Adult dental pulp cells (DPCs) isolated from human third molars have the capability to differentiate into keratocytes. After inducing differentiation in vitro, DPCs expressed molecules characteristic of keratocytes, keratocan, and keratan sulfate proteoglycans at the gene and protein levels. DPCs maintain the keratocyte phenotype after in vivo transplantation, demonstrating a potential for clinical application in cellular or tissue engineering therapies for corneal stromal blindness.
Keywords: Stem cells, Dental pulp, Corneal stroma, Corneal keratocytes, Cornea
Abstract
Corneal blindness afflicts millions of individuals worldwide and is currently treated by grafting with cadaveric tissues; however, there are worldwide donor tissue shortages, and many allogeneic grafts are eventually rejected. Autologous stem cells present a prospect for personalized regenerative medicine and an alternative to cadaveric tissue grafts. Dental pulp contains a population of adult stem cells and, similar to corneal stroma, develops embryonically from the cranial neural crest. We report that adult dental pulp cells (DPCs) isolated from third molars have the capability to differentiate into keratocytes, cells of the corneal stoma. After inducing differentiation in vitro, DPCs expressed molecules characteristic of keratocytes, keratocan, and keratan sulfate proteoglycans at both the gene and the protein levels. DPCs cultured on aligned nanofiber substrates generated tissue-engineered, corneal stromal-like constructs, recapitulating the tightly packed, aligned, parallel fibrillar collagen of native stromal tissue. After injection in vivo into mouse corneal stroma, human DPCs produced corneal stromal extracellular matrix containing human type I collagen and keratocan and did not affect corneal transparency or induce immunological rejection. These findings demonstrate a potential for the clinical application of DPCs in cellular or tissue engineering therapies for corneal stromal blindness.
Introduction
The cornea constitutes the transparent outermost tissues of the eye. It serves as a physical and biological barrier against the external environment and provides refractive power to focus light onto the retina. The corneal stroma is a dense, avascular connective tissue constituting 90% of the cornea thickness and providing the mechanical integrity and transparency of the cornea. The transparency of the corneal stroma is attributed to its highly organized extracellular matrix [1].The stroma is composed predominately of continuous bundles of type I collagen with a uniform fibril diameter, evenly distributed and highly organized into a lamellar structure. The stromal collagen fibrils are arranged parallel within each lamella, and the lamellae are layered with an orthogonal collagen orientation with respect to each other [2]. The corneal stroma is maintained by neural crest-derived cells, keratocytes. Trauma to the stromal tissue will result in activation of the keratocytes to fibroblasts and the deposition of unorganized scar tissue [3]. This disruption of the organized stromal matrix diminishes corneal transparency and can result in blindness.
Millions of individuals worldwide develop bilateral corneal blindness from trauma, infections, or genetic disease [4]. Because corneal scarring is largely irreversible, the most common method of treatment is penetrating keratoplasty with allogeneic cadaveric tissue. However, there is a global shortage of donor tissue. Also, although the cornea has been regarded as an immune privileged tissue, corneal grafts have had a failure rate of around 38% after 10 years, mainly because of tissue rejection [5]. The likelihood of rejection is increased by the infiltration of corneal vasculature, which is often associated with scar tissue, or by a history of graft rejection [6]. The development of an autologous cellular therapy in which cells remodel the corneal tissue into the proper structure or the generation of an engineered corneal stroma-like tissue construct from autologous cells to replace scarred tissue could bypass the limitations of current treatments.
Adult stem cells have been identified in several tissues in the body, including limbal corneal stromal tissues [7]. These corneal stromal stem cells (CSSCs) can differentiate into functional keratocytes and produce extracellular matrix in vitro similar to that of native stromal tissue [8]. Stem cell therapy also restores transparency in mouse corneal disease models, replacing disorganized stromal matrix with tissue indistinguishable from that of native stroma [9, 10]. These animal studies support the idea of stem cell-based therapy as a viable clinical approach to treat human corneal scarring. Because the cornea develops embryonically from the cranial neural crest, it has been postulated that autologous stem cells derived from other cranial neural crest tissues would have a strong affinity for differentiating toward corneal cell types.
