Abstract
Genome-wide expression quantitative trait locus (eQTL) mapping may reveal common genetic variants regulating gene expression. In addition to mapping eQTLs, we systematically evaluated the heritability of the whole blood transcriptome in 5626 participants from the Framingham Heart Study. Of all gene expression measurements, about 40% exhibit evidence of being heritable (, (p<0.05]), the average heritability was estimated to be 0.13, and 10% display . In order to identify the role of eQTLs in promoting phenotype differences and disease susceptibility, we investigated the proportion of cis/trans eQTLs in different heritability categories and discovered that genes with higher heritability are more likely to have cis eQTLs that explain large proportions of variance in the expression of the corresponding genes. Single cis eQTLs explain 0.33–0.53 of variance in transcripts on average, whereas single trans eQTLs only explain 0.02–0.07. The top cis eQTLs tend to explain more variance in the corresponding gene when its is greater. Taking body mass index (BMI) as a case study, we cross-linked cis/trans eQTLs with both GWAS SNPs and differentially expressed genes for BMI. We discovered that BMI GWAS SNPs in 16p11.2 (e.g., rs7359397) are associated with several BMI differentially expressed genes in a cis manner (e.g. SULT1A1, SPNS1, and TUFM). These BMI signature genes explain a much larger proportion of variance in BMI than do the GWAS SNPs. Our results shed light the impact of eQTLs on the heritability of the human whole blood transcriptome and its relations to phenotype differences.
Keywords: heritability, eQTL, transcriptome, gene expression
Introduction
Genome-wide association studies (GWAS) have identified numerous loci associated with complex phenotypes and disease traits, such as coronary heart disease (Lotta and Peyvandi 2011; Schunkert et al. 2011), blood pressure (Ehret et al. 2011; Levy et al. 2009; Newton-Cheh et al. 2009), lipids (Teslovich et al. 2010), body mass index (Guo et al. 2013; Monda et al. 2013; Speliotes et al. 2010), and type 2 diabetes (Morris et al. 2012; Voight et al. 2010). Although GWAS have provided valuable insights into the genetic basis of complex traits, they explain only a small proportion of the heritability of most traits (Tenesa and Haley 2013). For the majority of loci from GWAS, the mechanistic underpinnings for association with the corresponding traits are unknown. Gene expression may mediate genotype-phenotype association, and as such may be considered an intermediate molecular phenotype (Cheung and Spielman 2009). Dissecting the genetic determinants of gene expression can provide insight into mechanisms of disease and the functional consequences of genetic variation. Genome-wide expression quantitative trait locus (eQTL) mapping has been widely used to identify common genetic variants regulating gene expression (Myers et al. 2007; Schadt et al. 2008; Stranger et al. 2005; Stranger et al. 2007; Zeller et al. 2010). Although numerous studies (Myers et al. 2007; Schadt et al. 2008; Stranger et al. 2005; Stranger et al. 2007; Westra et al. 2013; Zeller et al. 2010) have revealed tens of thousands of cis and trans eQTLs, many cis and trans eQTLs remain unrecognized due to sample size limitations and tissue specificity (Grundberg et al. 2012; Price et al. 2011). Further investigations are needed to answer additional questions, such as, are these cis or trans components related to the heritability of gene expression and what is the proportion of variance in gene expression that can be explained by single cis or trans eQTLs?
In this study, we systematically investigated the heritability of the human whole blood transcriptome using Framingham Heart Study (FHS) pedigrees. Our goals were three fold. First, we explored the distribution of heritability of genome-wide gene expression levels using a large sample with well-defined extended pedigrees. Second, we investigated how cis and trans eQTLs are related to transcript heritability levels. Third, we sought to understand how the genetic basis of gene expression relates to phenotype differences and disease susceptibility via cis and trans components. To accomplish these aims, we estimated the overall heritability of approximately 18,000 genes in 5626 participants from the FHS, and assessed the proportion of variance in gene expression that is attributable to cis and trans eQTLs. By cross-linking the eQTLs with GWAS results of metabolic traits including body mass index (BMI), blood pressure (BP), and lipid traits in the NHGRI GWAS Catalog (Hindorff et al. 2009), we discovered trait-associated single nucleotide polymorphisms (SNPs) that explain relatively large proportions of the genetic variance of multiple gene transcripts, despite the fact that these SNPs only explain a small proportion of phenotypic variance for the same metabolic traits. Last, taking BMI as an example, by cross-linking the eQTLs with trait-associated SNPs (GWAS SNPs (Hindorff et al. 2009)), we discovered that some GWAS SNPs are cis eQTLs of certain gene transcripts and can explain large proportions of variance in expression of these transcripts. In addition, some of the corresponding cis eQTL gene transcripts show differential expression for BMI. Although the GWAS SNPs only explain a small proportion of phenotypic variance in BMI, these differentially expressed cis eQTL related gene transcripts explain a larger proportion of variance in BMI.
Materials and Methods
Study population description
In 1971, the offspring and offspring spouses (offspring cohort, N=5124) of the original FHS cohort participants were recruited and have been examined approximately every four years (except for the interval between examinations 1 and 2 with an intervening 8 years) (Feinleib et al. 1975). From 2002 to 2005, the adult children (third generation cohort, N=4095) of the offspring cohort participants were recruited and examined (Splansky et al. 2007). A total of 5626 participants from the offspring (N=2446) and third generation (N=3180) cohorts were included in this study. Whole blood samples were collected at the eighth examination of the offspring cohort and the second examination of the third generation cohort. All participants provided written consent for genetic research.
Gene expression profiling
Fasting peripheral whole blood samples (2.5ml) in PAXgene™ tubes (PreAnalytiX, Hombrechtikon, Switzerland) were collected and the Affymetrix Human Exon Array ST 1.0 (Affymetrix, Inc., Santa Clara, CA) was utilized to measure mRNA expression levels genome wide (N=~18,000 genes). Details of the design, sampling, RNA isolation, and mRNA measurement have been described previously (Huan et al. 2013; Joehanes et al. 2013). All data used herein are available online in dbGaP (http://www.ncbi.nlm.nih.gov/gap; accession number phs000007).
Genotyping and quality control
DNA isolation, and genotyping with the Affymetrix 500K mapping array and the Affymetrix 50K gene-focused MIP array have been described previously (Levy et al. 2009). A total of 503,551 SNPs with successful call rate >0.95 and Hardy-Weinberg Equilibrium (HWE) P>10−6 were retained. Imputation of ~36.3 million SNPs in 1000 Genomes Phase 1 SNP data was conducted using MACH (Li et al. 2010). In this study, we used the 1000-genome resource imputed SNPs with minor allele frequency (MAF) >0.01 and imputation ratio >0.3, yielding approximately 8 million SNPs for eQTL analysis.
eQTL identification
The eQTL list was generated using the 5257 individuals that had genome-wide genotypes and gene expression profiling. A cis eQTL was defined as an eQTL within a 1 megabase (Mb) flanking the gene. A trans eQTL was defined as an eQTLs in a different chromosome from the gene. The remaining eQTLs residing in the same chromosome but extending 1Mb distance were defined as long-range cis eQTLs. The imputed SNPs were coded in an additive genetic model of allele dosage that was continuous and ranged from 0 to 2. Linear mixed effects (LME) regression models were used to determine the association between gene expression (adjusting for age, sex, 10 technical covariates, cell types, the first principle component [PC1], and familial relatedness) and the imputed SNP dosage from the 1000-Genomes resource (Abecasis et al. 2012). Supplementary Table S1 shows the 10 technical covariates and PC1. Cell types included white blood cells [WBC], red blood cells [RBC], platelets, neutrophils, lymphocytes, monocytes, eosinophils, and basophils, which were imputed by partial least squares (PLS) regression (Joehanes R, PhD, unpublished data). Genomic coordinates were based on NCBI human reference genome build 37/hg19. The Benjamini-Hochberg method (BH) (Benjamini and Hochberg 1995) was used to calculate false discovery rates (FDR) of cis, trans, and long-range cis eQTLs separately.
