Abstract
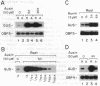
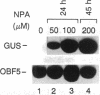
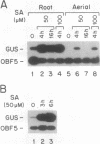
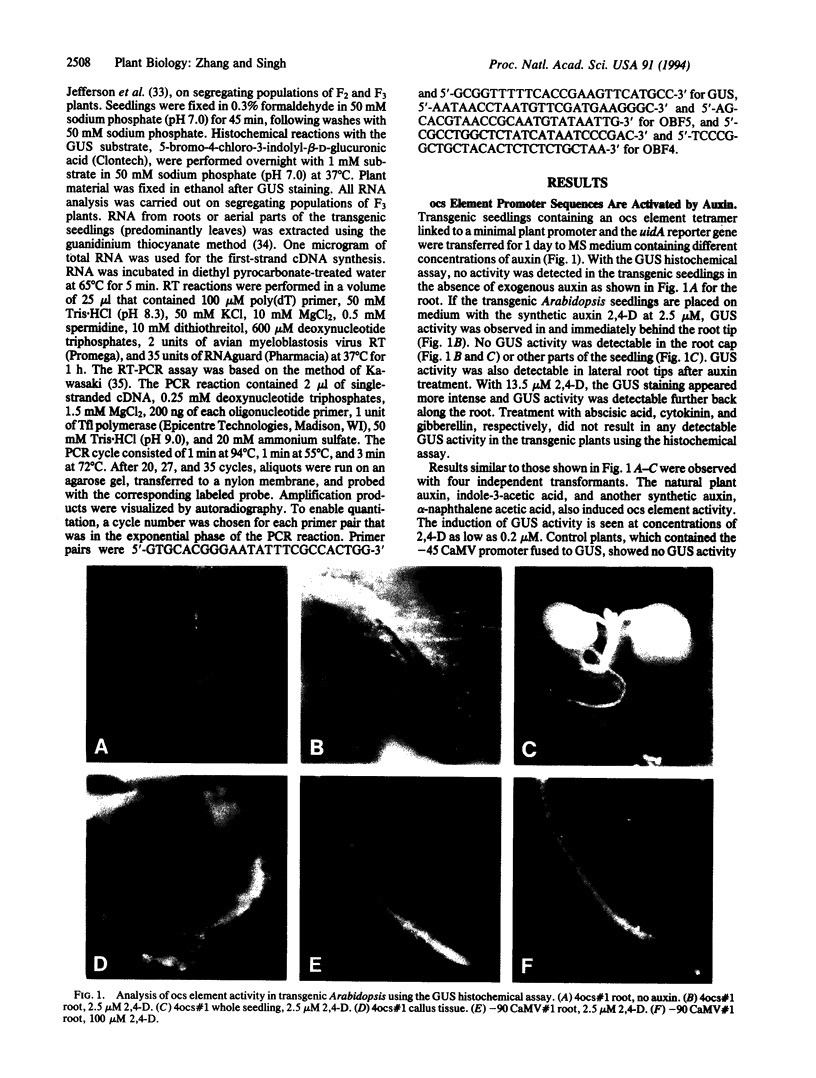
ocs elements are a group of promoter elements that have been exploited by two distinct groups of plant pathogens, Agrobacterium and certain viruses, to express genes in plants. We examined the activity of single and multiple ocs elements linked to a minimal plant promoter and the uidA reporter gene in transgenic Arabidopsis. beta-Glucuronidase activity was detected only in root tips and in callus tissue after auxin treatment. A more sensitive assay revealed that auxin treatment also increased ocs element activity in aerial parts of the plant, although the absolute levels of ocs element activity were greater in roots. The response of ocs elements to exogenous auxin began within 1 h. Salicylic acid, a disease-resistance signal in plants, also increased ocs element activity in both roots and aerial parts of the plant. The question of whether the induction in ocs element activity is mediated through auxin and/or salicylic acid signal transduction pathways or is part of a more general stress response is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Costa M. A., Ha S. B. Nopaline synthase promoter is wound inducible and auxin inducible. Plant Cell. 1990 Mar;2(3):225–233. doi: 10.1105/tpc.2.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P. N., Ren L., Chua N. H. The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J. 1989 Aug;8(8):2195–2202. doi: 10.1002/j.1460-2075.1989.tb08342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang J., Macdonald H., King P. J., Sturm A. A soluble auxin-binding protein from Hyoscyamus muticus is a glutathione S-transferase. Plant Physiol. 1993 May;102(1):29–34. doi: 10.1104/pp.102.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot KJM., Van Der Zaal B. J., Velterop J., Quint A., Mennes A. M., Hooykaas PJJ., Libbenga K. R. Further Characterization of Expression of Auxin-Induced Genes in Tobacco (Nicotiana tabacum) Cell-Suspension Cultures. Plant Physiol. 1993 Jun;102(2):513–520. doi: 10.1104/pp.102.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchez D., Tokuhisa J. G., Llewellyn D. J., Dennis E. S., Ellis J. G. The ocs-element is a component of the promoters of several T-DNA and plant viral genes. EMBO J. 1989 Dec 20;8(13):4197–4204. doi: 10.1002/j.1460-2075.1989.tb08605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecka E., Nagao R. T., Key J. L., Gurley W. B. Characterization of Gmhsp26-A, a stress gene encoding a divergent heat shock protein of soybean: heavy-metal-induced inhibition of intron processing. Mol Cell Biol. 1988 Mar;8(3):1113–1122. doi: 10.1128/mcb.8.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V. Glutathione S-transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28(3):173–207. doi: 10.3109/10409239309086794. [DOI] [PubMed] [Google Scholar]
- Dominov J. A., Stenzler L., Lee S., Schwarz J. J., Leisner S., Howell S. H. Cytokinins and auxins control the expression of a gene in Nicotiana plumbaginifolia cells by feedback regulation. Plant Cell. 1992 Apr;4(4):451–461. doi: 10.1105/tpc.4.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droog F. N., Hooykaas P. J., Libbenga K. R., van der Zaal E. J. Proteins encoded by an auxin-regulated gene family of tobacco share limited but significant homology with glutathione S-transferases and one member indeed shows in vitro GST activity. Plant Mol Biol. 1993 Mar;21(6):965–972. doi: 10.1007/BF00023595. [DOI] [PubMed] [Google Scholar]
