against Streptococcus mutans—the main bacteria that causes carie—using ASE and chemometric calculations.
Materials and Methods
Ethics Statement
The experiments were performed in accordance with the ethical principles for animal research adopted by the Ethics Committee on Animal Experimentation of the UFMS (CEUA/UFMS n°202/2009). Efforts were made to minimize suffering and after experimental procedures, the animals were euthanized in a CO2 chamber.
Acquisition, drying and stabilization of plant material
Achyrocline alata (Kunth) DC was cultivated in the Universidade Federal da Grande Dourados, Dourados, MS, Brazil. A voucher specimen was deposited under registration number 40310 in the CGMS herbarium, Campo Grande, MS, Brazil. The drying process was performed at 40°C and the plant was powdered with a knife mill (20 mesh). Inflorescences of A. satureioides were bought from Chileno Chás e Ervas (Laboratório Industrial Vida e Saúde Ltda—Chapecó, Brazil) and were used without pretreatment.
Hexane, ethanol, infusion and trichome extracts from A. alata
The hexane extract (HExt) was obtained from 0.6 g of the inflorescences using a model ASE-150 (Dionex) accelerated solvent extraction system with a 5 mL capacity cell using a hexane solvent at 130°C for a static extraction time of 4 minutes across 5 cycles with a 150% rinse. The ethanolic extract (EExt, 70% ethanol) was prepared using the same parameters sequentially from the same sample. The HExt was partitioned into a separatory funnel with hexane and 70% ethanol to obtain two phases: a hexane phase (HExt-HP) and ethanolic phase (HExt-EP). The trichome extract (TExt) was obtained using the method described by Schorr et al. [12]. The infusion extraction (IExt) involved pouring boiling water over the drug plant in a proportion of 2% for five minutes and then lyophilizing. All chemicals were analytical grade purchased from Vetec, Rio de Janeiro, Brazil.
Ethanolic phase of A. satureioides and the steam/leaves extract from A. alata
The ethanolic phase extract from the A. satureioides inflorescences (HExt-EPsat) and steam/leaves extract (SLExt) from A. alata were obtained via an ASE using the final optimized method (95:5 hexane:ethyl acetate as extractor solvent, 150°C, one cycle, 1 minute static time and partition with 90% ethanol).
Antibacterial activity against Streptococcus mutans
The antibacterial assay was conducted using HExt, EExt and HExt-EP. The S. mutans strain (UA159) was reactivated in a brain heart infusion broth (BHI, Sigma-Aldrich, São Paulo, Brazil) and the inoculum was standardized using a spectrophotometer at a wavelength of 625 nm to acquire a suspension containing approximately 1.5 × 106 CFU/mL [13]. The extract samples were dissolved in 1% of dimethylsulfoxide (DMSO) (Vetec, Rio de Janeiro, Brazil) and diluted from 1000 μg/mL to 15.6 μg/mL by two-fold serial dilutions. The positive control used was 0.12% (v/v) chlorhexidine in water (Sigma-Aldrich, St. Louis, MO, USA) and 1% DMSO as the negative control. The microplates were incubated with BHI at 37°C in jars containing 5% CO2 for 24 hours.
HPLC-DAD analysis
The HPLC-DAD analyses were performed using an SPD-M20A (Shimadzu, Kyoto, Japan) with a monolithic C-18 column (100 × 3 mm, Onyx/Phenomenex). Both water (A) and acetonitrile (B) (HPLC grade, Vetec, Rio de Janeiro, Brazil) containing 1% acetic acid were used as the mobile phases. This method began with 28% B for 5 minutes and increasing to 80% B over 11 minutes with a further 5.5 minutes to wash and stabilize the column at 1.8 mL/min flow.
Analytical method validation
The method was validated in accordance with the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH-Q2B [14]. The specificity, linearity, range and accuracy were evaluated based on the gnaphaliin and helipyrone response. Standard curve had the correlation coefficient measured and specificity evaluated with the pick purity in HPLC and NMR 1H. Accuracy was determined by recovery of gnaphaliin and helipyrone added in triplicates on plant material previously cleaned, in concentrations of 10μg/ml, 25μg/ml and 50μg/ml, Reproducibility was determined by successive injections (6) in three different days and should not differ by more than 5%.
Determination of Hydrogen peroxide and NO produced by peritoneal murine macrophages
The EExt and HExt-EP extracts were tested. Adult, male Swiss mice weighing 18–25 g were obtained from the animal colony in the Universidade Federal de Mato Grosso do Sul (UFMS), Campo Grande, MS, Brazil, maintained under controlled temperature (22 ± 2°C) and lighting (12/12 light/dark cycle), with water and food ad libitum. The six animals were kept in collective cages (40x 35x17 cm/ 3 animals/cage).
The macrophages were collected after pre-treating the mice with 3% thioglycolate 96 hours before harvesting. These cells were obtained by washing the cavities and a total cell count was obtained using a Neubauer hemocytometer. The cells were resuspended in an RPMI 1640 medium (Sigma Aldrich, St. Louis, USA) supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin and 10% fetal bovine serum (Sigma-Aldrich, St. Louis, USA).
The macrophage suspension (2×105 cells/100 μL) was added to the bottom of 96 wells and incubated for 1 hour at 37°C in a CO2 incubator for cell adhesion. Subsequently, the non-adherent cells were removed, and 100 μL of a phenol red solution (140 mM NaCl, 10 mM potassium-phosphate buffer pH 7.0, 5.5 mM dextrose, 0.56 mM phenol red) containing 8.5 U/ml of horseradish peroxidase (Sigma Aldrich, St. Louis, USA) and the EExt and HExt-EP in the presence or absence of phorbol myristate acetate (PMA, 50 ng/well, Sigma Aldrich, St. Louis, USA, 10 μL) was added, and the cells were incubated for 60 minutes at 37°C in a 5% CO2 atmosphere. The absorbance was determined using an ELISA reader at a wavelength of 620 nm [15].