We evaluated if the effect sizes of cis and trans eQTLs in independent individual sets showed concordance of allele effect. We split the overall samples into the discovery and replication sets (1:1 ratio) that included independent families between the two sets. Using the same methods described above, we identified eQTLs in the discovery and replication sets respectively and compared the effect sizes of eQTLs from the discovery and replication set.
To determine if differences in cell type distributions in different samples affected the identification of cis and trans eQTLs, we applied regression models without including cell types as covariates to repeat the eQTL analysis for the cis and trans eQTLs at FDR<1e-4. We then compared the t-test statistics of cis and trans eQTLs with and without adjusting for cell types.
By design, the Affymetrix Human Exon 1.0 ST array platform contains SNPs in some probes for targeting mRNA sequence; this SNP-on-probe phenomenon can affect the binding affinity of microarray probe sequences and may lead to false-positive and false-negative results (Ramasamy et al. 2013). To address this problem, we further limited eQTLs to the 6059 transcripts without any SNPs-on-probes. We reported the eQTL results for all 18,000 transcripts in the main body of the manuscript and the results for the 6059 transcripts without SNP-on-probes in the Supplementary Files.
Estimation of the additive heritability for a gene expression trait
The narrow-sense heritability estimate of a gene expression trait (denoted as ) was the proportion of the additive polygenic genetic variance of the total phenotypic variance of a gene expression trait: , here denotes the additive polygenic genetic variance and denotes the total phenotypic variance of a gene expression trait. In order to obtain both variance components and p-values for heritability estimates, the estimate was obtained using variance-component methodology implemented in SOLAR (Almasy and Blangero 1998) and in the lmekin() function of Kinship Package (Abecasis et al. 2001) (http://cran.r-project.org/web/packages/kinship/).
Heritability estimation for all gene expression traits was performed using the combined sample (N=5626). To determine whether heritability estimates were consistent in individual cohorts, we conducted analysis in the offspring cohort (N=2446) and the third generation cohort (N=3180) separately. The average heritability estimate was calculated as , where n = number of gene expression traits.
We further investigated if the heritability estimates in relation to cohorts, sample size and blood cell type portions (see Supplementary Files).
The proportion of variance in gene expression attributable to peak eQTLs
The proportion of variance in a single gene expression trait that is attributable to a single eQTL (or eSNP) was denoted as and was calculated as follows:
| (Equation 1) |
where was the total phenotypic variance of a gene expression trait; and were the polygenic variance and the residual error, respectively, when modeling with the tested eQTL; and were the polygenic variance and the residual error when modeling without the tested eQTL. The relative proportion of variance explained by a peak eQTL with respect to the heritability of a gene expression trait was calculated by . The lmekin() function in the Kinship package was used to estimate .
The proportion of variance in a clinical trait attributable to a signature gene
The proportion of the variance in a clinical trait attributable to a signature gene was calculated as follows:
| (Equation 2) |
where was the total phenotypic variance of the clinical trait, and were the polygenic variance and the residual error when modeling with the tested signature gene, and were the polygenic variance and the residual error when modeling without the tested signature gene. The lmekin() function in the Kinship package was used to estimate .
GWAS resources for metabolic traits
We cross-linked the eQTLs (or eSNPs) identified in this study with top SNPs reported from the GWAS for several metabolic traits, including hypertension/blood pressure (BP), obesity/body mass index (BMI) and lipid traits (High-density lipoprotein [HDL], Low-density lipoprotein [LDL], Total cholesterol [TC] and Triglycerides [TG]), in the NHGRI GWAS Catalog (accessed Dec 2013) (Hindorff et al. 2009). We used a p value ≤5e-8 to define a trait-specific significant result.
Identification of differentially expressed genes for BMI
To determine the proportion of variance that can be explained by differentially expressed genes or by eQTLs of these differentially expressed genes for a clinical trait, we used BMI as an example for proof-of-concept. We identified differentially expressed genes for BMI which was measured at the same examinations of the offspring and the third generation cohorts at which gene expression was assessed. Residuals for genes (after adjusting for technical covariates) were used to identify differentially expressed genes associated with BMI after accounting for age, sex, and family structure in LME models implemented in lmekin() (Abecasis et al. 2001). We applied Benjamini–Hochberg’s FDR<0.05 to denote significant signals and calculated FDR for selected BMI signature genes (Benjamini and Hochberg 1995). The proportion of the variance in BMI attributable to multiple differentially expressed signature genes was calculated using Equation (2).
Results
Study participants
Table 1 summarizes the characteristics of the 5626 FHS participants used in this study. At the baseline examination, the offspring cohort is, on average ~20 years older than the third generation cohort (66 versus 46 years). Supplementary Table S2 displays the family sizes in the 5626 individuals. Among the participants, 399 are unrelated individuals, and 5227 are from 704 families (family size ≥2). Among the 5227 individuals, 2352 are from 570 small families (family size 2–9), 1283 are from 101 medium size families (family size 10–19), 940 are from 29 relatively large families (family size 20–99), and 651 are from large families (size ≥100).
Table 1.
Characteristics of the 5,626 Framingham Heart Study Participants
| Offspring Cohort N=2,446 (examination cycle 8: 2005–2008) |
Third Generation Cohort N=3,180 (examination cycle 2: 2008–2011) |
|
|---|---|---|
| Phenotypes/Covariates | Mean (SD) | Mean (SD) |
| Male (%) | 45 | 47 |
| Age (yrs) | 66 (9) | 46 (9) |
| Body mass index (kg/m2) | 28.4 (5.4) | 28.0 (5.9) |
Heritability estimation of genome-wide gene expression measurements
The narrow sense heritability of ~18,000 transcripts was estimated using the FHS pedigrees. For simplicity, we refer to “narrow sense heritability” as “heritability” in the rest of this manuscript. Figure 1 displays the distribution of heritability estimates for 18,000 genes based on all 5626 participants (Supplementary Figure S1 displays the results for 6059 genes without SNPs-on-probes [see Methods]). The average heritability of global gene expression was estimated to be 0.072 (the median heritability estimate was 0.037). Among all genes, 7161 (~40% of the genome) were estimated to have (p<0.05), 1,730 (~10%) with (p<1.8e-6), 286 (~2%) with (p<6.3e-41), and 50 (~0.3%) with (p<3.7e-105).
Figure 1. Heritability Distribution in Framingham Cohorts.
A) Heritability distribution of transcripts; B) Summary of genes in different sub-categories.
