Abstract
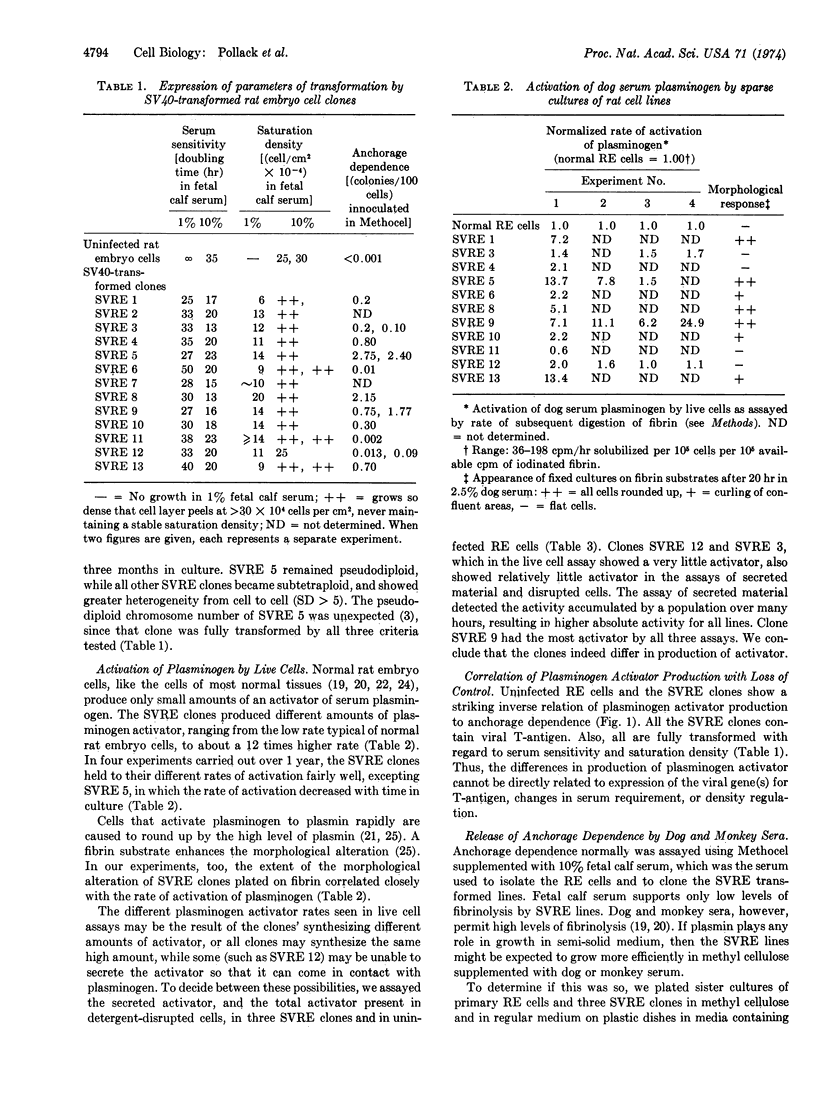
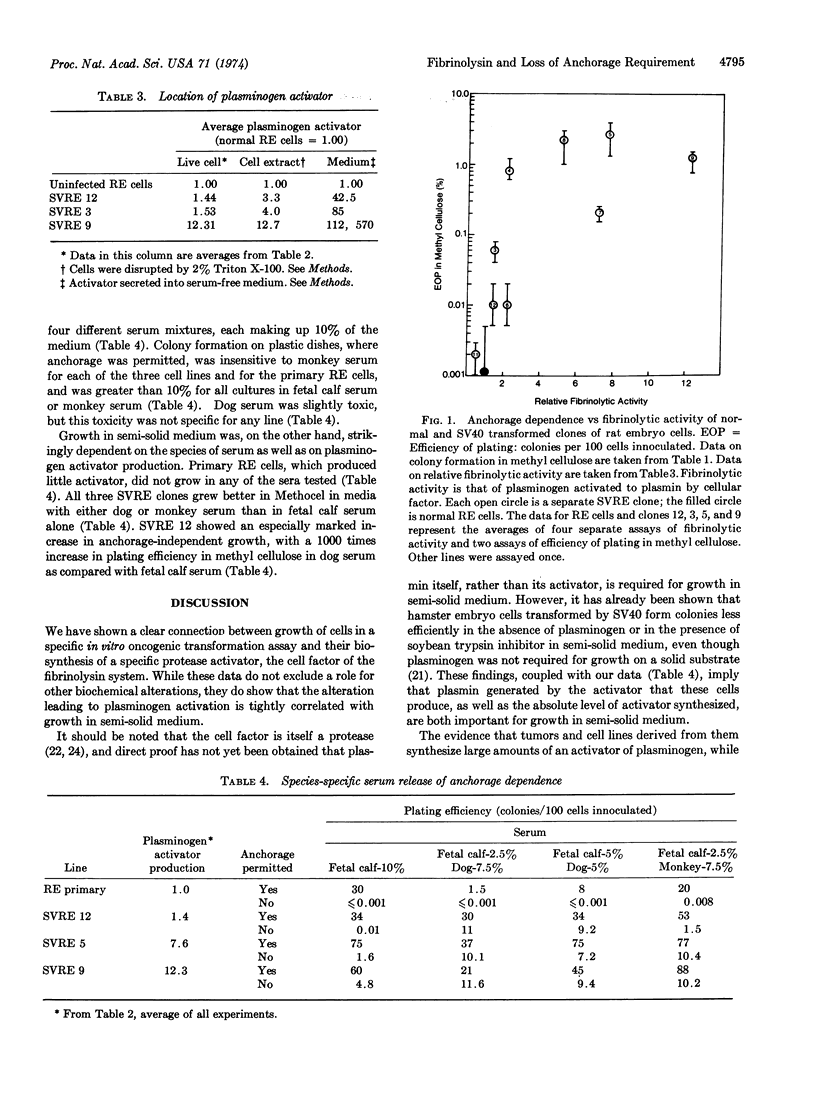
We have isolated several lines of rat embryo cells transformed by simian virus 40. All these lines are fully transformed with regard to saturation density and serum sensitivity, but they differ greatly in their anchorage dependence, as assayed by efficiency of plating in methyl cellulose suspension. This set of lines reveals a consistent relation of plasminogen activator production to plating efficiency in methyl cellulose. T-antigen-positive transformed lines that synthesize activator grow in methyl cellulose suspension, while T-antigen-positive transformed lines that do not synthesize activator fail to form colonies in suspension. Normal rat embryo cells produce very little plasminogen activator and do not grow in methyl cellulose. Sera that permit high levels of plasmin formation and activity support growth in semi-solid medium better than sera whose plasminogen is activated poorly and/or sera that contain inhibitors to plasmin.