The technique used to determine the release of nitric oxide was described by Ding et al. [16]. The non-adherent cells were removed and LPS (1μg/mL) and/or the EExt and HExt-EP were diluted in supplemented RPMI 1640 medium and added to the plate. The cells were incubated for 48 h, and at the end of this period, the production of NO was determined by the accumulation of nitrite in the cell culture supernatants. The absorbance was determined using an ELISA reader at a wavelength of 540 nm. These assays were performed in triplicate while simultaneously maintaining control cells without any PMA and lipopolysaccharide (LPS) for analysis of the spontaneous release of hydrogen peroxide and nitric oxide, respectively.
The cell viability was performed by mitochondrial-dependent reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma-Aldrich, St. Louis, USA) to formazan [17]. The peritoneal macrophages were obtained as previously described, incubated with the same conditions for 24 hours. Then, cells were incubated with EExt (4mg/mL, 8 mg/mL, 0.16 mg/mL) and with HExt-EP (4 mg/mL, 0.8 mg/mL, 0.16 mg/mL) for 16h. After this period the MTT (20μL, 5mg/mL) was added, incubed for 2 hours and absorbance was determined using an ELISA reader at a wavelength of 540 nm.
Variables screening by factorial design
The factorial design was built in a 25 factorial model, resolution III and in duplicates to optimize the follow variables: extractor solvent (hexane/acetone), temperature (70 to 130°C), cycles (1 to 5), time (1 to 5 min) and ethanol/water partition (50–90%). The response monitoring was based on the HExt-EP extract yields for active and inactive compounds (S1 Table).
Temperature and solvent optimization via RSM experimental design
The temperature (100°C to 180°C) and extractor solvent (hexane:ethyl acetate 50:50–100:0) were optimized via the RSM experimental design using a larger amplitude and a different solvent polarity to a fine-tuning of these parameters. The others parameters previously optimized via the factorial design were kept. These response analyses were based on the gnaphaliin and helipyrone concentration—quantified via HPLC—and extract yield (S2 Table).
Scale-up and exhaustive extraction of active chemicals
The exhaustive extraction and scale-up analyses were performed sequentially three times using 0.6 g of plant in a 5 mL cell and 5 g in a 100 mL cell according to the standard method. A comparison was made using the gnaphaliin and helipyrone concentration.
Isolation of HExt-EP compounds by semi-preparative HPLC-DAD and analysis by NMR and ESI-MS
The HExt-EP (600 mg) was fractionated in an HPLC-DAD Shimadzu, model LC-6AD, with a C-18 column (250 mm x 20 mm, Shimadzu), water (A) and acetonitrile (B), both with 0.05% TFA as mobile phase. The methodology started with 70% B for 6 minutes increasing to 95% B until 26 minutes and 7 minutes to stabilization of column, at 10.5 mL/min flow rate. The peaks with retention time (RT) 9.8 min; 15.7 min; 20 min and 22.23 min were manually collected.
The compounds were identified by 1H, 13C and DEPT-135° NMR, Bruker DPX-300 (300/75 MHz) diluted in deuterated chloroform or deuterated methanol (Merck, Darmstadt, Germany) and mass spectrometry, high resolution (microTOF II-ESI-TOF—Bruker Daltonics) with positive and negative ionization mode.
DPPH antioxidant assay
The antioxidant assay was made in 96-well plate described by Herald et al. [18]. EExt, HExt-EP and quercetin (400 μg/mL) as positive control were tested. The aqueous solution of DPPH (0.1 mMol) was prepared and 50 μL was added to all wells except the solvent wells, in which 50 μL of methanol was added. In the blank wells were added 25 μL of methanol and 50 μL of DPPH. In the sample wells were added 25 μL of the sample and 50 μL of DPPH in at least ten dilutions. Absorbance was measured at 517 nm using a microplate reader, SpectraMax Plus384, Molecular Devices, after 1.5 hours. The percentage of DPPH quenched was determined according to the equation: [(Asample-Ablank)x100/ADPPH blank].
Statistics
The factorial design, RSM design and Probit analysis of antioxidant test were performed using Minitab 16, Minitab Software Inc., USA. Statistical analyzes evaluating the production of reactive oxygen and nitrogen species by murine macrophages were analyzed by ANOVA followed by Bonferroni test using GraphPad Prism 5, GraphPad Software Inc., USA. The P values less than 0.05 were considered statistically significant.
Results
Extracts, fraction yields and microbiological assays
The EExt and HExt yields were 9.89 ± 3.5% and 4.35 ± 0.3%, respectively. After partitioning HExt, the HExt-EP and HExt-HP had yields of 1.8 ± 0.5% and 1.34 ± 0.5%, respectively. The IExt, TExt, HExt-HPsat and SLExt yielded 8.8%, 5.14 ± 1.1%, 2.1 ± 0.4% and 0.8%, respectively. All the results were based on the dry drug vegetable.
HExt exhibited antimicrobial activity with an MIC of 500 μg/mL, while the EExt extract exhibited an MIC of 1000 μg/mL. Thus, constituents present in the HExt were investigated to establish the chemical profile responsible for this antimicrobial activity. The HExt was partitioned using hexane and ethanol, and the HExt-HP and HExt-EP phases were obtained. Only the HExt-EP extract could be tested because HExt-HP was insoluble. HExt-EP had an MIC of 62.5 μg/mL (S3 Table).