We further investigated if the heritability estimates differed in relation to cohort (offspring vs. third generation), sample size, and blood cell type portions. We discovered that the overall heritability distribution is similar in the FHS offspring and third generation cohorts. In addition, differences in cell type proportions are not likely to significantly affect the heritability of gene expression levels. We also discovered that larger sample size and more comprehensive family structure provide more accurate heritability estimates (see Supplementary Files, Supplementary Figure S2–S3, and Supplementary Table S3).
Effects of cis and trans eQTLs’ on gene expression
We performed a genome-wide association analysis of global gene expression levels and identified cis, trans and long-range cis eQTLs. The proportion of gene transcripts with significant cis, trans and long-range cis eQTLs is much smaller at than at . At FDR<1e-4; 43% of genes have cis eQTLs at , compared to 93% of genes at . Similarly, 3% of genes have trans eQTLs at , compared to 21% of genes at ; and 1% of genes have long-range cis eQTLs at , compared to 10% of genes at .
We further computed the proportion of variance explained by a single cis or trans eQTL in the study samples for genes with expression heritability levels (1,730 genes or 10% of the total gene expression traits). By splitting the overall samples into independent discovery and replication sets (see Methods), we discovered that cis and trans eQTLs show consistent allelic directional associations both in the discovery and replication sets (Supplementary Figure S4). We evaluated if cell types affected the identification of eQTLs. The t-test statistics remained very similar with or without adjusting for cell types for both cis (Pearson r = 0.99) and trans (Pearson r = 0.98) eQTLs, suggesting that the differences of cell type distributions in different samples did not have large impact on the identification of either cis or trans eQTLs (Supplementary Figure S5). A similar finding was also reported by Westra et al. in a trans eQTL study (Westra et al. 2013).
We further applied a series of FDR thresholds (FDR<1e-4, 1e-6, 1e-8 and 1e-10) to select significant eQTLs. Figure 2 summarizes the proportions of gene transcripts with significant cis, trans or long-range cis eQTLs at different levels = (0.2, 0.3), (0.3, 0.4), (0.4, 0.5) and (0.5, 0.6). We observed that gene expression traits with higher heritability estimates tend to have higher proportions of significant cis eQTLs. For example, at FDR<1e-4 (corresponding p=3.2e-6), 49 of the 50 genes at of (0.6, 1) are associated with cis eQTLs. For the other four heritability categories, i.e. , (0.3, 0.4), (0.4, 0.5), and (0.5, 0.6), the proportions of genes with cis eQTLs are 92%, 91%, 96%, and 100%, respectively (Figure 2A). Compared to the proportions of significant cis eQTLs, in general, the proportions of significant trans eQTL are much smaller across all heritability categories. The transcripts of trans eQTLs are associated with heritability values in an opposite manner. For FDR thresholds, gene expression traits with higher heritability estimates tend to have lower proportions of significant trans eQTLs (Figure 2B). For example, at FDR threshold <1e-4 (corresponding p=1.6e-10), the proportions of trans eQTLs genes are 22%, 24%, 16%, 16%, and 2%, respectively, for the 5 heritability categories. The long-range cis eQTLs exhibit characteristics similar to those cis eQTLs rather than trans eQTLs (Figure 2C). For all FDR thresholds, gene expression traits with higher heritability estimates tend to have higher proportions of significant long-range cis eQTLs.
Figure 2. Relationship Between Heritability Estimates and Proportion of Genes with cis, trans or long-range cis eQTLs.
A) Proportion of transcripts having cis eQTLs in different heritability levels; B) Proportion of transcripts having trans eQTLs in different heritability levels; C) Proportion of transcripts having long-range cis eQTLs in different heritability levels.
Through investigating the peak cis, trans, or long-range cis eQTL for every gene, we discovered that the peak cis eQTLs explain a large proportion of variance in their respective gene expression level (Supplementary Table S4 and Figure 3A). On average the proportion of variance in the transcript with explained by a peak cis eQTL is about one third. Moreover, when the heritability of gene expression increases from of (0.2, 0.3) to (0.6, 1), the average increases from 0.33 to 0.53 (Figure 3A). In contrast, the peak trans eQTLs only explain a small proportion of variance in their respective gene expression levels. The average proportion of variance in the transcript explained by a single peak trans eQTL ranged from 0.02 to 0.07 in all of the five categories (Supplementary Table S5 and Figure 3B). For long-range cis eQTLs, when the heritability of gene expression increases from of (0.2, 0.3) to (0.6, 1), the average increases from 0.07 to 0.18 (Supplementary Table S6 and Figure 3C).
Figure 3. Proportion of Transcript Variance Explained by Peak eQTLs.
A) Variance proportion of a transcript explained by a single peak cis eQTL; B) Variance proportion of a transcript explained by a single peak trans eQTL; C) Variance proportion of a transcript explained by a single peak long-range cis eQTL.
In order to avoid the SNP-on-probe effect that may lead false-positive and false-negative results, we repeated analyses limited to 6,059 gene transcripts without any SNPs-on-probes (see Methods). Supplementary Figures S6–S7 present results for these 6,059 genes. The results are similar for these 6,059 genes vs. all 18,000 genes (Figures 2–3).
Metabolic traits GWAS SNPs that explained a large proportion of variability in eQTL genes
We cross-linked the GWAS results for several metabolic traits with the cis and trans eQTLs for genes with (the threshold for choosing eQTLs is FDR<1e-3). Table 2 summarizes a list of cis eQTLs that explain relatively large proportions of heritability in the corresponding gene expression levels . These cis eQTLs are among the peak signals for the corresponding genes (eQTL p<1e-40). Although these significant GWAS SNPs (that also are cis eQTLs) explain only very small proportions of genetic variance in the metabolic traits with which they are associated, these SNPs (cis eQTLs) explain relatively large proportions of the genetic variance in gene expression levels, and more strikingly, several cis eQTLs display large estimates (>0.5). Although some cis eQTLs display higher values of for a transcript, the transcript do not necessary correspond to the closest gene to the cis eQTL. For example, rs486416 (associated with total cholesterol and triglycerides in GWAS (Teslovich et al. 2010)) is located in the intron of EHMT2, but this SNP is associated with expression of SLC44A4 in a cis manner with for the SLC44A4 transcript. Additionally, we discovered that some cis eQTLs are associated with multiple gene expression traits and display relatively high . For example, rs12936231 (associated with HDL in GWAS (Teslovich et al. 2010)) is a cis eSNP of both GSDMB (, 28KB away from rs12936231) and ORMDL3 (, 48KB away from rs12936231) (Figure 4A).
