The dose-effect safety profile of skeletal muscle precursor cell (skMPC) therapy was tested in a dog model of intrinsic urinary sphincter deficiency. Injection of different doses of skMPCs had no effects on the body weight, blood cell count, or kidney or liver function test results. Urinary sphincter injection of skMPCs resulted in no significant local or systemic pathologic features within the dose range that improves sphincter pressures.
Keywords: Maximal urethral pressure, Skeletal muscle, Urinary incontinence, Stem cells, Pathology
Abstract
Locally injected skeletal muscle precursor cells (skMPCs) integrate into and restore the muscle layers, innervation, vasculature, and function of the sphincter complex in animal models of intrinsic urinary sphincter deficiency (ISD). The goal of the present study was to test the dose-effect safety profile of skMPC therapy in a dog model of ISD. Sphincter deficiency was created in 20 adult female dogs by surgically removing the skeletal muscle layer of the urinary sphincter complex. skMPCs isolated from the hind leg were expanded in culture and injected 4 weeks later into the sphincter complex at a dose of 25 million cells (n = 5), 50 million cells (n = 5), or 100 million cells (n = 5) per milliliter in a 2-ml volume. Five dogs received no sphincter injection. The measures of maximal sphincter pressure, complete blood count, and blood chemistry were performed monthly until their sacrifice at 9 months. At that point, full necropsy was performed to assess the safety of the skMPC injections. Injection of different doses of cells had no effects on the body weight, blood cell count, or kidney or liver function test results (p > .05 among the skMPC doses). Some incidental pathologic features were found in the lower urinary tract in all groups and were most likely associated with repeat catheterization. The maximal urinary sphincter pressure was higher in the 50 million cells per milliliter treatment group than in the other experimental groups (p < .05). The findings of the present study have confirmed that urinary sphincter injection of skMPCs results in no significant local or systemic pathologic features within the dose range that improves sphincter pressures.
Introduction
Urinary incontinence, including stress urinary incontinence (SUI), is a major urological/gynecological problem. As many as 25% of women older than 20 years have urinary incontinence, and 50% and 36% of these women have SUI alone or with urge incontinence, respectively [1–4]. The risk of SUI increases at or after middle age, and the prevalence of SUI peaks during the perimenopausal years [4]. SUI imposes substantial financial burden on the individual and society both, with treatment costs estimated to be up to $16 billion annually in the United States alone [5]. Current treatments of SUI include conservative options such as physical therapy [6] and biofeedback [7], pharmacological therapy [8], bulking agents [9], and surgical approaches [10], all of which have limitations, and alternatives are needed.
Cell therapy is currently proposed to restore functional muscle cells and aid in closure of the sphincter in women with intrinsic urinary sphincter deficiency (ISD) that results from aging and trauma-induced loss of sphincter innervation and muscle cells [1–4]. The cell types considered for use include bone marrow mesenchymal cells [11], adipose-derived stem cells [12], and amniotic fluid-derived stem cells [13]. The present study used skeletal muscle-derived precursor cells (skMPCs), shown in rodents, dogs, and nonhuman primates to directly integrate into the sphincter musculature and restore its volume and function, restoring somatic and adrenergic innervation and vasculature [14–18]. Although providing beneficial structural and functional efficacy, an important task is to test its safety at doses shown to improve sphincter structure and function [14].
Adult female dogs with experimentally produced ISD were used in the present study. Autologous skMPCs were injected into the urinary sphincter complex at a dose similar to that previously shown to be efficacious [14] and at one half that amount and twice that amount. We can now report that the original dose is the most effective and that none of the doses of cells produced local or systemic pathologic features.
Materials and Methods
Study Design
Young adult female Beagle dogs (age 12–15 months) were used in the present experiment. The Wake Forest University Institutional Animal Care and Use Committee approved the study, which was performed in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Euthanasia was performed according to the standards of the American Veterinary Medical Association. The overall study design is depicted in Figure 1.
Figure 1.
Overall study design. “Injury” indicates the skeletal muscle stripping of urinary sphincter complex. “Cells” indicates autologous skeletal muscle-derived precursor cells collected from a muscle biopsy. “Carrier” indicates vehicle (Dulbecco’s modified Eagle’s medium without added serum). Maximal urethral pressures and blood samples were taken before the injury procedure, immediately before the sphincter injections, and once each month for 9 months. Euthanasia and tissue collection were performed 9 months after the sphincter injections. Abbreviation: H&E, hematoxylin and eosin.
Twenty dogs started the experiment (5 dogs per study group), and 18 completed the study. Two dogs were euthanized (1 in the no injection group and 1 in the 25 million cells per milliliter injection group); 1 because of an unresponsive urinary tract infection and 1 because of postoperative bleeding complications. None were euthanized as a consequence of cell injection.
ISD Model
The sphincter was microsurgically removed in all 20 study animals. Anesthesia was induced with 4–6 mg of i.v. propofol and maintained with 1%–3% isoflurane. All the dogs were placed in supine position, intubated, and ventilated. The anesthesia was maintained using 1%–3% isoflurane. The surgical area was shaved and scrubbed with Dermabond povidone-iodine solution (Ethicon Inc., Somerville, NJ, http://www.ethicon.com). According to our recently established model of irreversible sphincter damage [14], approximately 25% of the circumferential muscle fibers in the sphincter were removed. At the same time, a muscle biopsy was taken via a 2-cm-long incision over the quadriceps muscle of the hind leg for collection of skMPCs. One month after model generation, urodynamic analysis was used to confirm the sphincter damage, and the treatment group received an injection of autologous muscle-derived precursor cells (MPCs).