Isolation and identification of the HExt-EP compounds
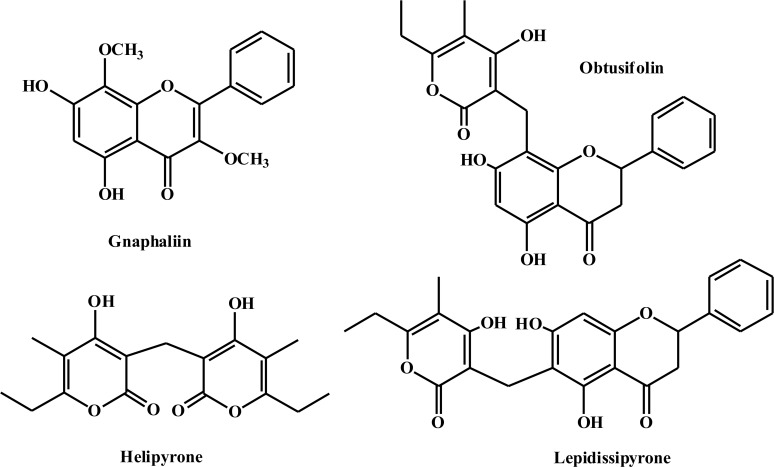
The compound with a retention time (RT) of 9.69 min was identified as the flavonol gnaphaliin (99 mg). The compounds with RTs of 10.1 min and 11.3 min were identified as the flavanones lepidissipyrone (30.9 mg) and obtusifolin (21.9 mg), respectively. The structural difference between these molecules is the position of the pyrone group—in lepidissipyrone this group is linked at C-6, while this group is attached to C-8 in obtusifolin. The compound with an RT of 10.5 min was identified as the polyketide helipyrone (65.2 mg), which contains the same pyrone unit as the flavanones (Fig. 1) (NMR data see S1 File).
Fig 1. Chemical structures of the main identified compounds.
Extract standardization and analytical method validation
A standardized A. alata extract for use against S. mutans must contain at least 14 ± 5% of gnaphaliin and at least 20 ± 4% of helipyrone. This profile was established based on the HExt-EP analyses (the fraction with the best activity against S. mutans). The specificity was confirmed via UV peak purity calculations for gnaphaliin and helipyrone in the extract and isolate. The corresponding linear regression founded had an R of 0.9998 for both of the isolated compounds. The range maintained a coefficient of variation below 5% and the accuracy was acceptable with coefficients of variation below 5%.
Variables screening by factorial design
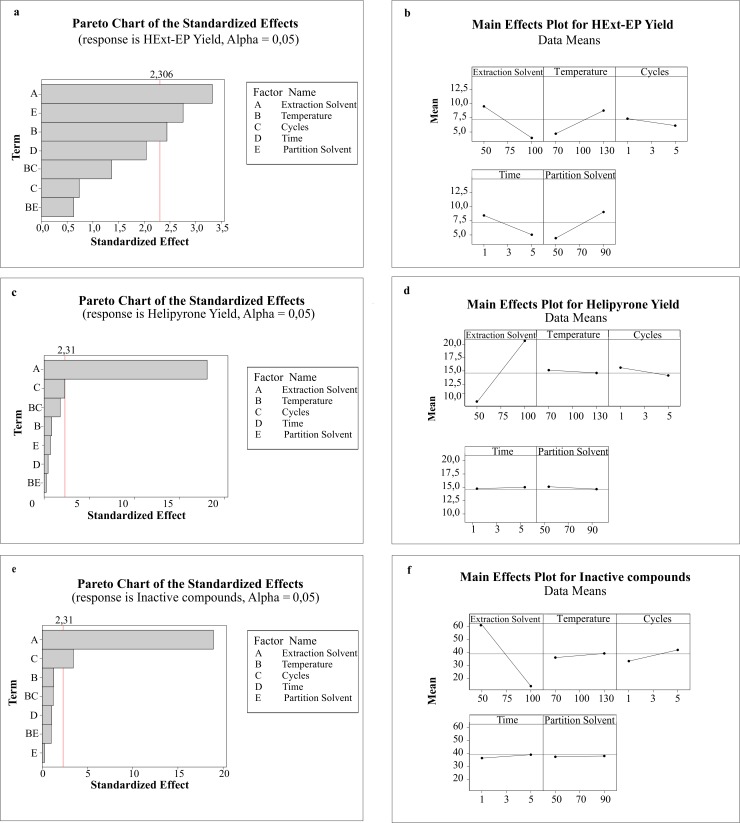
Of the five analyzed factors, the following were significant to the extract yield: the extractor solvent, temperature and ethanol percentage during partitioning, as shown on the Pareto chart of standardized effects (Fig. 2A). The effects plot figure (Fig. 2B) shows a negative influence of hexane (−5.55) on the extract yield. Increasing the temperature and ethanol percentage in the partition also positively contributes to the yield.
Fig 2. Pareto Chart of the standardized effects and main effects plot for HExt-EP yield (A and B), helipyrone yield (C and D) and inactive compound yield (E and F).
The Pareto chart of standardized effects in response to the active compounds indicated the solvent is the only significant parameter (Fig. 2C). However, this effect is opposite that found for the HExt-EP yield (Fig. 2D). Consequently, HExt-EP yield is was favored by acetone in the extractor solvent, while helipyrone and other active compounds yield require more hexane.