- Dudler R., Hertig C., Rebmann G., Bull J., Mauch F. A pathogen-induced wheat gene encodes a protein homologous to glutathione-S-transferases. Mol Plant Microbe Interact. 1991 Jan-Feb;4(1):14–18. doi: 10.1094/mpmi-4-014. [DOI] [PubMed] [Google Scholar]
- Ellis J. G., Tokuhisa J. G., Llewellyn D. J., Bouchez D., Singh K., Dennis E. S., Peacock W. J. Does the ocs-element occur as a functional component of the promoters of plant genes? Plant J. 1993 Sep;4(3):433–443. doi: 10.1046/j.1365-313x.1993.04030433.x. [DOI] [PubMed] [Google Scholar]
- Enyedi A. J., Yalpani N., Silverman P., Raskin I. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2480–2484. doi: 10.1073/pnas.89.6.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley R. C., Grossman C., Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J., Singh K. B. Isolation of a maize bZIP protein subfamily: candidates for the ocs-element transcription factor. Plant J. 1993 May;3(5):669–679. [PubMed] [Google Scholar]
- Franco A. R., Gee M. A., Guilfoyle T. J. Induction and superinduction of auxin-responsive mRNAs with auxin and protein synthesis inhibitors. J Biol Chem. 1990 Sep 15;265(26):15845–15849. [PubMed] [Google Scholar]
- Fromm H., Katagiri F., Chua N. H. An octopine synthase enhancer element directs tissue-specific expression and binds ASF-1, a factor from tobacco nuclear extracts. Plant Cell. 1989 Oct;1(10):977–984. doi: 10.1105/tpc.1.10.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm H., Katagiri F., Chua N. H. The tobacco transcription activator TGA1a binds to a sequence in the 5' upstream region of a gene encoding a TGA1a-related protein. Mol Gen Genet. 1991 Oct;229(2):181–188. doi: 10.1007/BF00272154. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B. Crown gall disease and hairy root disease : a sledgehammer and a tackhammer. Plant Physiol. 1990 Feb;92(2):281–285. doi: 10.1104/pp.92.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988 Jul 15;241(4863):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F., Lam E., Chua N. H. Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature. 1989 Aug 31;340(6236):727–730. doi: 10.1038/340727a0. [DOI] [PubMed] [Google Scholar]
- Kawata T., Imada T., Shiraishi H., Okada K., Shimura Y., Iwabuchi M. A cDNA clone encoding HBP-1b homologue in Arabidopsis thaliana. Nucleic Acids Res. 1992 Mar 11;20(5):1141–1141. doi: 10.1093/nar/20.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. R., Kim Y., An G. Identification of methyl jasmonate and salicylic acid response elements from the nopaline synthase (nos) promoter. Plant Physiol. 1993 Sep;103(1):97–103. doi: 10.1104/pp.103.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononowicz H., Wang Y. E., Habeck L. L., Gelvin S. B. Subdomains of the octopine synthase upstream activating element direct cell-specific expression in transgenic tobacco plants. Plant Cell. 1992 Jan;4(1):17–27. doi: 10.1105/tpc.4.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Benfey P. N., Gilmartin P. M., Fang R. X., Chua N. H. Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7890–7894. doi: 10.1073/pnas.86.20.7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Langridge W. H., Fitzgerald K. J., Koncz C., Schell J., Szalay A. A. Dual promoter of Agrobacterium tumefaciens mannopine synthase genes is regulated by plant growth hormones. Proc Natl Acad Sci U S A. 1989 May;86(9):3219–3223. doi: 10.1073/pnas.86.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J., Carr J. P., Klessig D. F., Raskin I. Salicylic Acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990 Nov 16;250(4983):1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Schindler U., Beckmann H., Cashmore A. R. TGA1 and G-box binding factors: two distinct classes of Arabidopsis leucine zipper proteins compete for the G-box-like element TGACGTGG. Plant Cell. 1992 Oct;4(10):1309–1319. doi: 10.1105/tpc.4.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Dennis E. S., Ellis J. G., Llewellyn D. J., Tokuhisa J. G., Wahleithner J. A., Peacock W. J. OCSBF-1, a maize ocs enhancer binding factor: isolation and expression during development. Plant Cell. 1990 Sep;2(9):891–903. doi: 10.1105/tpc.2.9.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Tokuhisa J. G., Dennis E. S., Peacock W. J. Saturation mutagenesis of the octopine synthase enhancer: correlation of mutant phenotypes with binding of a nuclear protein factor. Proc Natl Acad Sci U S A. 1989 May;86(10):3733–3737. doi: 10.1073/pnas.86.10.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Nakayama T., Mikami K., Iwabuchi M. HBP-1a and HBP-1b: leucine zipper-type transcription factors of wheat. EMBO J. 1991 Jun;10(6):1459–1467. doi: 10.1002/j.1460-2075.1991.tb07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Nagata T. parB: an auxin-regulated gene encoding glutathione S-transferase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):56–59. doi: 10.1073/pnas.89.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Niwa Y., Machida Y., Nagata T. Location of the cis-acting auxin-responsive region in the promoter of the par gene from tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8013–8016. doi: 10.1073/pnas.87.20.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhisa J. G., Singh K., Dennis E. S., Peacock W. J. A DNA-binding protein factor recognizes two binding domains within the octopine synthase enhancer element. Plant Cell. 1990 Mar;2(3):215–224. doi: 10.1105/tpc.2.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida S., Sato K. Glutathione transferases and cancer. Crit Rev Biochem Mol Biol. 1992;27(4-5):337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- Valvekens D., Van Montagu M., Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Foley R. C., Singh K. B. Isolation and characterization of two related Arabidopsis ocs-element bZIP binding proteins. Plant J. 1993 Oct;4(4):711–716. doi: 10.1046/j.1365-313x.1993.04040711.x. [DOI] [PubMed] [Google Scholar]
- van der Zaal E. J., Droog F. N., Boot C. J., Hensgens L. A., Hoge J. H., Schilperoort R. A., Libbenga K. R. Promoters of auxin-induced genes from tobacco can lead to auxin-inducible and root tip-specific expression. Plant Mol Biol. 1991 Jun;16(6):983–998. doi: 10.1007/BF00016071. [DOI] [PubMed] [Google Scholar]