Dental pulp is a vascularized connective tissue in the center of the tooth containing fibroblasts, nerves, blood vessels, and a population of neural crest-derived, mesenchymal stem cells [11]. This self-renewing tissue maintains the surrounding hard tissue, dentin. The stem cells in the dental pulp are thought to respond to injury, such as cavity formation, by migrating to regions of damage, differentiating and depositing reparative dentin [12]. These stem cells are clonogenic and multipotent [13], maintaining the ability to differentiate into dental and nondental cells such as odontoblasts, hair follicle cells, hepatocytes, neural cells, islets, myocytes, cardiomyocytes, and corneal epithelium [14–21].
The objective of the present study was to assess the feasibility of using adult DPCs for therapies to repair the corneal stroma. The ability of DPCs to differentiate into keratocytes in vitro was assessed, the use of DPCs to engineer a structurally organized corneal stroma-like construct was evaluated, and the effects of the in vivo microenvironment on human DPC function after injection into mouse corneal stroma was analyzed. The present study provides promising data on the potential of adult dental pulp as an autologous stem cell source for corneal stroma regeneration.
Materials and Methods
Dental Pulp Cell Isolation
Dental pulp cells were isolated using a procedure similar to that previously described [13, 22]. Human adult third molars were collected after routine extraction at the University of Pittsburgh School of Dental Medicine. The surrounding soft tissues were scraped from the teeth, the molars were cracked open, and the pulp was removed. The pulp tissue was minced and then digested for 1.5 hours at 37°C in a solution containing 1.6 × 103 U/ml type I collagenase (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and 3.12 U/ml Dispase II (ZenBio, Inc., Research Triangle Park, NC, http://zenbio.com). The digest was then passed through a cell strainer, and the dental pulp cells were plated at a density of 3,000 to 10,000 cells per cm2 and cultured in a growth medium containing high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, http://www.invitrogen.com) with 20% fetal bovine serum (Life Technologies, Carlsbad, CA, http://lifetechnologies.com), 10,000 U/ml penicillin, and 10 mg/ml streptomycin (Gibco). The DPCs were passaged at 80% confluence using TrypLE Express enzyme solution (Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com). Experiments were performed using cells at passages 2–5.
In Vitro DPC Pellet Culture for Induction of Keratocyte Differentiation
Keratocyte differentiation of DPCs used methods similar to those previously described [23]. DPC pellets were formed by centrifuging 200,000 DPCs suspended in growth medium in 15-ml conical tubes at 1,500 rpm for 5 minutes. After 2 days, the culture medium was switched from growth medium to keratocyte differentiation medium containing advanced Dulbecco’s modified Eagle’s medium (Gibco) with 1 mM ascorbate-2-phosphate (Sigma-Aldrich), 10 ng/ml fibroblast growth factor 2 (Gibco), and 0.1 ng/ml transforming growth factor-β3 (Sigma-Aldrich) [24]. The culture medium was changed every 2–3 days, and conditioned medium was collected for Western blot analysis for a total of 21 days. The pellets were homogenized, and the RNA was isolated for quantitative real-time polymerase chain reaction (qPCR) analysis after 2 weeks of culture. The pellets were embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, USA, Inc., Torrance, CA, http://www.sakura-americas.com) for frozen sectioning after 2 or 14 days of culture.
Engineered Corneal Stroma-Like Tissue Construct Formation From DPCs
Corneal stroma constructs were engineered using similar methods to those previously described [8, 24]. DPCs were plated at a density of 2.5 × 105 cells per cm2 on polycaprolactone, 24-well inserts with parallel, aligned 700-nm-diameter nanofibers (Nanofiber Solutions, Columbus, OH, http://www.nanofibersolutions.com) in growth medium. After 48 hours, the medium was switched to keratocyte differentiation medium, which was replenished every 2–3 days for 4 weeks. The samples were then fixed for immunostaining, two-photon microscopy, or transmission electron microscopy.
DPC Injection Into Mouse Corneal Stroma
The University of Pittsburgh institutional animal care and use committee approved the animal studies, which were performed according to the guidelines provided by the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Ophthalmic and Vision Research. DPCs were injected into mouse corneal stroma similar to the procedure described previously [9]. DPCs were cultured on collagen-coated plastic in keratocyte differentiation medium for 7 days. The DPCs were then labeled with membrane dye Vybrant DiO (benzoxazolium, 3-octadecyl-2-[3-(3-octadecyl-2(3H)-benzoxazolylidene)-1-propenyl]-, perchlorate; Life Technologies). The cells were suspended in a solution of DMEM containing 5 μM DiO at a concentration of 2 × 106 cells per milliliter for 30 minutes at 37°C. The cells were washed twice in DMEM before injection.