The third analyzed response was the inactive metabolites. In the Pareto chart of standardized effects (Fig. 2E), the most significant factors were the extractor solvent and cycle. Fig. 2F shows that the extractor solvent had a negative effect (−47.32), which proves increasing the percent acetone and cycles increases the inactive product yield.
The best parameters for increasing the yield and selectivity for the compounds of interest are 100% hexane as the extractor solvent, 130°C, one cycle and 90% ethanol partition. The time was not a statistically significant parameter.
Temperature and solvent optimization via the RSM experimental design
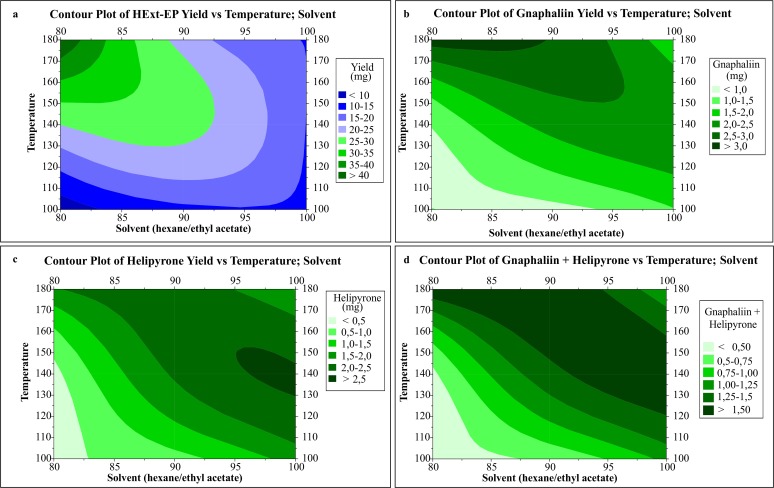
While optimizing the temperature and solvent via the RSM, the solvent extractor was changed from acetone to ethyl acetate because of its lower relative polarity and due to a sealing ring in the ASE extractor cell that does not support acetone at temperatures above 130°C. The maximum percentages of compounds were calculated from these results both alone and together (Fig. 3).
Fig 3. Contour plot of responses analysied on during the RSM experiment for HExt-EP yield (A), Gnaphaliin yield (B), Helipyrone yield (C) and the sum of gnaphaliin and helipyrone (D).
The HExt-EP (Fig. 3A) had the best yield when the temperature and solvent were 180°C and 80% hexane, respectively. This result was expected based on the responses previously observed during the factorial design, where higher temperatures and more polar extractor solvents increased the final extract yield.
The Fig. 3B shows the gnaphaliin yield, which resembling more to HExt-EP optimum yield. The combination of high temperatures and polar solvents tends to extract more polar compounds. Fig. 3C shows the ideal helipyrone region was between 95–100% hexane with a temperatures range from 130 to 150°C.
Fig. 3D shows the total gnaphaliin and helipyrone yield, and the darker region indicates where the maximum yields of both compounds can be obtained. Therefore, the final method for maximizing the yield and selectivity of the active compounds was 95% hexane as the extractor solvent, 150°C, one cycle, 1 minute of static time and at 90% ethanol partition.
Scale-up and exhaustive extraction of active chemicals
The present work established the ideal production conditions for a standardized extract with a maximum yield, selectivity and activity against S. mutans. The exhaustive extraction and scale-up analysis proves the optimal parameters adopted by the final methodology founded on the chemometric analysis. The first extraction yielded 98.83% of gnaphaliin and 99.05% of helipyrone. The scale-up, changing the cell by 100 mL cell, provided on the first extraction 94% of yield for both substances monitored (Table 1).
Table 1. Gnaphaliin and Helipyrone yield in successive extractions with the optimized method and in scale-up.
| 5ml Cell (0.6g) | Gnaphaliin yield (%) | Helipyrone yield (%) |
|---|---|---|
| Extraction 1 | 98.83 | 99.05 |
| Extraction 2 | 0.96 | 0.72 |
| Extraction 3 | 0.19 | 0.22 |
| 100ml Cell (5g) | ||
| Extraction 1 | 94.29 | 94.18 |
| Extraction 2 | 2.74 | 2.85 |
| Extraction 3 | 2.95 | 2.96 |
Hydrogen peroxide and NO production by peritoneal murine macrophages
The EExt did not change the H2O2 produced by the macrophages or PMA and potentiated NO liberation due to LPS stimulation at 0.16 mg/mL (Table 2). The HExt-EP extract potentiates H2O2 liberation at 0.16 mg/mL but only in the presence of PMA. The NO liberation concentrations of 0.16 mg/mL and 0.8 mg/mL indicate the effect of LPS on the stimulated macrophage. The optimized extract HExt-EP was not cytotoxic to the cells during the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay, where EExt 4mg/mL shows 19% of viable; 0.8 mg/mL (44%); 0.16 mg/mL (91%) and for HExt-EP 4 mg/mL (90%); 0.8 mg/mL (91%); 0.16 mg/mL (90%).