Cell Harvest/Culture and Injection
The muscle biopsy tissue was placed in sterile Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, http://www.invitrogen.com), and isolation of skMPCs was completed as previously described [14]. The skeletal muscle biopsy was minced into small pieces (≤1 mm3), incubated for 2 hours at 37°C in DMEM containing 0.2% (wt/vol) type I collagenase (Worthington Biochemical Corp., Lakewood, NJ, http://www.worthington-biochem.com), and exposed to gentle trituration for isolation of single skeletal muscle fibers (confirmed by microscopy). The isolated muscle fibers were then washed 3 times with phosphate-buffered saline and transferred onto Matrigel (1 mg/ml; Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com) precoated 35-mm dishes containing 3 ml of myogenic medium (20% fetal bovine serum, 10% horse serum [Gibco], 0.5% chick embryo extract, and 1% penicillin/streptomycin in DMEM) and incubated at 37°C and 5% CO2/95% O2 until confluent. MPCs migrating from the plated myofibers were trypsinized and plated in 10-cm culture dishes with complete medium containing 10% fetal bovine serum and 1% penicillin/streptomycin in DMEM. The cell surface markers of canine skMPCs have been previously published by our group [14].
Cell Injection
After 4–6 weeks in culture, 50 × 106, 100 × 106, or 200 × 106 cells were trypsinized and transferred into a conical tube for labeling and subsequent mixing with 2 ml of DMEM without serum for injection. Subsequently, a final concentration of 25, 50, or 100 cells × 106/ml cells was injected into the sphincter complex of the dogs and is how the data are presented. A low midline abdominal incision was made in all but the 5 noninjected (control) dogs to expose the damaged sphincter region. The exact location of the damage was determined using the marking sutures left during model generation. The cells were carefully injected at the depth of the remaining muscle and at the 3-, 6-, 9-, and 12-o’clock positions around the circumference of the sphincter complex using a 25-guage needle [14]. Care was taken not to release the cells into the urethral lumen or the peritoneal cavity. After all surgeries, observations were made every 15 minutes until the dogs were fully awake and active. The dogs were carefully monitored for signs of bladder infection, and urine samples were taken if any concern was present.
Urodynamic Evaluation
All examinations were performed with the dogs under general anesthesia and in the supine position. The anesthesia depth was carefully monitored by evaluation of the eye reflex, heart rate, and systolic blood pressure. We used the Life-Tech Urolab Opus System V (Life-Tech Inc., Stafford, TX, http://www.life-tech.com) combined with a Millar microtip transducer catheter (Millar Instruments, Inc., Houston, TX, http://www.millar.com) and a rectal balloon catheter [14]. Static urethra pressure profilometry (pressure curve along the length of the urethra) was used to measure the maximal resting urethral sphincter pressure (MUP) at the site of sphincter damage. These were performed at a speed of 0.5 mm/s using a pulling device at one third of the maximal bladder volume. The average of 3 closure pressures within the sphincter region (2 cm) was used for statistical analysis. MUP measurements were taken at baseline, immediately after the sphincter injury, 4 weeks after collection of the cells, immediately before cell injection, and then monthly for 9 months.
Body Weights/Collection of Blood for Cell Counts and Kidney/Liver Enzyme Testing
The body weights of all dogs were measured at the same time points as the MUP values. Similarly, 5 ml of blood was collected from the saphenous vein into EDTA tubes and serum separator heparinized tubes, and the plasma was used for analysis of the red and white blood cell counts using the ADVIA 120 Hematology System (Siemens Healthcare, Terrytown, NY, http://www.healthcare.siemens.com) and the hemoglobin, blood urea nitrogen, creatinine, alanine transaminase (ALT), and aspartate aminotransferase (AST) using the Synchron CX5CE Clinical Systems (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com).
Necropsy and Collection of Tissues
The dogs were first heparinized (100 IU/kg i.v.) and then euthanized using sodium pentobarbital (80–100 mg/kg i.v.). Before the removal of any tissues, a general assessment was made on any visible abnormalities that might be present externally or internally. The entire urinary sphincter complex was then removed and placed in 4% paraformaldehyde for 48 hours. The sections were placed in 70% ethanol and stained with hematoxylin and eosin (H&E). The sphincter muscle cells were identified via immunocytochemistry using an anti-desmin antibody (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) at a dilution of 1:50. All sections were counterstained with Gill’s hematoxylin. The remaining tissue removed at necropsy was placed in 4% neutral buffered formalin, sectioned, and stained with H&E. Representative sections of the urethra, urinary bladder, uterine body, uterine horns, large intestine, regional lymph nodes, liver, spleen, adrenal glands, kidneys, heart, caudal lobe of the lungs, and ovaries were collected and assessed by an independent veterinary pathologist.
Statistical Approach
Data are expressed as the mean ± SEM. Statistical analyses were performed using GraphPad Prism, version 6.0 (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). The initial analysis use one-way analysis of variance. If significance was attained, a post hoc analysis was performed with Dunnett’s multiple comparison testing. A two-tailed Student’s t test was used in the two-group comparisons. Comparisons were made between the control and the 25 million cells per milliliter (50 million cells total), 50 million cells per milliliter (100 million cells total), and 100 million cells per milliliter (200 million cells total) groups.