Table 2.
cis eQTLs among GWAS Results for Metabolic Traits
| SNP -- Gene | SNP -- Trait | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Chr. | eQTL | SNP location |
* |
eSNP-Pval |
† |
‡GWAS-p value |
§ |
GWAS- Trait |
|||
| GSTM4 | chr1 | rs4970843 | Intronic (SORT1) |
0.55 | 7.26E-63 | 0.11 | 9.77E-23 | 0.002 | LDL/TC | |||
| RHD | chr1 | rs926438 | Intronic (RHCE) |
0.52 | 5.19E-289 | 0.47 | 1.68E-09 | 5.01E-7 | LDL/TC | |||
| DOCK7 | chr1 | rs1748195 | Intronic (DOCK7) |
0.23 | 2.73E-162 | 0.60 | 2.43E-42 | 0.004 | LDL/TG/TC | |||
| GSTM4 | chr1 | rs4970843 | Intronic (SORT1) |
0.55 | 7.26E-63 | 0.11 | 9.77E-23 | 0.001 | LDL/TC | |||
| CLCN6 | chr1 | rs1256713 6 |
Intronic (CLCN6) |
0.21 | 6.68E-52 | 0.21 | 3.41E-10 | 0.007 | SBP/DBP | |||
| C4B | chr6 | rs2072633 | Intronic (CFB) |
0.21 | 1.56E-40 | 0.16 | 2.67E-08 | 0 | TC | |||
| HLA-B | chr6 | rs437179 | Coding(SKI V2L) |
0.46 | 1.38E-59 | 0.11 | 6.98E-12 | 5.60E-4 | TC | |||
| HLA-B | chr6 | rs2524095 | Unknown | 0.46 | 4.88E-113 | 0.22 | 3.02E-08 | 0.001 | TG | |||
| LOC10051 0059 |
chr6 | rs2894254 | Intronic (C6orf10) |
0.74 | 1.65E-218 | 0.24 | 8.92E-10 | 0.004 | TG | |||
| HCP5 | chr6 | rs2596565 | Unknown | 0.29 | 5.63E-28 | 0.13 | 1.55E-10 | 0.004 | TG/TC | |||
| NCRNA00 243 |
chr6 | rs3130544 | Unknown | 0.26 | 3.70E-100 | 0.37 | 4.84E-10 | 0.003 | TG/TC | |||
| FLOT1 | 0.25 | 1.39E-59 | 0.23 | |||||||||
| SLC44A4 | chr6 | rs486416 | Intronic (EHMT2) |
0.56 | 0 | 0.87 | 1.99E-14 | 0.006 | TG/TC | |||
| CSGALNA CT1 |
chr8 | rs1967103 | Intronic (CSGALNA CT1) |
0.55 | 5.97E-218 | 0.35 | 5.78E-09 | 0.006 | HDL/TG | |||
| GRINA | chr8 | rs6985603 | 5' upstream (SPATC1) |
0.26 | 1.10E-104 | 0.35 | 3.39E-10 | 0.002 | LDL | |||
| C11orf10 | chr11 | rs174534 | Intronic (C11orf9) |
0.32 | 2.01E-255 | 0.65 | 2.68E-18 | 0.005 | HDL/LDL/T G/TC |
|||
| FADS2 | chr11 | rs174549 | Intronic (FADS1) |
0.74 | 0 | 0.54 | 2.08E-20 | 0.004 | HDL/LDL/T G/TC |
|||
| SIDT2 | chr11 | rs6589597 | Unknown | 0.53 | 2.17E-297 | 0.46 | 7.22E-14 | 0.003 | TC | |||
| SIDT2 | chr11 | rs7107152 | Intronic (SIDT2) |
0.53 | 0 | 0.68 | 7.13E-10 | 0.001 | TG | |||
| POC1B | chr12 | rs1110532 8 |
Intergenic | 0.35 | 2.53E-41 | 0.11 | 1.08E-09 | 0.003 | SBP/DBP | |||
| MAP2K5 | chr15 | rs1290281 2 |
Intronic (MAP2K5) |
0.24 | 1.40E-198 | 0.73 | 1.33E-08 | 1.49E-4 | BMI | |||
| APOB48R | chr16 | rs1244388 1 |
Intronic (ATXN2L) |
0.60 | 0 | 0.72 | 5.32E-10 | 2.06E-4 | BMI | |||
| GSDMB | chr17 | rs1293623 1 |
Intronic (ZPBP2) |
0.35 | 0 | 0.92 | 2.47E-09 | 1.48E-4 | HDL | |||
| ORMDL3 | 0.24 | 1.34E-92 | 0.34 | |||||||||
| LILRB3 | chr19 | rs431420 | Unknown | 0.44 | 3.50E-160 | 0.33 | 7.51E-13 | 8.22E-5 | HDL | |||
| LILRB2 | 0.34 | 5.93E-124 | 0.31 | |||||||||
| SLC44A2 | chr19 | rs2288904 | Coding (SLC44A2) |
0.27 | 2.37E-48 | 0.16 | 5.72E-10 | 0.005 | LDL | |||
| PVRL2 | chr19 | rs8104483 | Intronic (PVRL2) |
0.58 | 1.13E-271 | 0.40 | 1.94E-20 | 2.90E-4 | LDL/TC | |||
is the proportion of total phenotypic variance that can be explained by the additive genetic components
is the ratio of the proportion of variance explained by a peak eQTL to the heritability of a gene expression trait
GWAS-p value: the minimum trait-associated SNP p value in NHGRI GWAS Catalog (Hindorff et al. 2009)
is the ratio of the proportion of variance explained by a SNP to the heritability of a clinical trait. is calculated from FHS samples along.
Figure 4. Genotype-specific Gene Expression and a Proposed Schematic of Master cis- and trans- eQTL Regulation of Transcripts.
A) An example of master cis eQTL model; B) An example of master trans eQTL model. The number above the directed curve line indicated the heritability proportion of transcripts explained by the SNP. TF indicated transcription factor. The y-axis geneExp_resid indicated the gene expression values were adjusted for age, sex and technique covariates.
Table 3 summarizes trans eQTLs for genes with . In contrast to cis eQTLs, most trans eQTLs display smaller (≤ 0.1) with only one exception trans eQTL rs289754 for BTN3A1 . Although having smaller average , some trans eQTLs are also associated with multiple gene transcripts. Table 3 displays two such trans eSNPs: one example is rs3184504, which is nonsynonymous in SH2B3 and is associated in GWAS with SBP, DBP (Levy et al. 2009) and LDL (Teslovich et al. 2010). rs3184504 is a trans eQTL associated with five gene transcripts including FCGR1A , ARHGEF40 , MYADM , IDO1 and IFIT3 . Another SNP, rs289754, is a trans eSNP associated with three genes located in the MHC Cluster I – BTN3A1 , HCP5 and HLA-F (Figure 4B).
Table 3.