Table 2. Effect of the EExt and HExt-EP extracts on hydrogen peroxide (H2O2) production and nitric oxide (NO) production by mouse peritoneal macrophages.
| Treatment | μM H2O2 | Treatment | μM NO |
|---|---|---|---|
| Macrophages (MF) MF + PMA 50ng | 0.6 ± 0.37 30.10 ± 6.23 * | Macrophages (MF) MF + LPS 1μg/mL | 5.27±0.01 16.79±0.04** |
| Eext | |||
| MF + 0.16mg/mL | 0.51 ± 0.51 | MF + 0.16mg/mL | 5.92± 3.03 |
| MF + 0.8mg/mL | 0.51 ± 0.51 | MF + 0.8mg/mL | 3.85± 0.76 |
| MF + 4mg/mL | 10.65 ± 2.30 | MF + 4mg/mL | 4.40±0.98 |
| MF + PMA + 0.16mg/mL | 21.60 ± 5.73 | MF + LPS + 0.16mg/mL | 24.67±3.56** |
| MF + PMA + 0.8mg/mL | 24.60 ± 3.95 | MF + LPS + 0.8mg/mL | 7.58±3.62 |
| MF + PMA + 4mg/mL | 39.65 ± 6.65 | MF + LPS + 4mg/mL | 5.70±1.63 |
| HExt-EP | |||
| MF + 0.16mg/mL | 4.81 ± 1.64 | MF + 0.16mg/mL | 14.69±8.13 |
| MF + 0.8mg/mL | 3.41 ± 1.92 | MF + 0.8mg/mL | 9.93±5.75 |
| MF + 4mg/mL | 0 ± 0 | MF + 4mg/mL | 6.37±1.11 |
| MF + PMA + 0.16mg/mL | 62.46 ± 1.94 * | MF + LPS + 0.16mg/mL | 39.18±0.76** |
| MF + PMA + 0,8mg/mL | 38.20 ± 3.82 | MF + LPS + 0.8mg/mL | 43.52±0.11** |
| MF + PMA + 4mg/mL | 30.70 ± 4.16 | MF + LPS + 4mg/mL | 24.12±3.25 |
n = 3 performed in triplicate;
* P<0.05 compared to macrophages (MF).
** P<0.05 compared to macrophages estimulated with LPS. ANOVA and Bonferroni’s test. PMA (phorbol myristate acetate); lipopolysaccharide (LPS); Macrophages (MF).
Antioxidant assay
The DPPH assay indicates that EExt (IC50 = 19.8) and quercetin (IC50 = 3.4) shows good antioxidant activity, while the standardized HExt-EP did not has the same potential (IC50 = 609) (S3 Table).
Chemical profiles of HExt-EP, STExt, TExt and IExt extracts from A. alata and HExt-EPsat from A. satureioides
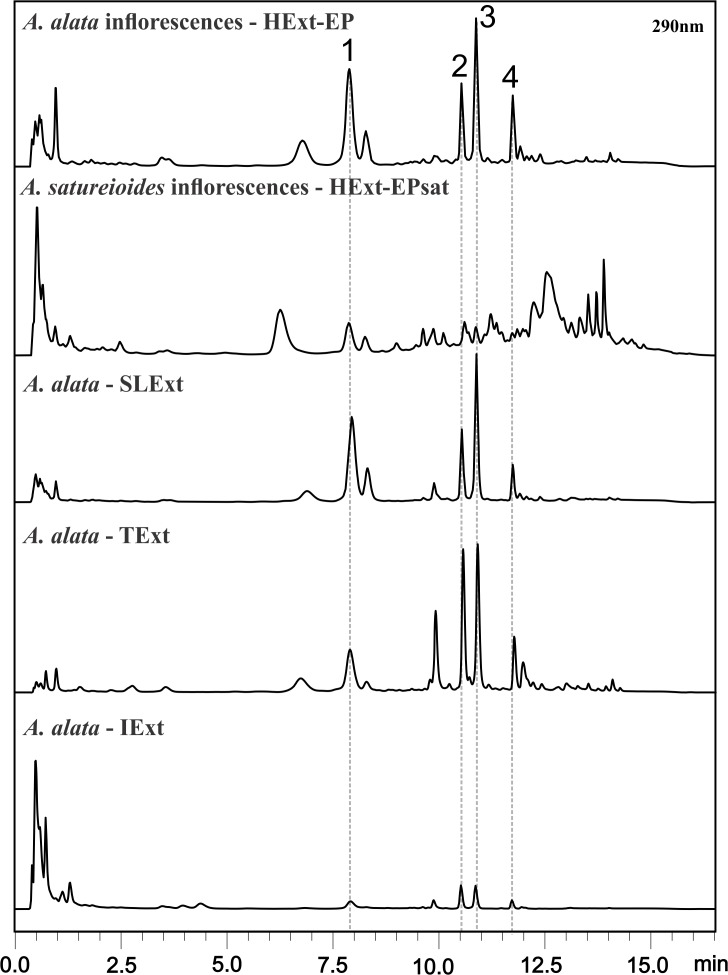
HExt-EP, TExt and STExt had similar chemical profiles. The 4 compounds identified in this study are also present in IExt, although they only consist of 4% of the extract. A. satureioides shows a different and more complex chemical profile than A. alata.
Discussion
The results demonstrated the efficacy of accelerated solvent extraction with the aid of chemometric calculations and bioactivity assays for obtaining an A. alata optimized extract with potent antimicrobial activity.
Chlorhexidine exhibits a MIC of 2.7 to 80 μg/mL [19] for different types of bacteria and for S. mutans (UA159) was founded a MIC of 1.25 μg/mL [20]. Other compounds used in oral products like triclosan has a MIC ranging 0.1–20 μg/mL for some strains of S. mutans [21] and HExt-EP had a MIC of 62.5 μg/mL. Palombo [21] stated that vegetable extracts with MIC above 100 μg/mL has potential to develop products with oral applications. And the present results demonstrates the potential usefulness of HExt-EP against S. mutans. Other bacteria must be tested for new applications of the optimized extract.
