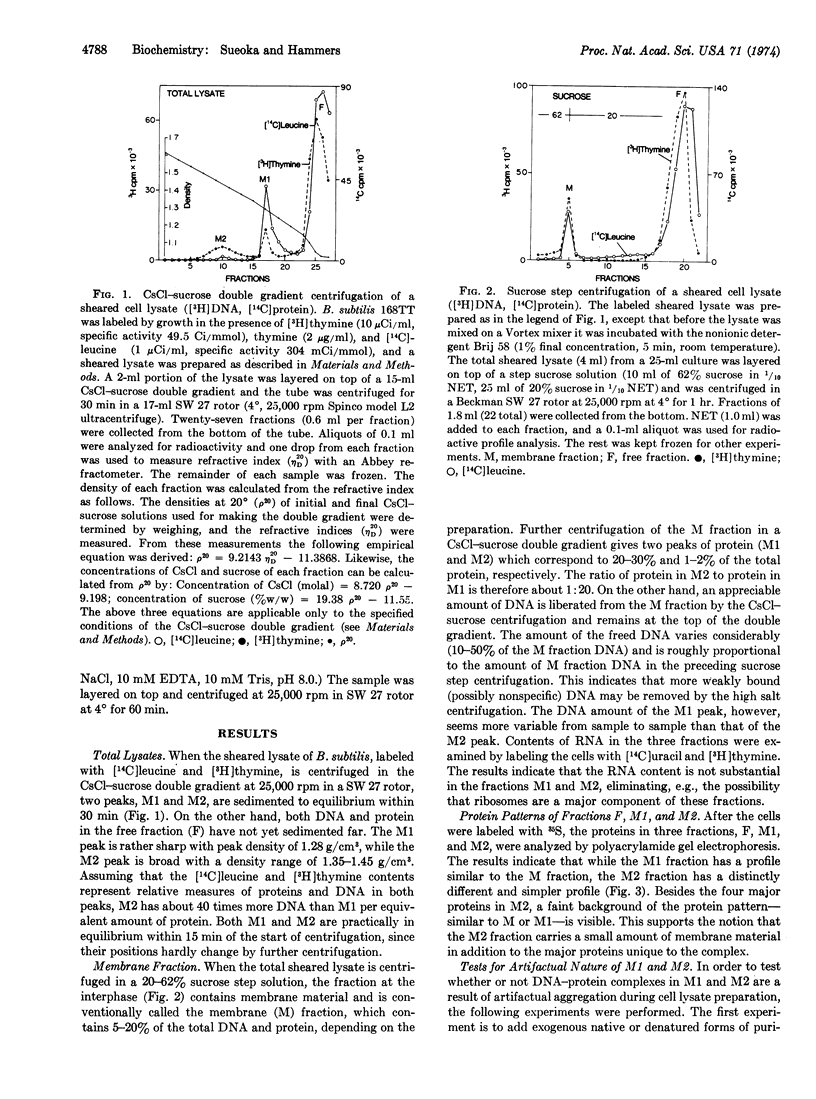
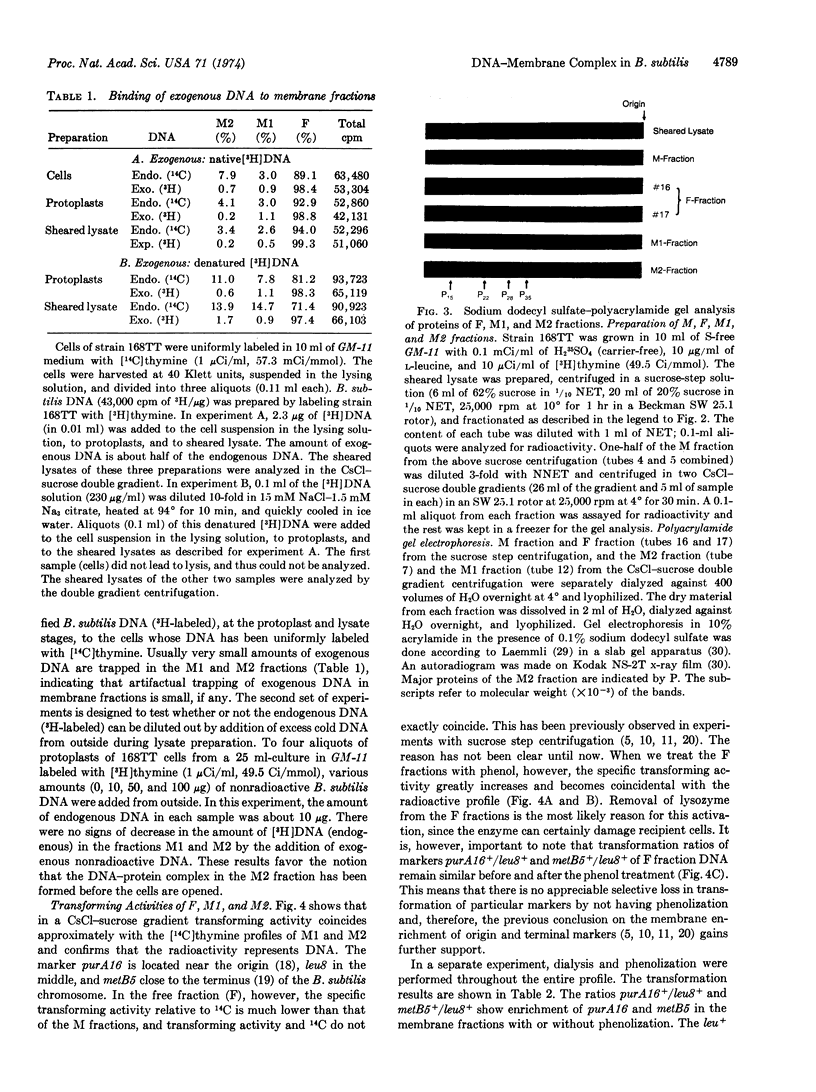
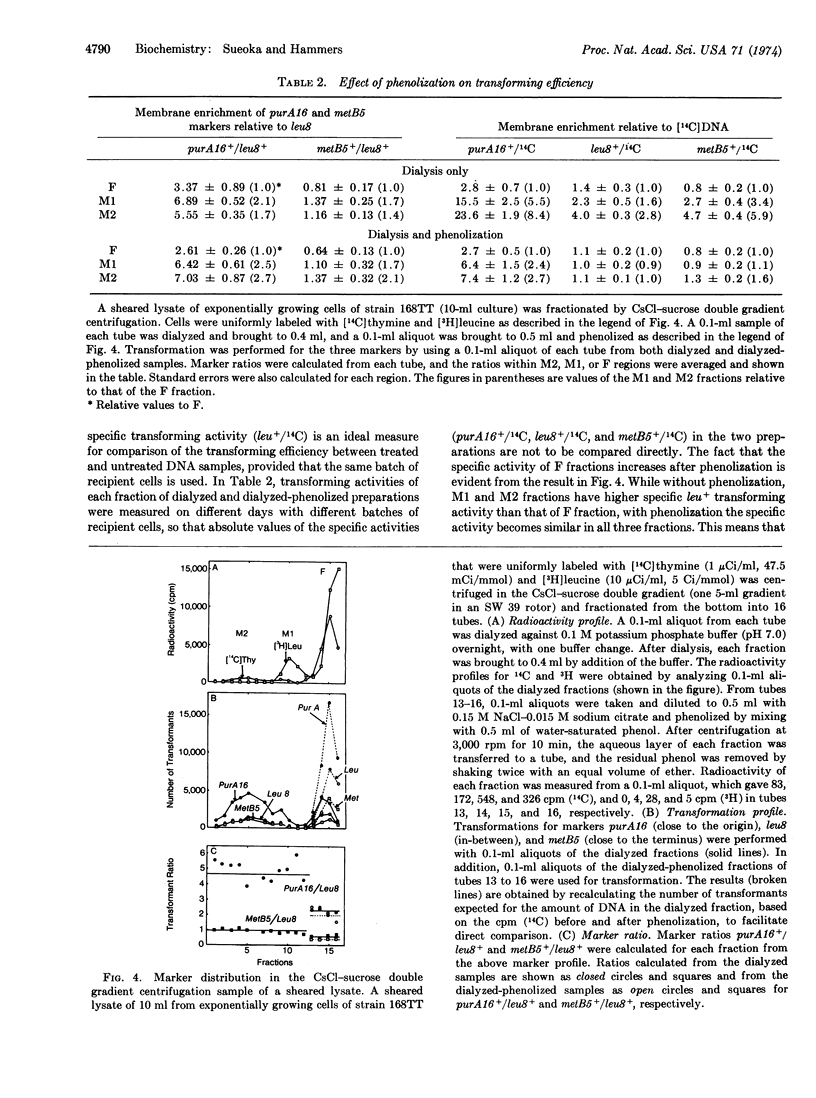
Abstract
A DNA-membrane complex of Bacillus subtilis was separated from the bulk of the membrane by CsCl-sucrose double gradient centrifugation. The complex has a CsCl-sucrose buoyant density of 1.35-1.45 g/cm3. Gel electrophoretic analyses show that the complex has about 5% of the whole membrane proteins and contains proteins unique to the complex as well as a small amount of membrane proteins. DNA in the complex is enriched for a genetic marker close to the origin (pur A16) and for one near the terminus (metB5). Artifactual formation of the complex during the lysate preparation was shown to be unlikely.