trans eQTLs among GWAS for Metabolic Traits
| SNP -- Gene | SNP - Trait | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Chr. for gene |
eQTL | SNP Location |
* |
eSNP-Pval |
† |
‡GWAS-p value |
§ |
GWAS-Trait | |||
| SELP | chr1 | rs10761741 | Intronic (JMJD1C) | 0.37 | 4.84E-09 | 0.02 | 1.81E-11 | 0.002 | TG | |||
| AQP10 | chr1 | 0.21 | 9.04E-14 | 0.06 | ||||||||
| ITGA2B | chr17 | 0.4 | 1.22E-10 | 0.02 | ||||||||
| CXCL5 | chr4 | rs10761779 | Intergenic | 0.29 | 2.37E-13 | 0.04 | 4.13E-11 | 0.002 | TG | |||
| PKHD1L1 | chr8 | 0.32 | 3.60E-10 | 0.03 | ||||||||
| PPBP | chr4 | 0.30 | 1.28E-08 | 0.02 | ||||||||
| EBF1 | chr5 | rs11078927 | Intronic (GSDMB) | 0.28 | 2.76E-08 | 0.02 | 1.28E-08 | 1.88E-4 | HDL | |||
| CPA3 | chr3 | rs1178977 | Intronic (BAZ1B) | 0.39 | 1.44E-08 | 0.01 | 2.90E-55 | 0.03 | TG | |||
| ALAS2 | chrX | rs1800562 | Coding (HFE) | 0.37 | 2.73E-13 | 0.03 | 6.07E-10 | 0.005 | LDL/TC | |||
| ALPL | chr1 | rs2872507 | Intergenic | 0.25 | 5.10E-09 | 0.05 | 2.83E-08 | 1.89E-4 | HDL | |||
| BTN3A1 | chr6 | rs289754 | Intronic (NLRC5) | 0.23 | 2.22E-49 | 0.19 | 8.98E-10 | 1.17E-4 | HDL | |||
| HCP5 | chr6 | 0.29 | 2.57E-35 | 0.1 | ||||||||
| HLA-F | chr6 | 0.26 | 8.53E-14 | 0.04 | ||||||||
| FCGR1A | chr1 | rs3184504 | Coding (SH2B3) | 0.27 | 9.82E-14 | 0.04 | 2.33E-14 | 0.002 | SBP/DBP/LDL/TC | |||
| ARHGEF40 | chr14 | 0.28 | 8.82E-11 | 0.03 | ||||||||
| MYADM | chr19 | 0.25 | 3.88E-09 | 0.03 | ||||||||
| IDO1 | chr8 | 0.26 | 2.02E-08 | 0.02 | ||||||||
| IFIT3 | chr10 | 0.22 | 2.82E-08 | 0.02 | ||||||||
| PLA2G7 | chr6 | rs3808456 | Intronic (TRPS1) | 0.45 | 2.18E-10 | 0.03 | 1.28E-09 | 0 | HDL | |||
| ESPN | chr1 | rs634869 | Intergenic | 0.32 | 2.29E-11 | 0.03 | 4.94E-08 | 4.17E-4 | HDL | |||
| GUCY1A3 | chr4 | rs7077256 | Intronic (REEP3) | 0.27 | 2.31E-08 | 0.03 | 1.70E-10 | 0.001 | TG | |||
| ITGB3 | chr17 | 0.54 | 4.64E-12 | 0.02 | ||||||||
| CLU | chr8 | 0.47 | 3.84E-09 | 0.02 | ||||||||
| GPR109A | chr12 | rs7832357 | Intergenic | 0.22 | 3.83E-11 | 0.03 | 1.84E-11 | 0.004 | TG | |||
| MME | chr3 | 0.23 | 4.34E-09 | 0.03 | ||||||||
is the heritability of the transcript
is the heritability proportion of the transcript explained by a single eSNP
GWAS-p value: the minimum trait-associated SNP p value in NHGRI GWAS catalog (Hindorff et al. 2009)
is the ratio of the proportion of variance explained by a SNP to the heritability of a clinical trait (). is calculated from FHS samples along.
Identification of “master” eQTLs associated with BMI signatures genes
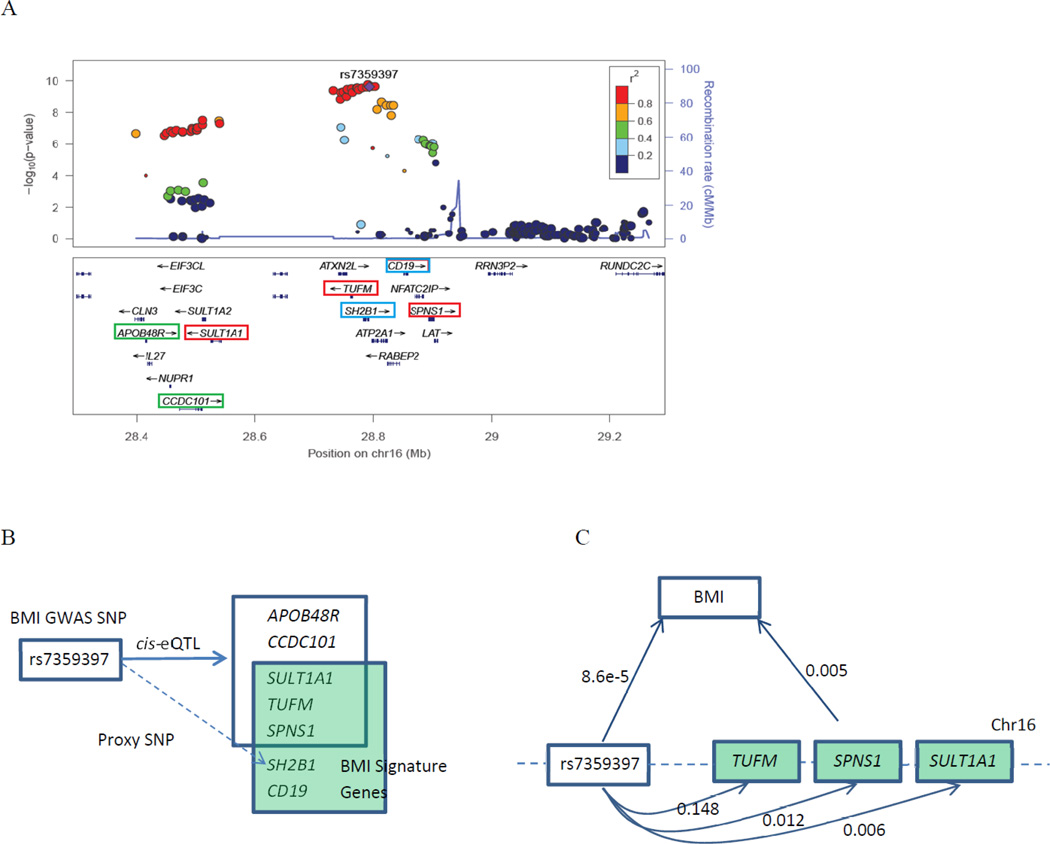
Based on our finding that some eQTLs explain large proportions of variation in gene expression levels -- although they only explain small proportions of variance in metabolic traits -- we investigated the relations between the trait-specific differential expression signature genes and the genes with the eQTLs that also are GWAS SNPs for the same trait. Using BMI as a proof-of-concept example, at first, we identified BMI differentially expressed genes at FDR<0.05 (i.e. BMI signature genes, Supplementary Table S7). Second, we cross-linked these signature genes with significant BMI GWAS SNPs as well as with eQTLs. By doing so we discovered that 9 BMI signature genes are associated with 6 BMI GWAS SNPs (Speliotes et al. 2010) in a cis manner (Table 4). Among a total of 18,000 genes measured by the Affymetrix Exon Array, 18 genes had cis eQTLs with p<5e-8 in BMI GWAS. Comparison of the two ratios 9/5409 and 18/18000 by hypergeometric test yielded p=0.02, indicating the BMI transcripomic signatures were enriched for BMI GWAS SNP-associated transcripts at p=0.02 by hypergeometric test. rs7359397 is a cis eQTL for three BMI signature genes – SULT1A1 (FDR adjusted p=0.05), SPNS1 (FDR adjusted p=5.6e-4), and TUFM (FDR adjusted p=0.03). Other two genes located near rs7359397, CD19 and SH2B1, also are BMI signature genes at FDR adjusted p=0.04 and 1e-4, however, rs7359397 was not recognized as a cis eQTL for these two genes (Figure 5A–B). We further discovered groups of differentially expressed genes that are associated with the same GWAS SNPs and jointly explain greater proportions of the variance in BMI than do the SNPs associated with the trait in GWAS. For instance, rs7359397 only explained 0.0086% of phenotypic variance in BMI (calculated using FHS data alone), but the three BMI signature genes (SULT1A1, SPNS1, and TUFM) that are associated with this SNP in cis manner, jointly explained 0.5% of the interindividual variability in BMI (Figure 5C).