Results
Body Weights
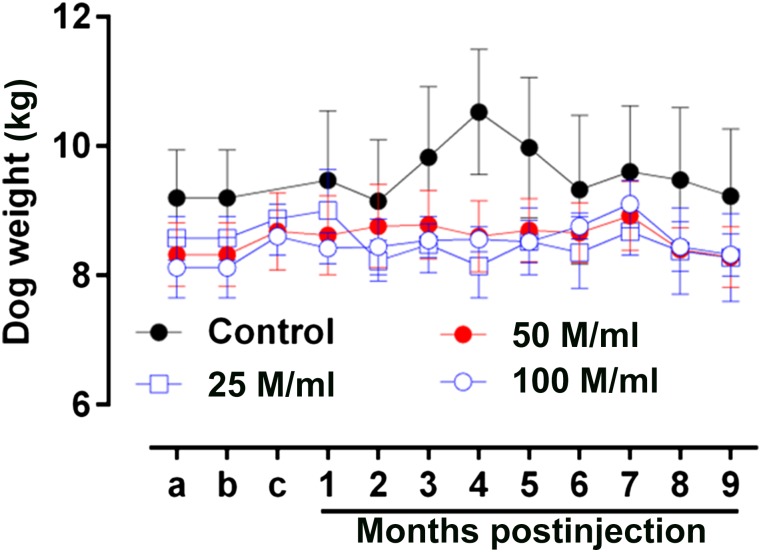
The baseline values for the dog weights were similar at the onset of the experiment (control 9.2 ± 0.7 kg, n = 4, vs. 25 million cells per milliliter 8.5 ± 0.3 kg, n = 4, 50 million cells per milliliter 8.3 ± 0.4 kg, n = 5, or 100 million cells per milliliter 8.1 ± 0.5, n = 5; p = .531; Fig. 2). Furthermore, no significant change was found in the body weight of the dogs throughout the study as assessed by the within-group analysis (control, n = 4, p = .996; 25 million cells per milliliter, n = 4, p = .986; 50 million cells per milliliter, n = 5, p = .999; and 100 million cells per milliliter, n = 5, p = .819).
Figure 2.
Body weights. The baseline values for the animal weights were similar at the onset of the experiment (control: 9.2 ± 0.7 kg, n = 4; vs. 25 million cells per milliliter: 8.5 ± 0.3 kg, n = 4; 50 million cells per milliliter: 8.3 ± 0.4 kg, n = 5; and 100 million cells per milliliter: 8.1 ± 0.5 kg, n = 5; p = .531). Furthermore, no significant change was seen in the body weights of the dogs throughout the study, as assessed by the within-group analysis (control, n = 4, p = .996; 25 million cells per milliliter, n = 4, p = .986; 50 million cells per milliliter, n = 5, p = .999; and 100 million cells per milliliter, n = 5, p = .819). Abbreviation: M, million.
Serum Markers
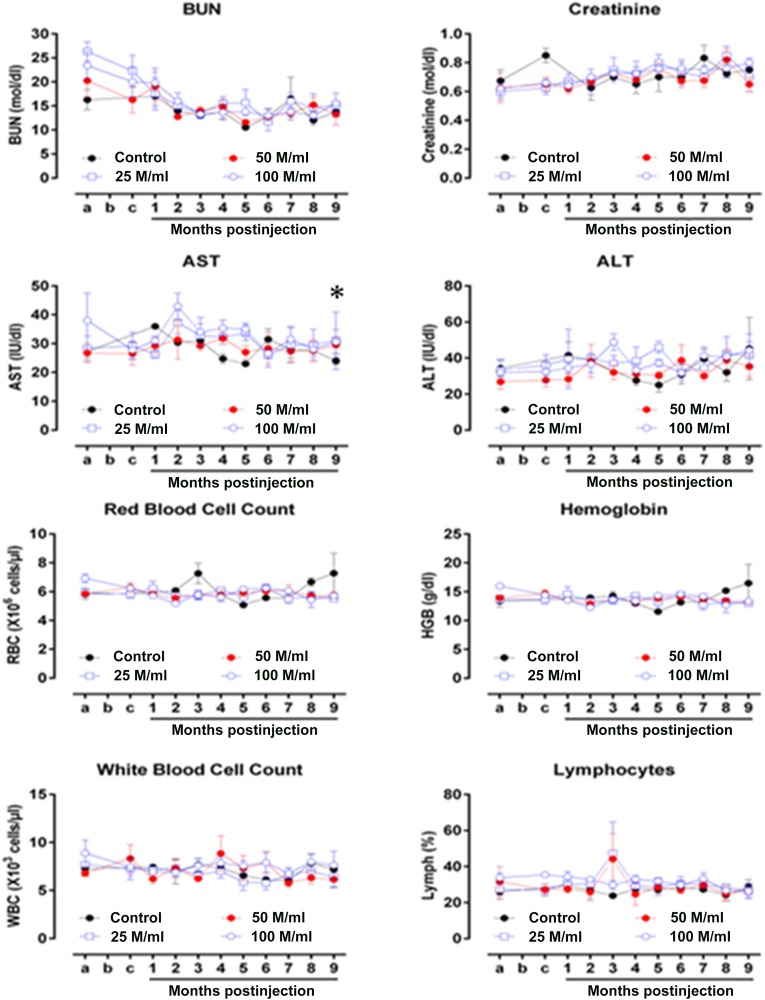
The blood urea nitrogen (BUN) and creatinine concentrations (serum markers of kidney function) were similar among experimental groups throughout the study (BUN, p = .254; creatinine, p = .601, n = 4–5) (Fig. 3). AST, a serum marker of liver function, showed a modest difference between the 100 million cells per milliliter versus control and 50 million cells per milliliter (p = .039, n = 4–5; and p = .048, n = 5, respectively). In contrast, no change was seen in ALT (p = .061, n = 4–5). Finally, the blood hematologic values (hemoglobin [Hgb], red blood cell [RBC] counts, total white blood cell [WBC] count, and lymphocyte count) were similar among the groups throughout the study (Hgb, p = .927; RBC count, p = .569; WBC count, p = .089; and lymphocyte count, p = .100; n = 4–5).