Keywords: replication
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Amodio F. J., Jenkins M., Gutmann E. D., Ferris F. L. Studies with DNA-cellulose chromatography. I. DNA-binding proteins from Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:289–305. doi: 10.1101/sqb.1968.033.01.033. [DOI] [PubMed] [Google Scholar]
- Anthony D. D., Zeszotek E., Goldthwait D. A. Initiation by the DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1966 Sep;56(3):1026–1033. doi: 10.1073/pnas.56.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta J. P., Cundliffe E., Daniels M. J., Silverstein J. L., Susskind M. M., Schaechter M. Some unique properties of the deoxyribonucleic acid-bearing portion of the bacterial membrane. J Bacteriol. 1972 Oct;112(1):195–199. doi: 10.1128/jb.112.1.195-199.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Some features of the bacterial membrane studied with the aid of a new fractionation technique. Biochem J. 1971 Apr;122(2):197–207. doi: 10.1042/bj1220197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P., Fox C. F. Evidence for stable attachment of DNA to membrane at the replication origin of Escherichia coli. Biochem Biophys Res Commun. 1970 Oct 9;41(1):157–162. doi: 10.1016/0006-291x(70)90482-1. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Ishihama A. Isolation of an RNA polymearse-DNA complex by CsCl equilibrium centrifugation. Biochem Biophys Res Commun. 1971 Dec 3;45(5):1255–1261. doi: 10.1016/0006-291x(71)90153-7. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Pène J. J. Association of the Bacillus subtilis chromosome with the cell membrane: resolution of free and bound deoxyribonucleic acid on renografin gradients. J Bacteriol. 1970 Nov;104(2):839–850. doi: 10.1128/jb.104.2.839-850.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Sueoka N. Gene expression during outgrowth of Bacillus subtilis spores. The relationship between gene order on the chromosome and temporal sequence of enzyme synthesis. J Mol Biol. 1971 Aug 28;60(1):31–44. doi: 10.1016/0022-2836(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leif R. C. Density gradient system. I. Formation and fractionation of density gradients. Anal Biochem. 1968 Oct 24;25(1):271–282. doi: 10.1016/0003-2697(68)90102-4. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. A., Sueoka N. Membrane attachment of the replication origins of a multifork (dichotomous) chromosome in Bacillus subtilis. J Mol Biol. 1972 Aug 21;69(2):237–248. doi: 10.1016/0022-2836(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Ohi S., Sueoka N. Adenosine triphosphate-dependent deoxyribonuclease in Bacillus subtilis. J Biol Chem. 1973 Nov 10;248(21):7336–7344. [PubMed] [Google Scholar]
- Olsen W. L., Heidrich H. G., Hannig K., Hofschneider P. H. Deoxyribonucleic acid-envelope complexes isolated from Escherichia coli by free-flow electrophoresis: biochemical and electron microscope characterization. J Bacteriol. 1974 May;118(2):646–653. doi: 10.1128/jb.118.2.646-653.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DE LA LIAISON ENTRE NOYAU ET M'ESOSOME CHEZ BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1964 Sep;107:384–400. [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Snyder R. W., Young F. E. Association between the chromosome and the cytoplasmic membrane in Bacillus subtilis. Biochem Biophys Res Commun. 1969 May 8;35(3):354–362. doi: 10.1016/0006-291x(69)90506-3. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Murakami S., Yoshikawa H. Chromosome-membrane association in Bacillus subtilis. I. DNA release from membrane fraction. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1559–1565. doi: 10.1016/s0006-291x(71)80264-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yoshikawa H. Topography of chromosome membrane junction in Bacillus subtilis. Nat New Biol. 1973 Aug 15;244(137):204–206. doi: 10.1038/newbio244204a0. [DOI] [PubMed] [Google Scholar]