The four main components in HExt-EP constituted approximately 44% of the extract. The flavonoid gnaphaliin was characterized as being antifungal against Botrytis cinerea [22], reduced both edema and leukocytes infiltration [23], which modulated the anti-inflammatory activity, and inhibited human low-density lipoproteins [24]. In present work, three new compounds were characterized from this genus: the flavanones obtusifolin and lepidissipyrone and the polyketide helipyrone. Obtusifolin was first described by Hänsel et al. [25] while studying Gnaphalium obtusifolium (Asteraceae), and lepidissipyrone was identified in the genus Helichrysum (Asteraceae) [26]. Helipyrone was first found in Helichrysum italicum [27].
Flavonoid prenylations or substitutions at the C-6 and C-8 positions may increase the antimicrobial activity [28] by rendering the molecule less polar and enhancing the bacterial penetration. Tsuchiya and Iinuma [29] found the antimicrobial mechanism for this molecule type/class could be involved in reducing the membrane fluidity of gram-positive bacteria.
Helipyrone polyketide exhibited an MIC of 6 μg/mL against Bacillus subtilis, Staphylococcus aureus, S. epidermidis (gram-positives) and the yeast Mycobacterium phlei and an MIC of 100 μg/mL for the gram-negative bacterias Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Salmonella typhimurium [30]. The selectivity for gram-positive bacteria supports the importance of helipyrone to HExt-EP and its monitoring when optimizing the extraction for use against S. mutans. Studies utilizing bioactivity assays as a tool for standardization are successful because they can find better parameters to improve the yield of interesting compounds. Herrero et al [31] show that this method is not only applicable to plants. These authors use a factorial design and accelerated solvent extraction to monitor antioxidants compounds extracted from the microalga Spirulina platensis.
The hydrogen peroxide/NO production by peritoneal murine macrophages shows an atypical result for HExt-EP, where at higher concentrations induces a decreasing response (Table 2). The literature has reported several compounds that induce a biphasic response, like the agonist agent PMA [32], LPS [33,34], garlic extract [35], IL-1 [36] and others [37]; and in all these researches, increasing the dose of stimulators a decreasing effect is observed. Therefore, HExt-EP may contain compounds that induce this response. In addition, the extracts concentration ranged, keeping the stimulus concentration uniform and this must contribute to biphasic response. When this occurs may, perhaps, exist a synergic effect between the via involved with counterregulation response and the system protects itself against cytotoxic agents.
The antibacterial activity of HExt-EP can be better understood using the results from the hydrogen peroxide/NO production of peritoneal murine macrophages and antioxidant assays. Increasing the reactive oxygen species added with lower antioxidant activity suggests that HExt-EP may act in vivo directly as a bacteriostatic and indirectly by stimulating the immunologic responses of the macrophages [38, 39]. These results are complementary considering the necessity of immune responses for eliminating pathogens via bacteriostatic agents [40].
The standardized HExt-EP was more effective for eliminating S. mutans than the IExt, which had 4% of the identified compounds versus 44% for HExt-EP. Moreover, the compounds identified in this study represent 0.4% of the dry plant weight in IExt, whereas they constitute 0.9% of the dry plant weight in HExt-EP. In addition to the improved selectivity, the HExt-EP extracted twice as many compounds of the infuse extraction, reduced the antioxidant activity and stimulated the macrophages to produce reactive oxygen species, which are important to the immune response against bacteria [41].
Comparing the results with Toffoli-Kadri et al. [42] evidences the importance of selective extraction for a specific activity apparent. The hydromethanolic extract from A. alata, which has a similar chemical profile to EExt (data not shown), inhibited the reactive oxygen species and has potent antioxidant activity [43]. Thus, the standardizing the extracts for biological purposes may ensure their therapeutic success considering using different extraction methods on the same drug vegetable may generate distinct chemical profiles and even opposite biological responses.
A comparison of HExt-EP from A. alata and HExt-EPsat from A. satureioides shows distinct chemical profiles. The infusion of A. alata inflorescences has been used as substitute of A. satureioides to treat infections and inflammations and A. satureioides was used to evaluate the interchangeable of the species for the optimized extraction method. A. satureioides has a complex chemical profile, while A. alata shows only four major compounds (Fig. 4), which differ in similarity between their polar compounds [10]. This extraction can be used to effectively identify these species.
Fig 4. Chromatograms HPLC-DAD of HExt-EP, HExt-EPsat, SLExt, TExt and HExt at 290 nm. The compounds Gnaphaliin, Lepidissipyrone, Helipyrone e Obtusifolin is indicated by the numbers 1, 2, 3 and 4, respectively.
The MTT assay is essential for evaluating the cellular response against different treatment parameters because cell viability below 80% is able to effectively respond to a specific stimulus and leads to differentiation in inflammatory macrophages [44]. The optimized extract HExt-EP proves the cell viability of HExt-EP at all concentrations. The use ASE extractor coupled with chemometric calculations was effectively to optimized yield, selectivity, antimicrobial activity and allows obtaining a safety extract compared to the EExt, which has a chemical profile similar to the forms with ethnopharmacological use.
Conclusions
ASE and chemometric calculations allowed us to obtain a standardized extract of gnaphaliin and helipyrone from A. alata. This extract has bacteriostatic properties and stimulates reactive oxygen species/nitric oxide, which is important to microbial and immunological responses. Relative to other extractive methodologies, such as infusion, the developed method was exhaustive and increased the concentrations for the compounds of interest more than eleven times. Furthermore, the chemical profiles of the different plant parts have similar compositions and differ from the A. satureioides substitute. This extract was not cytotoxic and has potential for use in oral hygiene product development, but requires pre-formulation studies.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors express their gratitude to Prof. Dr. Norberto Peporine Lopes for his collaboration in this research.