Table 4.
BMI GWAS SNPs for BMI Differentially Expressed Genes linking by eQTLs
| Gene -- Trait | SNP -- Gene | SNP -- Trait | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Chr. for gene |
eQTL | SNP Location | Signature P value |
Signature FDR |
Phenotypic Proportion (Gene for Trait) |
eSNP p value |
Cis Trans |
Phenotypic Proportion (SNP for Gene) |
‡GWAS - p value |
Phenotypic Proportion (SNP for Trait) |
||
| ETV5 | chr3 | rs9816226 | Intronic (DGKG) | 0.01 | 0.0002 | 0.001 | 0.003 | 4.44E-16 | cis | 0.012 | 7.61E-14 | 1.5E-4 | |
| AAGAB | chr15 | rs2278076 | 3' downstream (MAP2K5) |
0.04 | 0.0003 | 0.002 | 0.001 | 6.08E-06 | cis | 0.004 | 1.46E-10 | 0 | |
| CLN6 | chr15 | rs3784707 | Intronic (MAP2K5) |
0.17 | 1.35E-10 | 2.58E-09 | 0.008 | 9.66E-06 | cis | 0.004 | 1.46E-10 | 0 | |
| SULT1A1 | chr16 | rs7498665 | Coding (SH2B1) | 0.21 | 0.01 | 0.05 | 0.001 | 5.54E-07 | cis | 0.006 | 1.75E-10 | 8.6E-5 | |
| SPNS1 | 0.05 | 8.25E-05 | 5.57E-04 | 0.004 | 5.77E-15 | cis | 0.012 | ||||||
| TUFM | 0.19 | 0.007 | 0.03 | 0.002 | 5.16E-08 | cis | 0.148 | ||||||
| PTPRJ | chr11 | rs2290851 | Intronic (FNBP4) | 0.19 | 1.28E-17 | 5.79E-16 | 0.014 | 3.88E-07 | cis | 0.005 | 2.77E-10 | 4.5E-4 | |
| MTCH2 | 0.08 | 1.13E-05 | 9.26E-05 | 0.003 | 3.60E-14 | cis | 0.011 | ||||||
| LIN7C | chr11 | rs1519480 | 3' downstream (BDNF) |
0.08 | 2.55E-06 | 2.39E-05 | 0.004 | 2.22E-14 | cis | 0.012 | 2.12E-09 | 4.4E-4 | |
GWAS-p value: for BMI, the p values are from GIANT (Speliotes et al. 2010);
Figure 5. cis/trans eQTLs linking BMI GWAS SNPs and BMI signature genes.
A) Regional association plots (drawn by LocusZoom (Pruim et al. 2010)) of BMI GWAS SNPs (peak SNP rs7359397) and its proxy genes. The y-axis is –log10-transformed BMI GWAS p values from GIANT (Speliotes et al. 2010); the genes marked by green rectangle indicating eQTL genes, by blue rectangle indicating BMI signature genes and by red rectangle indicating the overlap of eQTL genes and BMI signature genes. The genes marked by green rectangle indicating eQTL genes and by blue rectangle indicating BMI signature genes. B) Schematic figure depicting BMI GWAS SNP (peak SNP rs7359397) is associated with multiple genes in cis-manner, and some of those genes are differentially expressed for BMI; C) rs7359397 driving BMI differential gene expression signatures. The number above the directed curved line indicated the phenotypic proportion of transcripts explained by the SNP, the phenotypic proportion of traits explained by the SNP, or the phenotypic proportion of traits explained by trait signatures.
Discussion
We systematically investigated the heritability of global gene expression using whole blood samples from 5626 FHS participants. To our knowledge, this is the largest heritability study of global gene expression. We observed that approximately 40% of the genome (7161 gene) display and ~10% displayed . We confirmed that transcripts with higher heritability estimates are more likely to be associated with cis eQTLs, but this is not the case for trans eQTL (Dixon et al. 2007; Goring et al. 2007; Grundberg et al. 2012). Moreover, we found that single cis eQTLs explain on average 0.33–0.53 of variance in expression levels for genes with . Single trans eQTLs, however, only explain 0.02–0.07 of variance in transcripts with . Interestingly we discovered that the top cis eQTLs tend to explain more variance in the respective transcript when increased, but there is no obvious trend for the top trans eQTLs. Taking BMI as a case study, by cross-linking the cis/trans eQTLs with BMI GWAS SNPs (Hindorff et al. 2009; Speliotes et al. 2010) and BMI signature genes, we observed that some BMI GWAS SNPs are actually the cis eQTLs for multiple BMI signature genes. Although these SNPs (also cis eQTLs) explain only very small proportions of variance in BMI, they explain relatively larger proportions of variance in gene expression levels. These BMI signature genes -- driven by the BMI GWAS SNPs -- jointly explain a larger proportion of the variability in BMI than do the GWAS SNPs.
We found that 40% of gene transcript levels are heritable and the average heritability of global gene expression genome-wide is estimated to be 0.07 (for all 18,000 genes) and 0.13 (for 7,161 genes with ). Several studies reported that 40–70% of gene transcripts are heritable with (Emilsson et al. 2008; Goring et al. 2007; Price et al. 2011; Stranger et al. 2007). Dixon et al reported that the average heritability for global gene expression is 0.2 (Dixon et al. 2007), and the average heritability for transcripts with range between 0.15 and 0.30 (Emilsson et al. 2008; Price et al. 2011; Stranger et al. 2007). These heritability estimates are considerable larger than our findings. By sub-sampling FHS families, we discovered that small sample sizes and relatively simple family structures display a wider range of average heritability estimates (Supplementary Figures S3) than large and complex family structures, which may partly explain the different heritability estimates reported in several previous studies and in our sample. Heritability is the proportion of phenotypic variation attributable to genetic variation; varying levels of pedigree structures are needed to obtain accurate variance parameters from genetic and environmental components in order to get more accurate estimation of heritability. The current study sample (N=5626) that consists of extended families of two-generations of FHS participants is five times larger than the sample sizes of prior studies (Emilsson et al. 2008; Goring et al. 2007; Price et al. 2011; Stranger et al. 2007). In addition, the heritability estimates are further compared between the offspring cohort (N=2446) and the third generation cohort (N=3180), and between samples with higher vs. lower WBC, neutrophil, or lymphocyte proportions. With a large sample size, complex family structure, and careful study design, we believe that the heritability estimates of global gene expression in the current study are more stable and reliable than previous findings.