After general anesthesia via ketamine/xylazine (IVX Animal Health, Inc., St. Joseph, MO, http://www.bayer.com) intraperitoneal injection, the eyes of C57BL/6 mice were anesthetized using topical 0.5% proparacaine (Falcon Pharmaceuticals, Fort Worth, TX, http://www.alcon.com). An intrastromal tunnel was made in the center of each cornea using a 33-gauge needle. The right eye of each mouse was injected with 2 μl of DPC solution containing 2.5 × 104 cells per microliter in DMEM, and the left eye was injected with medium only as a control. The mice were euthanized after up to 5 weeks.
In Vivo Imaging and Corneal Opacity Assessment
At 1 and 5 weeks after injection, the mouse corneas were visualized using a Leica MZFLIII high-resolution stereo fluorescence biomicroscope with a vertical fluorescence illuminator (Leica Microsystems, Buffalo Grove, IL, http://www.leica-microsystems.com) to assess corneal clarity and localize the DiO-labeled DPCs. The mice were anesthetized and stabilized using a three-point stereotactic mouse restrainer. A drop of either GenTeal Gel (Alcon, Hünenberg, Switzerland, http://www.alcon.com) or Gonak (Akorn, Inc., Somerset, NJ, http://www.akorn.com) was placed on the eye for lubrication before imaging.
Five weeks after injection, the mice (n = 6) were anesthetized, and the eyes were scanned using optical coherence tomography (OCT), as previously described [25]. The images were captured using a spectral domain-optical coherence tomography scanner (Bioptigen, Inc., Morrisville, NC, http://www.bioptigen.com) with an axial resolution of 4 μm and an A-scan acquisition rate of 20 kHz, scan area of 3.5 × 3.5 mm, with 250 A-scans × 250 frames × 1,024 samplings. The images were processed using Fiji Is Just ImageJ (FIJI, http://www.fiji.sc) software. A custom-built macro was used to register and preprocess the volumes. Next, a central button was punched from the cornea, and the epithelium was digitally removed. Corneal opacity was quantified in MetaMorph, version 7.7.8.0 (Molecular Devices, Inc., Sunnyvale, CA, http://www.moleculardevices.com) by setting a uniform threshold for segmentation and determining the average intensity of voxels in the stroma.
Reverse Transcription-Polymerase Chain Reaction and Quantitative Real Time Polymerase Chain Reaction
For RNA isolation, undifferentiated DPCs were first homogenized using a QiaShredder (Qiagen, Hilden, Germany, http://www.qiagen.com) per the manufacturer’s instructions. Cultured cell pellets were either homogenized using a bead homogenizer (MagNA Lyser, Roche Diagnostics Corp., Indianapolis, IN, http://www.lifescience.roche.com) for 2 cycles at 6,000 rpm for 20 seconds or flash frozen and homogenized using a handheld glass homogenizer.
Human keratocytes were isolated, as previously described [26]. Human central cornea was digested in 2.4 U/ml Dispase II overnight at 4°C to facilitate epithelial and endothelial tissue removal. The stroma was then minced into 2-mm cubes and digested in DMEM with 1 mg/ml collagenase type L (Sigma-Aldrich) for 3 hours at 37°C. The cells were collected by centrifugation, and RNA was isolated immediately.