Data Availability
All relevant data from the Ethics Committee on Animal Experimentation of the UFMS (CEUA/UFMS n°202/2009) are available in the paper and its Supporting Information files.
Funding Statement
The authors express their gratitude to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) and Instituto Nacional de Áreas Úmidas (INAU) for their support in carrying out the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Raynie DE. Modern Extraction Techniques. Analytical Chemistry. 2010; 82: 4911–4916. 10.1021/ac101223c [DOI] [PubMed] [Google Scholar]
- 2. Vilkhu K, Mawson R, Simons L, Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innovative Food Science & Emerging Technologies. 2008; 9: 161–169. [Google Scholar]
- 3. Gao FY, Hu YS, Ye XL, Li J, Chen Z, Fan G. Optimal extraction and fingerprint analysis of Cnidii fructus by accelerated solvent extraction and high performance liquid chromatographic analysis with photodiode array and mass spectrometry detections. Food Chemistry. 2013; 141: 1962–1971. 10.1016/j.foodchem.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 4. Zhao LC, He Y, Deng X, Yang GL, Li W, Liang J, et al. Response Surface Modeling and Optimization of Accelerated Solvent Extraction of Four Lignans from Fructus Schisandrae . Molecules. 2012; 17: 3618–3629. 10.3390/molecules17043618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richter BE, Jones BA, Ezzell JL, Porter NL, Avdalovic N, Pohl C. Accelerated solvent extraction: A technique for sample preparation. Analytical Chemistry. 1996; 68: 1033–1039. [Google Scholar]
- 6. Hossain MB, Barry-Ryan C, Martin-Diana AB, Brunton NP. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chemistry. 2011; 126: 339–346. [Google Scholar]
- 7. Zhou J, Zheng XX, Yang Q, Liang ZY, Li DH, Yang XB, et al. Optimization of Ultrasonic-Assisted Extraction and Radical-Scavenging Capacity of Phenols and Flavonoids from Clerodendrum cyrtophyllum Turcz Leaves. Plos One. 2013; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pezzuto JM. Plant-derived anticancer agents. Biochemical Pharmacology. 1997; 53: 121–133. [DOI] [PubMed] [Google Scholar]
- 9. de Souza GC, Haas APS, von Poser GL, Schapoval EES, Elisabetsky E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. Journal of Ethnopharmacology. 2004;90: 135–143. [DOI] [PubMed] [Google Scholar]
- 10. Grassi-Zampieron R, França LV, Carollo CA, Vieira MdC, Oliveros-Bastidas A, de Siqueira JM. Comparative profiles of Achyrocline alata (Kunth) DC. and A. satureioides (Lam.) DC., Asteraceae, applying HPLC-DAD-MS. Revista Brasileira de Farmacognosia. 2010; 20: 575–579. [Google Scholar]
- 11. Bohlmann F, Abraham WR, Robinson H, King RM. Naturally-Occurring Terpene Derivatives .290. A New Labdane Derivative and Geranylphloroglucinols from Achyrocline alata . Phytochemistry. 1980; 19: 2475–2477. [Google Scholar]
- 12. Schorr K, Da Costa FB. Quantitative determination of enhydrin in leaf rinse extracts and in glandular trichomes of Smallanthus sonchifolius (Asteraceae) by reversed-phase high-performance liquid chromatography. Phytochemical Analysis. 2005; 16: 161–165. [DOI] [PubMed] [Google Scholar]
- 13.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 2003; Document M7-A6.
- 14.ICH-4. International Conference on the Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Q2B. Validation of analytical procedures: Methodology; 1994.
- 15. Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. Journal of Immunological Methods. 1980; 38: 161–170. [DOI] [PubMed] [Google Scholar]
- 16. Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988; 141: 2407–2412. [PubMed] [Google Scholar]
- 17. Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival—Application to Proliferation and Cyto-Toxicity Assays. Journal of Immunological Methods. 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 18. Herald TJ, Gadgil P, Tilley A. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. Journal of the Science of Food and Agriculture. 2012; 92: 2326–2331. 10.1002/jsfa.5633 [DOI] [PubMed] [Google Scholar]
- 19. Amorim CVGd, Aun CE, Mayer MPA. Susceptibility of some oral microorganisms to chlorhexidine and paramonochlorophenol. Brazilian Oral Research. 2004; 18: 242–246. [DOI] [PubMed] [Google Scholar]
- 20. Liu J, Ling JQ, Zhang K, Huo LJ, Ning Y. Effect of Sodium Fluoride, Ampicillin, and Chlorhexidine on Streptococcus mutans Biofilm Detachment. Antimicrobial Agents and Chemotherapy. 2012; 56: 4532–4535. 10.1128/AAC.00885-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palombo EA. Traditional Medicinal Plant Extracts and Natural Products with Activity against Oral Bacteria: Potential Application in the Prevention and Treatment of Oral Diseases. Evidence-Based Complementary and Alternative Medicine. 2011; 1–15. [DOI] [PMC free article] [PubMed]
- 22. Cotoras M, Mendoza L, Munoz A, Yanez K, Castro P, Aguirre M. Fungitoxicity against Botrytis cinerea of a Flavonoid Isolated from Pseudognaphalium robustum. Molecules. 2011; 16: 3885–3895. [Google Scholar]