Figure 3.
Serum markers. BUN and creatinine values were similar among the experimental groups throughout the study (BUN: p = .254; creatinine: p = .601, n = 4–5). AST showed a modest difference between the 100 million cells per milliliter and control groups and the 50 million cells per milliliter (∗) (p = .039, n = 4–5; and p = .048, n = 5, respectively). No change was seen in ALT (p = .061, n = 4–5). The hemoglobin (Hgb), red blood cell (RBC) count, total white blood cell (WBC) count, and lymphocyte counts were similar among the groups throughout the study (Hgb: p = .927; RBC count: p = .569; WBC count: p = .089; and lymphocyte count: p = .100; n = 4–5). Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; M, million.
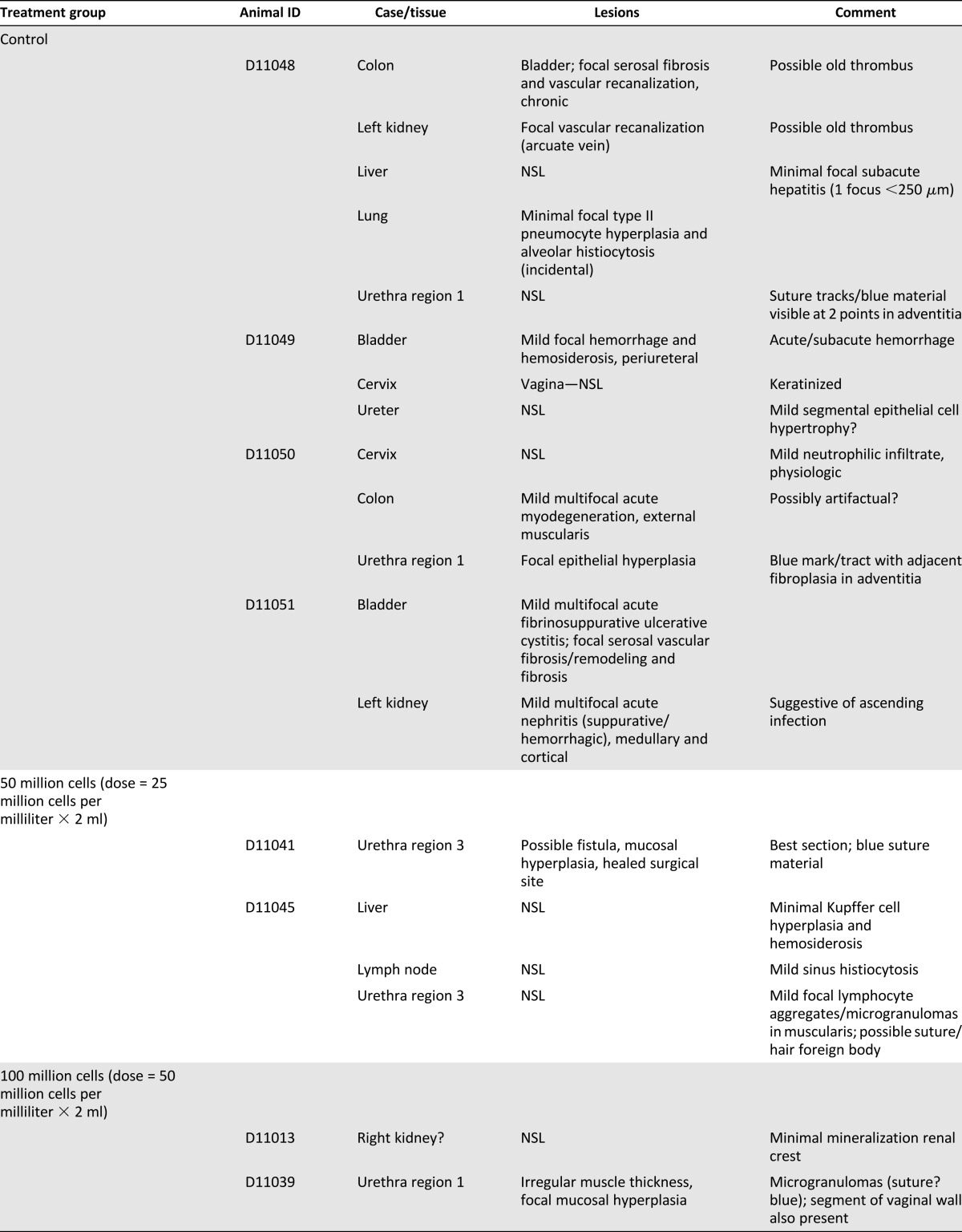
Gross and Tissue Pathologic Features
Unremarkable gross pathologic changes were seen in the dogs (some enlarged lymph nodes and abdominal adhesions in different dogs; Table 1). No patterns of gross pathologic features were found among the treatment groups.
Table 1.