In this study, we confirmed that cis components explain a larger proportion of variance in expression levels of genes than trans components (Grundberg et al. 2012; Price et al. 2011). In addition, we discovered that the proportion of genetic variance in a transcript explained by the peak eQTL is different for transcripts with different heritability estimates. On average, approximately 53% of genetic variance for transcripts with is explained by a single cis eQTL, and the proportion decrease to 33% for transcripts with to 0.3. This finding suggests that, in general, transcripts with higher heritability estimates are affected by fewer nearby genetic loci compared to transcripts with lower heritability. In contrast, the most significant trans eQTLs explain, on average, much smaller proportions of genetic variance in transcripts and do not show obvious trends in relation to heritability estimates, ( ranges from 0.02 to 0.07 for transcripts on average in all levels). Grundberg et al. estimated that at least 0.12 of total heritability of transcripts is “missed” when only considering common cis eQTL effects, and they proposed that the “missing heritability” might be due to low-frequency and rare variants (Grundberg et al. 2012). However, our findings suggest that the “missing heritability” may also be attributable in part to trans eQTLs with modest genetic effects that are not detected easily in smaller sized eQTL studies.
By cross-linking GWAS results for metabolic traits with transcripts and with cis and trans eQTLs, we identified a number of GWAS SNPs that explain large proportions of the variation in several genes (Table 2–3). For example, rs12936231, a cis eQTL for GSDMB, explains up to 92% of genetic variance in GSDMB. We also discovered that some GWAS SNPs, that we consider to represent master eQTLs, are associated with multiple transcripts. For example, rs3184504 is a trans eQTL for 5 genes, FCGR1A, ARHGEF40, MYADM, IDO1, and IFIT3. The mechanism underlying the associations of multiple transcripts with a single eQTL (a master eSNP) has not been elucidated to date. One possible mechanism is that transcripts sharing common cis/trans eQTLs may be tightly co-expressed and involved in the same biological process or pathway (Rotival et al. 2011). Another possible explanation is that the cis /trans eQTL and its proxy SNPs may have an effect on a regulatory component, such as transcription factors (TFs) or microRNAs (miRNAs) that regulate multiple transcripts, and this eQTL is thereby associated with multiple transcripts targeted by the TFs or the miRNAs. Figure 4B illustrates a “master” effect by a trans eQTL that is located in or near a TF or a key regulator. Different alleles of the trans eQTL may affect the transcription or translation of the TF or key regulator, giving rise to different isoforms of the TF or key regulation. Multiple genes whose expression levels are regulated by those TFs or key regulators may show association with this SNP. For example, rs289754 is located in the intron of NLRC5 (16q13) and is a trans eQTL for three genes: BTN3A1 (6p22), HCP5 (6p21.3) and HLA-F (6p21.3). Rs289754 explained 0.19, 0.10, and 0.04 of the heritability of BTN3A1, HCP5 and HLA-F, respectively (Table 4). NLRC5 has been newly recognized as a key regulator of MHC class I related immune functions (Kobayashi and van den Elsen 2012). However, NLRC5 itself does not have a DNA-binding domain and must interact with multiple proteins to activate the MHC class I dependent immune response (Kobayashi and van den Elsen 2012). The third possible explanation is that the common cis eQTLs in the nearby region display allelic effects on the nucleosome, chromatin structure, or TF binding affinity, and in this way govern the transcriptional activity of nearby genes. Figure 4A shows a mechanism by which a “master” cis eQTL affects transcription of multiple nearby genes. Supportive evidence exists for rs12936231 (17q21), which displays CTCF promoter binding in eight cell lines (Verlaan et al. 2009). CTCF is a well-characterized protein involved in chromatin looping and chromosome repositioning. The same study (Verlaan et al. 2009) reported that rs12936231 is a significant cis eQTL for GSDMB (distance ~28KB from transcription start site [TSS]) and ORMDL3 (distance ~48 KB from TSS) gene expression. We were able to confirm that rs12936231 is significantly associated with transcripts from the nearby genes GSDMB (p value=0) and ORMDL3 (p value = 1.34e-92). In addition, we discovered that rs12936231 explain 0.94 of heritability of GSDMB and 0.34 of the heritability of ORMDL3 expression levels (Table 3), thereby providing further evidence that rs12936231 regulates both genes. However, a functional study is needed to further elucidate the role of the eQTL in relation to gene regulation. We further linked the trait-GWAS SNPs and trait-signature genes via their eQTLs. Taking BMI as proof-of-concept case study, we found several BMI signature genes that are associated with BMI GWAS SNPs in a cis manner. For example, BMI GWAS SNP rs7359397 (p=1.64e-14 in BMI GWAS (Speliotes et al. 2010)) is a cis eQTL for 3 BMI signature genes (SULT1A1, SPNS1, and TUFM, Figure 4 and Table 4) among its six cis eQTL genes. These three BMI signature genes extend the proportion of phenotypic variance of BMI explained by rs7359397.
Conclusions
We systematically evaluated the heritability of global whole blood gene expression using a large sample size of FHS extended pedigrees. We discovered that with increasing heritability of transcripts, there is an increase in the contribution of cis, but not trans regulation. Future studies are needed to investigate if this finding holds true across different tissues. By cross-linking the eQTLs with trait-associated SNPs (i.e. BMI GWAS SNPs (Hindorff et al. 2009)), we discovered that some GWAS SNPs are cis eQTLs of certain gene transcripts and can explain large proportions of variance in expression of these transcripts. In addition, some of the corresponding cis eQTL gene transcripts show differential expression in relation to the same trait (i.e. BMI). Although the GWAS SNPs only explain a small proportion of phenotypic variance in BMI, the differentially expressed cis eQTL related gene transcripts explained a larger proportion of variance in BMI. Overall, our results shed light on the impact of eQTLs on the heritability of the human blood transcriptome, and the role of eQTLs in promoting phenotype differences and disease susceptibility.
Supplementary Material
Acknowledgements
D. L., C. L. and T.H. designed, directed, and supervised the project. D. L was responsible for funding of the project. T. H., C. L. and D. L. drafted the manuscript. P. C. organized the experiment material and data exchange. All authors participated in revising and editing the manuscripts. All authors have read and approved the final version of the manuscript.
Footnotes
Disclosure declaration
The authors declare no conflict of interest.