- 23. Sala A, Recio MC, Schinella GR, Manez S, Giner RM, Cerdá-Nicolás M, et al. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. European Journal of Pharmacology. 2003; 461: 53–61. [DOI] [PubMed] [Google Scholar]
- 24. Aiyegoro OA, Okoh AI. Phytochemical Screening and Polyphenolic Antioxidant Activity of Aqueous Crude Leaf Extract of Helichrysum pedunculatum . International Journal of Molecular Sciences. 2009; 10: 4990–5001. 10.3390/ijms10114990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansel R, Ohlendor D, Pelter A. Obtusifolin, a Flavonone with a Biogenetically Unusual C9-Unit. Zeitschrift Fur Naturforschung Part B-Chemie Biochemie Biophysik Biologie Und Verwandten Gebiete B. 1970; 25: 989. [PubMed] [Google Scholar]
- 26. Jakupovic J, Zdero C, Grenz M, Tsichritzis F, Lehmann L, Hashemi-Nejad SM, et al. 21 Acylphloroglucinol Derivatives and Further Constituents from South-African Helichrysum Species. Phytochemistry.1989; 28: 1119–1131. [Google Scholar]
- 27. Opitz L, Hansel R. Helipyrone, Methylene-Bis-Triacetic Acid Lactone from Helichrysum italicum . Tetrahedron Letters. 1970; 3369. [Google Scholar]
- 28. Sohn HY, Son KH, Kwon CS, Kwon GS, Kang SS. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine. 2004; 11: 666–672. [DOI] [PubMed] [Google Scholar]
- 29. Tsuchiya H, Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua . Phytomedicine. 2000; 7: 161–165. [DOI] [PubMed] [Google Scholar]
- 30. Rios JL, Recio MC, Villar A. Isolation and Identification of the Antibacterial Compounds from Helichrysum stoechas . Journal of Ethnopharmacology. 1991; 33: 51–55. [DOI] [PubMed] [Google Scholar]
- 31. Herrero M, Martin-Alvarez PJ, Senorans FJ, Cifuentes A, Ibanez E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chemistry. 2005; 93: 417–423. [Google Scholar]
- 32. Nothnick WB, Soloway PD. Novel implications in the development of endometriosis: Biphasic effect of macrophage activation on peritoneal tissue expression of tissue inhibitor of metalloproteinase-1. American Journal of Reproductive Immunology. 1998; 40: 364–369. [DOI] [PubMed] [Google Scholar]
- 33. O'Flaherty JT, Jacobson DP, Redman JF. Bidirectional effects of protein kinase C activators. Studies with human neutrophils and platelet-activating factor. Journal of Biological Chemistry. 1989; 264: 6836–6843. [PubMed] [Google Scholar]
- 34. O'Flaherty JT, Redman JF, Jacobson DP. Mechanisms involved in the bidirectional effects of protein kinase C activators on neutrophil responses to leukotriene B4. J Immunol.1990; 144: 1909–1913. [PubMed] [Google Scholar]
- 35. Adedapo AA, Jimoh FO, Koduru S, Masika PJ, Afolayan AJ. Assessment of the medicinal potentials of the methanol extracts of the leaves and stems of Buddleja saligna. Bmc Complementary and Alternative Medicine. 2009; 9: 21 10.1186/1472-6882-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Linner KM, Nicol SE, Sharp BM. IL-1 beta modulates the concanavalin-A-induced expression of proenkephalin A mRNA in murine thymocytes. J Pharmacol Exp Ther. 1993; 267: 1566–1572. [PubMed] [Google Scholar]
- 37. Calabrese EJ. Hormetic dose-response relationships in immunology: Occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Critical Reviews in Toxicology. 2005; 35: 89–295. [DOI] [PubMed] [Google Scholar]
- 38. Lopes FCM, Calvo TR, Colombo LL, Vilegas W, Carlos IZ. Immunostimulatory and cytotoxic activities of Indigofera suffruticosa (Fabaceae). Natural Product Research. 2011; 25: 1796–1806. 10.1080/14786419.2010.488624 [DOI] [PubMed] [Google Scholar]
- 39. Carlos IZ, Carli CBA, Maia DCG, Benzatti FP, Lopes FCM, Roese FM, et al. Immunostimulatory effects of the phenolic compounds from lichens on nitric oxide and hydrogen peroxide production. Revista Brasileira de Farmacognosia. 2009; 19: 847–852. [Google Scholar]
- 40. Czaika VA, Siebenbrock J, Czekalla F, Zuberbier T, Sieber MA. Reactive oxygen species and the bacteriostatic and bactericidal effects of isoconazole nitrate. Mycoses. 2013; 56: 16–22. 10.1111/myc.12055 [DOI] [PubMed] [Google Scholar]
- 41. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clinical Infectious Diseases. 2004; 38: 864–870. [DOI] [PubMed] [Google Scholar]
- 42. Toffoli-Kadri MC, Carollo CA, Lourenço LD, Felipe JL, Néspoli JHB, Wollf LG, et al. In vivo and in vitro anti-inflammatory properties of Achyrocline alata (Kunth) DC. Journal of Ethnopharmacology. 2014; 153: 461–468. 10.1016/j.jep.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 43. Grassi-Zampieron RF, Vieira MC, Siqueira JMd. Atividade antioxidante e captora de radicais livres dos extratos de Achyrocline alata (Kunth.) DC. em comparação com extratos de Achyrocline satureioides (Lam.) DC. Revista Brasileira de Farmacognosia. 2009; 19: 572–576. [Google Scholar]
- 44. Adams DO, Hamilton TA. The Cell Biology of Macrophage Activation. Annual Review of Immunology. 1984; 2: 283–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data from the Ethics Committee on Animal Experimentation of the UFMS (CEUA/UFMS n°202/2009) are available in the paper and its Supporting Information files.