Gross pathology observations
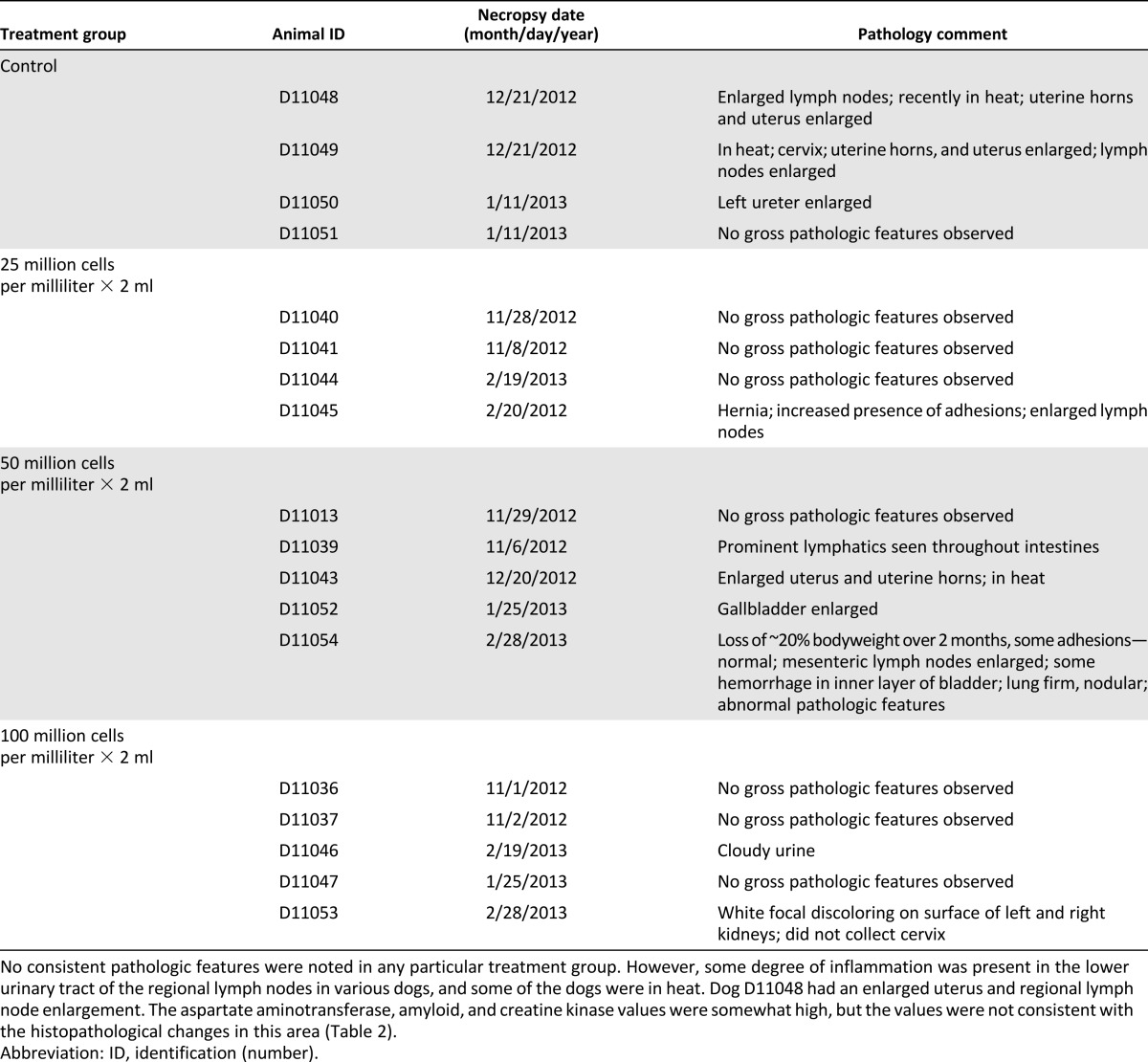
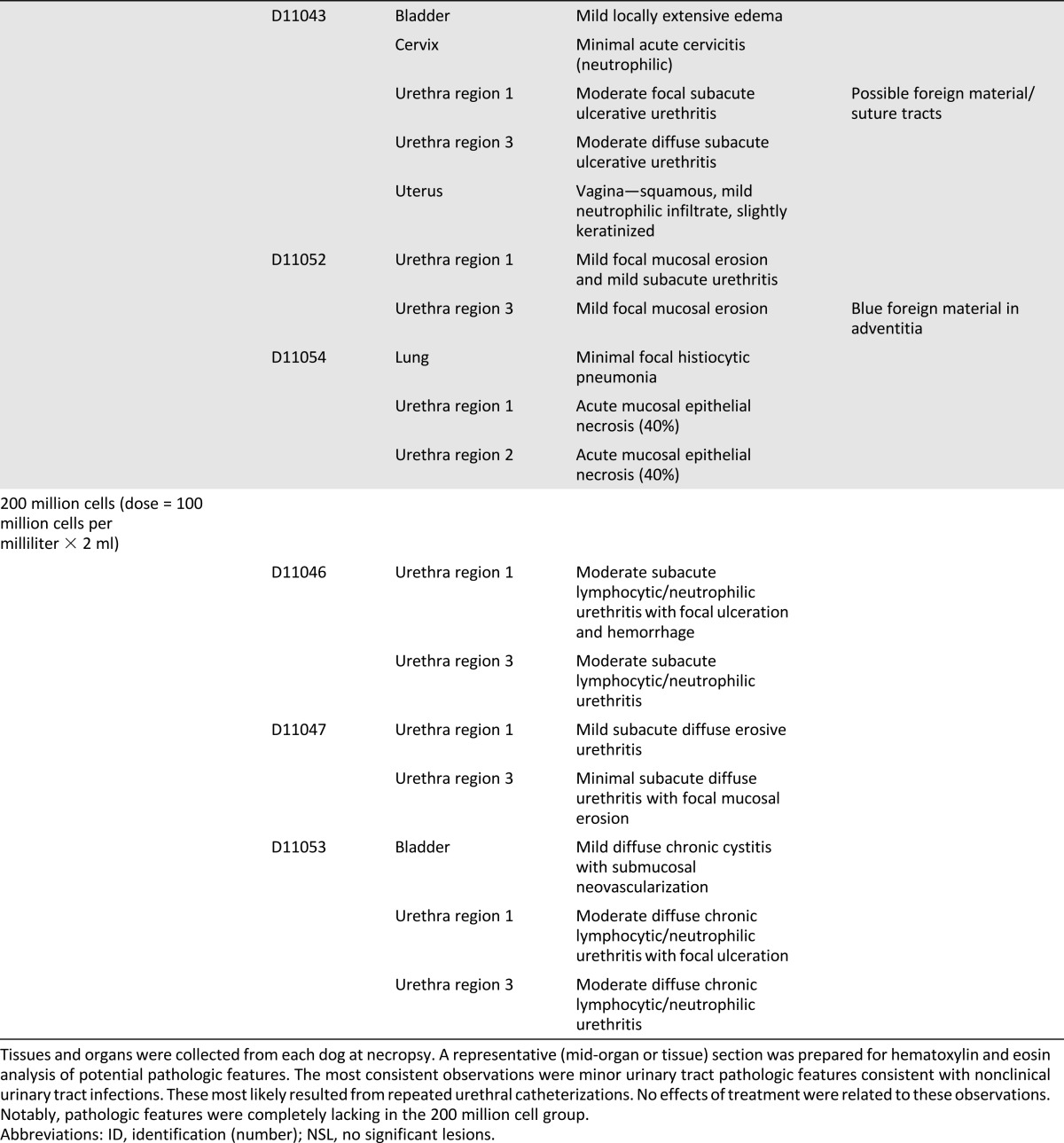
Similar to the gross pathologic findings, no treatment effect was found on tissue pathologic examination among the treatment groups (Table 2). However, histological evidence was present of subacute to chronic urethritis in several of the dogs, regardless of their treatment group, with some evidence of pre-existing infections in other parts of the urinary system (kidney, bladder). The blue suture material noted in several of the urethral samples was the blue Prolene suture (Ethicon) used to mark the cell injection sites. In addition to the rather consistent pathologic features seen in the urinary tract, ancillary pathologic features were noted in the colon and liver of a few dogs.
Table 2.
Histopathological observations
Efficacy
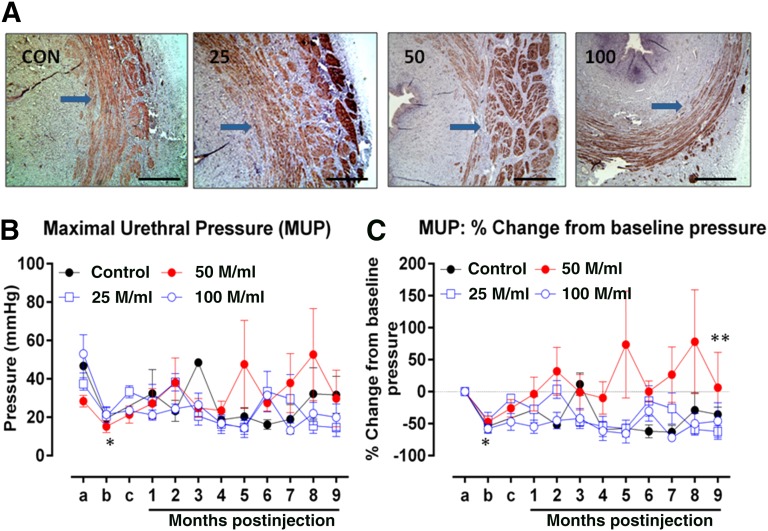
Desmin staining of the sphincter complex confirmed that the muscle layers of the complex in all treatment groups were intact 9 months after the skMPC injections (Fig. 4A). No marked differences were found in the amount of positive desmin staining among the groups. Surgical removal of the outer 25% of the muscle layer of the sphincter complex resulted in a decrease in MUP in all experimental groups (Fig. 4B; point a vs. point b, control: p = .037, n = 4; 25 million cells per milliliter: p = .036, n = 4; 50 million cells per milliliter: p = .007, n = 5; 100 million cells per milliliter: p = .020, n = 5). Furthermore, no difference was found in the postoperative MUPs among the experimental groups (Fig. 3; point b, p = .576, n = 4–5), supportive of equivalent sphincter injury among the groups. The baseline MUPs displayed variability among groups; thus, we chose to assess the effect of skMPCs on the MUPs as a change with respect to the baseline (Fig. 4C). These data indicated that the MUP values in the dogs treated with 50 million cells per milliliter were significantly greater than those in the control (p = .0004), 25 million cells per milliliter (p = .0014), and 100 million cells per milliliter (p < .0001) groups over time (Fig. 4C). However, the MUP values of the other 2 skMPC groups (25 million cells per milliliter and 100 million cells per milliliter) were not different than the control values (p = .956 and p = .871, respectively). No relationships were found between the observed pathologic features and the MUP valves at 9 months.