References
- Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cardon LR, Cookson WO, Sham PC, Cherny SS. Association analysis in a variance components framework. Genet Epidemiol. 2001;21(Suppl 1):S341–S346. doi: 10.1002/gepi.2001.21.s1.s341. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995:289–300. [Google Scholar]
- Cheung VG, Spielman RS. Genetics of human gene expression: mapping DNA variants that influence gene expression. Nature Reviews Genetics. 2009;10:595–604. doi: 10.1038/nrg2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KC, Taylor J, Burnett E, Gut I, Farrall M, Lathrop GM, Abecasis GR, Cookson WO. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O'Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjogren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimaki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, Aspelund T, Garcia M, Chang YP, O'Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, Carlson S, Helgason A, Walters GB, Gunnarsdottir S, Mouy M, Steinthorsdottir V, Eiriksdottir GH, Bjornsdottir G, Reynisdottir I, Gudbjartsson D, Helgadottir A, Jonasdottir A, Styrkarsdottir U, Gretarsdottir S, Magnusson KP, Stefansson H, Fossdal R, Kristjansson K, Gislason HG, Stefansson T, Leifsson BG, Thorsteinsdottir U, Lamb JR, Gulcher JR, Reitman ML, Kong A, Schadt EE, Stefansson K. Genetics of gene expression and its effect on disease. Nature. 2008;452:423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Goring HHH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, Cole SA, Jowett JBM, Abraham LJ, Rainwater DL, Comuzzie AG, Mahaney MC, Almasy L, MacCluer JW, Kissebah AH, Collier GR, Moses EK, Blangero J. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nature Genetics. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- Grundberg E, Small KS, Hedman AK, Nica AC, Buil A, Keildson S, Bell JT, Yang TP, Meduri E, Barrett A, Nisbett J, Sekowska M, Wilk A, Shin SY, Glass D, Travers M, Min JL, Ring S, Ho KR, Thorleifsson G, Kong A, Thorsteindottir U, Ainali C, Dimas AS, Hassanali N, Ingle C, Knowles D, Krestyaninova M, Lowe CE, Di Meglio P, Montgomery SB, Parts L, Potter S, Surdulescu G, Tsaprouni L, Tsoka S, Bataille V, Durbin R, Nestle FO, O'Rahilly S, Soranzo N, Lindgren CM, Zondervan KT, Ahmadi KR, Schadt EE, Stefansson K, Smith GD, McCarthy MI, Deloukas P, Dermitzakis ET, Spector TD, R MTHE. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nature Genetics. 2012;44 doi: 10.1038/ng.2394. 1084-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Lanktree MB, Taylor KC, Hakonarson H, Lange LA, Keating BJ, Fairfax BP, Elbers CC, Barnard J, Farrall M. Gene-centric meta-analyses of 108 912 individuals confirm known body mass index loci and reveal three novel signals. Human Molecular Genetics. 2013;22:184–201. doi: 10.1093/hmg/dds396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan T, Zhang B, Wang Z, Joehanes R, Zhu J, Johnson AD, Ying S, Munson PJ, Raghavachari N, Wang R, Liu P, Courchesne P, Hwang SJ, Assimes TL, McPherson R, Samani NJ, Schunkert H, Meng Q, Suver C, O'Donnell CJ, Derry J, Yang X, Levy D. A systems biology framework identifies molecular underpinnings of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1427–1434. doi: 10.1161/ATVBAHA.112.300112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joehanes R, Ying S, Huan T, Johnson AD, Raghavachari N, Wang R, Liu P, Woodhouse KA, Sen SK, Tanriverdi K, Courchesne P, Freedman JE, O'Donnell CJ, Levy D, Munson PJ. Gene expression signatures of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1418–1426. doi: 10.1161/ATVBAHA.112.301169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi KS, van den Elsen PJ. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol. 2012;12:813–820. doi: 10.1038/nri3339. [DOI] [PubMed] [Google Scholar]
- Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic epidemiology. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta LA, Peyvandi F. Addressing the complexity of cardiovascular disease by design. Lancet. 2011;377:356–358. doi: 10.1016/S0140-6736(10)62240-4. [DOI] [PubMed] [Google Scholar]
- Monda KL, Chen GK, Taylor KC, Palmer C, Edwards TL, Lange LA, Ng MC, Adeyemo AA, Allison MA, Bielak LF. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nature genetics. 2013 doi: 10.1038/ng.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nature genetics. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AJ, Gibbs JR, Webster JA, Rohrer K, Zhao A, Marlowe L, Kaleem M, Leung D, Bryden L, Nath P, Zismann VL, Joshipura K, Huentelman MJ, Hu-Lince D, Coon KD, Craig DW, Pearson JV, Holmans P, Heward CB, Reiman EM, Stephan D, Hardy J. A survey of genetic human cortical gene expression. Nat Genet. 2007;39:1494–1499. doi: 10.1038/ng.2007.16. [DOI] [PubMed] [Google Scholar]
- Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O'Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O'Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Helgason A, Thorleifsson G, McCarroll SA, Kong A, Stefansson K. Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals. Plos Genetics. 2011;7 doi: 10.1371/journal.pgen.1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy A, Trabzuni D, Gibbs JR, Dillman A, Hernandez DG, Arepalli S, Walker R, Smith C, Ilori GP, Shabalin AA. Resolving the polymorphism-in-probe problem is critical for correct interpretation of expression QTL studies. Nucleic acids research. 2013;41:e88–e88. doi: 10.1093/nar/gkt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotival M, Zeller T, Wild PS, Maouche S, Szymczak S, Schillert A, Castagne R, Deiseroth A, Proust C, Brocheton J, Godefroy T, Perret C, Germain M, Eleftheriadis M, Sinning CR, Schnabel RB, Lubos E, Lackner KJ, Rossmann H, Munzel T, Rendon A, Erdmann J, Deloukas P, Hengstenberg C, Diemert P, Montalescot G, Ouwehand WH, Samani NJ, Schunkert H, Tregouet DA, Ziegler A, Goodall AH, Cambien F, Tiret L, Blankenberg S. Integrating genome-wide genetic variations and monocyte expression data reveals trans-regulated gene modules in humans. PLoS Genet. 2011;7:e1002367. doi: 10.1371/journal.pgen.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt EE, Molony C, Chudin E, Hao K, Yang X, Lum PY, Kasarskis A, Zhang B, Wang S, Suver C, Zhu J, Millstein J, Sieberts S, Lamb J, GuhaThakurta D, Derry J, Storey JD, Avila-Campillo I, Kruger MJ, Johnson JM, Rohl CA, van Nas A, Mehrabian M, Drake TA, Lusis AJ, Smith RC, Guengerich FP, Strom SC, Schuetz E, Rushmore TH, Ulrich R. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Magi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segre AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpelainen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- Stranger BE, Forrest MS, Clark AG, Minichiello MJ, Deutsch S, Lyle R, Hunt S, Kahl B, Antonarakis SE, Tavare S, Deloukas P, Dermitzakis ET. Genome-wide associations of gene expression variation in humans. PLoS Genet. 2005;1:e78. doi: 10.1371/journal.pgen.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger BE, Nica AC, Forrest MS, Dimas A, Bird CP, Beazley C, Ingle CE, Dunning M, Flicek P, Koller D, Montgomery S, Tavare S, Deloukas P, Dermitzakis ET. Population genomics of human gene expression. Nature Genetics. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenesa A, Haley CS. The heritability of human disease: estimation, uses and abuses. Nat Rev Genet. 2013;14:139–149. doi: 10.1038/nrg3377. [DOI] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O'Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaan DJ, Berlivet S, Hunninghake GM, Madore AM, Lariviere M, Moussette S, Grundberg E, Kwan T, Ouimet M, Ge B, Hoberman R, Swiatek M, Dias J, Lam KC, Koka V, Harmsen E, Soto-Quiros M, Avila L, Celedon JC, Weiss ST, Dewar K, Sinnett D, Laprise C, Raby BA, Pastinen T, Naumova AK. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am J Hum Genet. 2009;85:377–393. doi: 10.1016/j.ajhg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segre AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bostrom KB, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney ASF, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PRV, Jorgensen T, Kao WHL, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JRB, Petersen A-K, Platou C, Proenca C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparso T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra H-J, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, Christiansen MW, Fairfax BP, Schramm K, Powell JE. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nature genetics. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, Maouche S, Germain M, Lackner K, Rossmann H, Eleftheriadis M, Sinning CR, Schnabel RB, Lubos E, Mennerich D, Rust W, Perret C, Proust C, Nicaud V, Loscalzo J, Hubner N, Tregouet D, Munzel T, Ziegler A, Tiret L, Blankenberg S, Cambien F. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.