Keywords: semi-solid medium, oncogenic viruses, fibrinolysis, serum
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- BARNETT E. V., BARON S. An activator of plasminogen produced by cell culture. Proc Soc Exp Biol Med. 1959 Nov;102:308–311. doi: 10.3181/00379727-102-25229. [DOI] [PubMed] [Google Scholar]
- Castor L. N. Control of division by cell contact and serum concentration in cultures of 3T3 cells. Exp Cell Res. 1971 Sep;68(1):17–24. doi: 10.1016/0014-4827(71)90581-7. [DOI] [PubMed] [Google Scholar]
- DEFENDI V., LEHMAN J., KRAEMER P. "Morphologically normal" hamster cells with malignant properties. Virology. 1963 Apr;19:592–598. doi: 10.1016/0042-6822(63)90058-8. [DOI] [PubMed] [Google Scholar]
- Defendi V., Lehman J. M. Transformation of hamster embryo cells in vitro by polyoma virus: morphological, karyological, immunological and transplantation characteristics. J Cell Physiol. 1965 Dec;66(3):351–409. doi: 10.1002/jcp.1030660313. [DOI] [PubMed] [Google Scholar]
- Eagle H., Foley G. E., Koprowski H., Lazarus H., Levine E. M., Adams R. A. Growth characteristics of virus-transformed cells. Maximum population density, inhibition by normal cells, serum requirement, growth in soft agar, and xenogeneic transplantability. J Exp Med. 1970 Apr 1;131(4):863–879. doi: 10.1084/jem.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Grady L. J., North A. B. Some effects of 5-bromodeoxyuridine on polyoma-transformed mouse cells. Exp Cell Res. 1974 Jul;87(1):120–126. doi: 10.1016/0014-4827(74)90532-1. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Macpherson I. The basis of the tumorigenicity of BHK 21 cells. Int J Cancer. 1968 Sep 15;3(5):654–662. doi: 10.1002/ijc.2910030514. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Maroudas N. G. Anchorage dependence: correlation between amount of growth and diameter of bead, for single cells grown on individual glass beads. Exp Cell Res. 1972 Oct;74(2):337–342. doi: 10.1016/0014-4827(72)90385-0. [DOI] [PubMed] [Google Scholar]
- Oey J., Vogel A., Pollack R. Intracellular cyclic AMP concentration responds specifically to growth regulation by serum. Proc Natl Acad Sci U S A. 1974 Mar;71(3):694–698. doi: 10.1073/pnas.71.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Requirement of plasminogen for correlated changes in cellular morphology, colony formation in agar, and cell migration. J Exp Med. 1973 Nov 1;138(5):1056–1064. doi: 10.1084/jem.138.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Quigley J. P., Reich E. Fibrinolysis associated with oncogenic transformation. Morphological correlates. J Biol Chem. 1974 Jul 10;249(13):4312–4320. [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Ossowski L., Reich E. Plasminogen, the serum proenzyme activated by factors from cells transformed by oncogenic viruses. J Biol Chem. 1974 Jul 10;249(13):4306–4311. [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., LIKELY G. D., EARLE W. R. The development of variations in transplantability and morphology within a clone of mouse fibroblasts transformed to sarcoma-producing cells in vitro. J Natl Cancer Inst. 1954 Oct;15(2):215–237. [PubMed] [Google Scholar]
- Stoker M. G., Thornton M., Riddle P., Birg F., Meyer G. Surface antigen and polypseudopodia in abortive transformation of BHK 21 cells by polyoma virus. Int J Cancer. 1972 Nov;10(3):613–618. doi: 10.1002/ijc.2910100321. [DOI] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J., Dano K., Kellerman G. M., Reich E. Fibrinolysis associated with oncogenic transformation. Partial purification and characterization of the cell factor, a plasminogen activator. J Biol Chem. 1974 Jul 10;249(13):4295–4305. [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of cells transformed by polyma virus. Cold Spring Harb Symp Quant Biol. 1962;27:367–374. doi: 10.1101/sqb.1962.027.001.034. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Veselý P., Sindelarova J. Growth regulation and tumour formation of normal and neoplastic rat cells. Int J Cancer. 1973 Jan 15;11(1):77–89. doi: 10.1002/ijc.2910110109. [DOI] [PubMed] [Google Scholar]
- Wiblin C. N., MacPherson I. A. The transformation of BHK 21 hamster cells by simian virus 40. Int J Cancer. 1972 Sep 15;10(2):296–309. doi: 10.1002/ijc.2910100210. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Till J. E. Formation of lung colonies by polyoma-transformed rat embryo cells. J Natl Cancer Inst. 1966 Aug;37(2):177–184. [PubMed] [Google Scholar]
- Wyke J. Phenotypic variation and its control in polyoma-transformed BHK21 cells. Exp Cell Res. 1971 May;66(1):209–223. doi: 10.1016/s0014-4827(71)80031-9. [DOI] [PubMed] [Google Scholar]