Figure 4.
Efficacy. (A): Desmin staining (nova red) of the urethral sphincter complex in the CON, 25, 50, and 100. Arrows point to the desmin staining in these cross-sections of the sphincter. Scale bars = 200 µm. (B, C): Sphincter pressures. The actual MUP values (B) and the percentage of change in MUP (C) taken before sphincter injury (point a), immediately after sphincter injury (point b), 4 weeks later immediately before cell injection (point c), and then monthly for 9 months (points 1–9). The mean MUP values for all experimental groups decreased from point a to point b (∗) (CON: p = .037, n = 4; 25: p = .036, n = 4; 50: p = .007, n = 5; 100: p = .020, n = 5). No difference was found in postoperative MUPs among the experimental groups (B) at point b (p = .576, n = 4–5). The MUP values in the dogs treated with 50 million cells per milliliter were significantly greater than those in the CON group (∗∗) (p = .0004) or 25 (p = .0014) and 100 group (p < .0001) (C). However, the MUP values of the other 2 skMPC groups (25 and 100) were not different from the CON values (p = .956 and p = .871, respectively). Abbreviations: 25, 25 million cells per milliliter; 50, 50 million cells per milliliter; 100, 100 million cells per milliliter; CON, control; M, million; MUP, maximal resting urethral sphincter pressure.
Discussion
The major finding of our study was that injection of skMPCs for the treatment of urinary sphincter deficiency, at doses within the therapeutic range, did not produce clinical or tissue pathologic features in the sphincter complex. Although some generalized tissue pathologic features were present within the lower urinary tract, it was not specific to the treatment groups and appeared to be related to the repeated urethral catheterization rather than the skMPCs themselves.
Cell therapy for urinary incontinence is rapidly moving from the preclinical to the clinical testing stages. As such, the major emphasis of testing has turned to optimization of the cell types and doses and, especially, of its safety [18–20]. Most preclinical studies have focused on the functional efficacy, sphincter regeneration, and mechanism of action [18]. The potential adverse effects on tissue have not been comprehensively explored, with the evaluation restricted to adverse events on efficacy and directly in the sphincter complex. Additionally, a great majority of the preclinical studies have been relatively short term (up to 3 months) and were not able assess any long-term potential pathologic features. The adverse effects in clinical studies have focused on clinical events, including a worsening of symptoms or other urinary problems [16, 18]. Obviously, they did not include reports of tissue pathology.
In the present study, we found no adverse effects (injection of 25–100 million skMPCs per milliliter) on gross or histological pathologic features, blood cell counts, or liver and kidney function markers up to 9 months after cell injection. This is probably equivalent to around 2–3 years of follow-up in humans. However, it does appear that repeated catheterization of the dog urethra might have caused inflammation and, in some cases, infection within the lower urinary tract, which can also be expected in the clinical setting. Although not caused by the cells, per se, one cannot rule out the possibility that testing the sphincter pressures could increase the risk of lower urinary tract infections. It is also possible that the inflammation from the urinary tract infections could affect the urethral pressures and explain some of the variability in our MUP values.
Several different cell types have been used in preclinical studies to test their ability to restore urinary sphincter function [11–16]. The most commonly used in clinical studies have been skMPCs [16, 18, 19]. In a review of both preclinical and clinical studies, the doses of cells given have varied greatly. They have been, with one exception [20], injected directly into the sphincter complex either periurethrally or transurethrally. Because of the differences in sphincter structure, size, and location within the abdomen, it is very difficult to calculate an “equivalent dose ratio” among all the studies, even within the clinical studies [21]. Doses of 250,000 to 5 million skMPCs have been given to rodents [18]. Also, 100 million skMPCs have been given to dogs [14]. Five million cells have been given to nonhuman primates [17], and 10–400 million to patients [18]. The only clinical study to test the different doses of skMPCs was reported by Sèbe et al. [16] and found no difference in efficacy with a dosage of 10–50 million cells. In our study, the cell dose of 50 million cells per milliliter was the only dose that demonstrated improved sphincter function, confirming the effective dose used in our previous study [14]. It remains unclear why dose-dependent improvement was not seen with cell therapy; however, we speculate that 25 million per milliliter might not be sufficient and 100 million cells per milliliter could have created crowded conditions for the cells to sustain viability owing to insufficient nutrients and oxygen. However, the difference was small, and a more likely explanation is that we did not test the full dose-response curve. A study by Carr et al. [22] tested a much wider dose range of skMPCs and found that the higher doses (32–228 million cells) provided better results (drier pads and fewer reported leaks) than the lower doses (1–16 million cells); thus, future studies should assess a broader dose-response relationship.
In contrast to our previous preclinical studies using rodents, dogs, and nonhuman primates [13–15, 17], the goal of the present experiment was to assess the safety within a dose range of skMPCs that improved sphincter function. It was not our goal to measure structural (muscle) regeneration, because we have previously reported that isolated skMPCs express markers for both mature skeletal muscle cells (desmin) and those of precursor cells (MyoD, PAX-7) in culture and restore smooth and skeletal muscle content, innervation, and vasculature [17]. In particular, we have demonstrated in nonhuman primates that labeled skMPCs incorporate into the sphincter musculature, urothelium, and vasculature of the sphincter complex, restoring the sphincter constrictor responses to somatic and adrenergic nerve responses [17]. Our objective in the present study was only to show that a muscle layer existed to support the MUP.
Conclusion
In this study, we could not identify any adverse effects of skMPCs on local or systemic pathologic features at a dose that improves sphincter function.
Acknowledgment
We thank the Crown Foundation for the funding to perform this study.
Author Contributions
J.K.W.: conception and design, data collection, data analysis, manuscript writing; D.E.: data analysis, manuscript writing; A.D.: conception and design, collection and analysis of data, administrative support; M.M.: data collection; J.A.: data analysis and interpretation, translational medicine support; J.M.C.: data collection, data analysis and interpretation; J.J.Y.: conception and design, manuscript writing; A.A.: conception and design, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.D. has compensated employment. J.M.C. is a compensated expert consultant for Pfizer and AbbVie. The other authors indicated no potential conflicts of interest.
References
- 1.Holroyd-Leduc JM, Straus SE. Management of urinary incontinence in women: Scientific review. JAMA. 2004;291:986–995. doi: 10.1001/jama.291.8.986. [DOI] [PubMed] [Google Scholar]
- 2.Luber KM. The definition, prevalence, and risk factors for stress urinary incontinence. Rev Urol. 2004;6(suppl 3):S3–S9. [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JS, Nyberg LM, Kusek JW, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Urinary Incontinence in Women. Am J Obstet Gynecol. 2003;188:S77–S88. doi: 10.1067/mob.2003.353. [DOI] [PubMed] [Google Scholar]
- 4.Hannestad YS, Rortveit G, Sandvik H, et al. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trøndelag. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 5.Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 6.Bø K. Pelvic floor muscle strength and response to pelvic floor muscle training for stress urinary incontinence. Neurourol Urodyn. 2003;22:654–658. doi: 10.1002/nau.10153. [DOI] [PubMed] [Google Scholar]
- 7.Mørkved S, Bø K, Fjørtoft T. Effect of adding biofeedback to pelvic floor muscle training to treat urodynamic stress incontinence. Obstet Gynecol. 2002;100:730–739. doi: 10.1016/s0029-7844(02)02160-9. [DOI] [PubMed] [Google Scholar]
- 8.Thor KB, Donatucci C. Central nervous system control of the lower urinary tract: New pharmacological approaches to stress urinary incontinence in women. J Urol. 2004;172:27–33. doi: 10.1097/01.ju.0000118381.04432.22. [DOI] [PubMed] [Google Scholar]
- 9.Kerr LA. Bulking agents in the treatment of stress urinary incontinence: History, outcomes, patient populations, and reimbursement profile. Rev Urol. 2005;7(suppl 1):S3–S11. [PMC free article] [PubMed] [Google Scholar]
- 10.Bullock TL, Ghoniem G, Klutke CG, et al. Advances in female stress urinary incontinence: Mid-urethral slings. BJU Int. 2006;98(suppl 1):32–40. doi: 10.1111/j.1464-410X.2006.06361.x. [DOI] [PubMed] [Google Scholar]
- 11.de la Garza-Rodea AS, van der Velde I, Boersma H, et al. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant. 2011;20:217–231. doi: 10.3727/096368910X522117. [DOI] [PubMed] [Google Scholar]
- 12.Lin G, Wang G, Banie L, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BS, Chun SY, Lee JK, et al. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med. 2012;10:94–108. doi: 10.1186/1741-7015-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberli D, Aboushwareb T, Soker S, et al. Muscle precursor cells for the restoration of irreversibly damaged sphincter function. Cell Transplant. 2012;21:2089–2098. doi: 10.3727/096368911X623835. [DOI] [PubMed] [Google Scholar]
- 15.Yiou R, Yoo JJ, Atala A. Restoration of functional motor units in a rat model of sphincter injury by muscle precursor cell autografts. Transplantation. 2003;76:1053–1060. doi: 10.1097/01.TP.0000090396.71097.C2. [DOI] [PubMed] [Google Scholar]
- 16.Sèbe P, Doucet C, Cornu JN, et al. Intrasphincteric injections of autologous muscular cells in women with refractory stress urinary incontinence: A prospective study. Int Urogynecol J. 2011;22:183–189. doi: 10.1007/s00192-010-1255-5. [DOI] [PubMed] [Google Scholar]
- 17.Badra S, Andersson KE, Dean A, et al. Long-term structural and functional effects of autologous muscle precursor cell therapy in a nonhuman primate model of urinary sphincter deficiency. J Urol. 2013;190:1938–1945. doi: 10.1016/j.juro.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 18.Lin C-S, Lue TF. Stem cell therapy for stress urinary incontinence: A critical review. Stem Cells Dev. 2012;21:834–843. doi: 10.1089/scd.2011.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stangel-Wojcikiewicz K, Jarocha D, Piwowar M, et al. Autologous muscle-derived cells for the treatment of female stress urinary incontinence: A 2-year follow-up of a Polish investigation. Neurourol Urodyn. 2014;33:324–330. doi: 10.1002/nau.22404. [DOI] [PubMed] [Google Scholar]
- 20.Cruz M, Dissaranan C, Cotleur A, et al. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gyncecol Int. 2012;2012:612946–612953. doi: 10.1155/2012/612946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boissier R, Karsenty G. [Cellular therapy and urinary incontinence] [Article in French] Prog Urol. 2012;22:454–461. doi: 10.1016/j.purol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Carr LK, Robert M, Kultgen PL, et al. Autologous muscle derived cell therapy for stress urinary incontinence: A prospective, dose ranging study. J Urol. 2013;189:595–601. doi: 10.1016/j.juro.2012.09.028. [DOI] [PubMed] [Google Scholar]