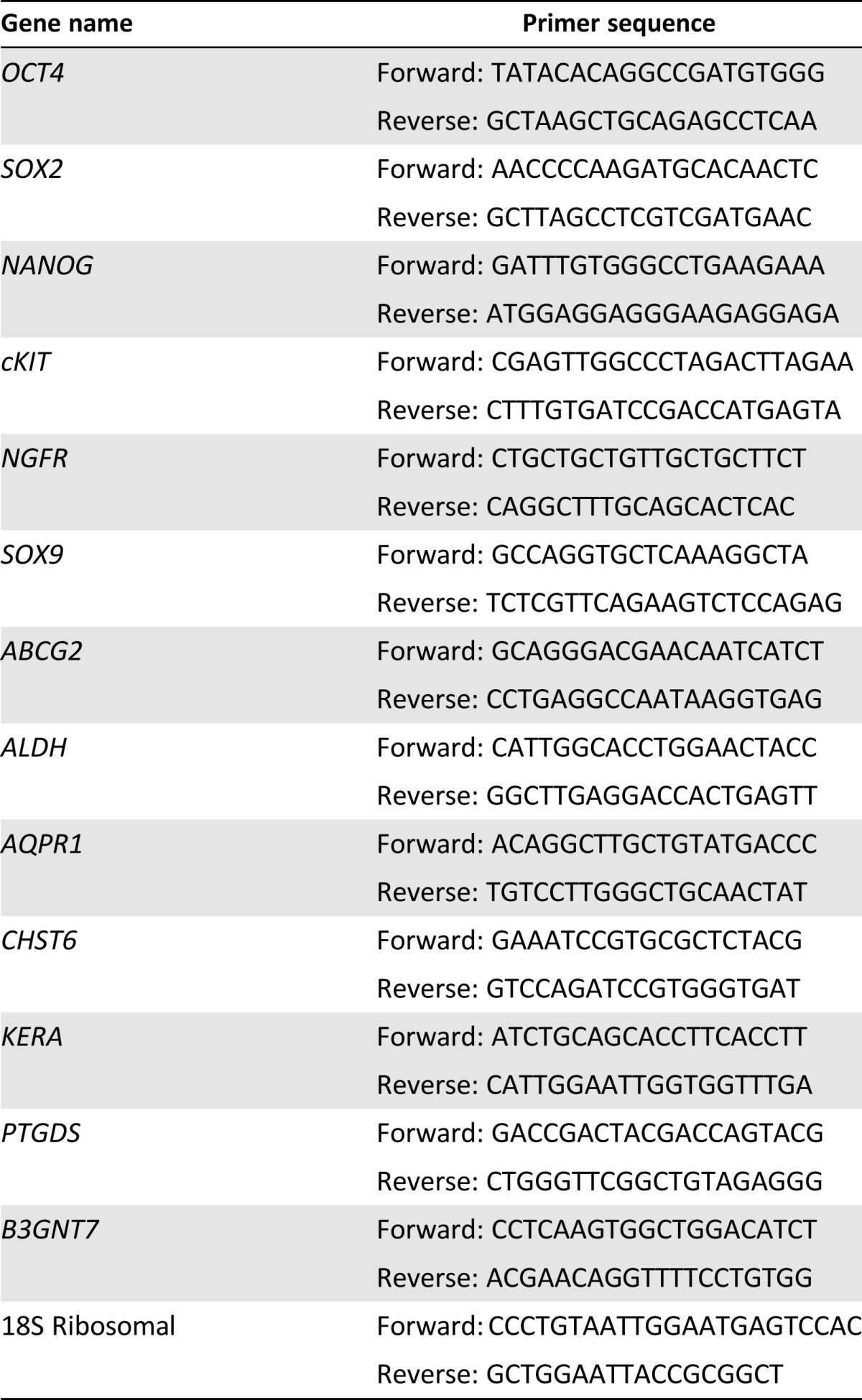
RNA was isolated using RNeasy Minikit (Qiagen) per the manufacturer’s instructions, treated with DNAse I (Ambion; Life Technologies) and concentrated by alcohol precipitation. RNA was transcribed to cDNA using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) per the manufacturer’s instructions. Qualitative reverse transcription polymerase chain reaction (RT-PCR) was performed as previously described [7]. The PCR products were separated on 5% Criterion TBE gel (BioRad Laboratories, Inc., Hercules, CA, http://www.bio-rad.com) and detected using SYBR Safe DNA gel stain (Life Technologies). qPCR was performed using direct dye binding (SYBR Green; Applied Biosystems, Life Technologies) using the primers listed in Table 1. RNA expression was normalized against the amplification of 18S rRNA for each sample. Relative gene expression was calculated using the 2−ΔΔCt method [27].
Table 1.
Polymerase chain reaction primer sequences
Western Blot for Keratan Sulfate-Containing Proteoglycans
Culture medium was collected throughout DPC pellet culture to assess the content of the secreted proteoglycans. Proteoglycans were isolated using SPEC 3 NH2-ion exchange columns (Agilent Technologies, Santa Clara, CA, http://www.aligent.com), dialyzed, and then dried, as previously described [28]. As a control, portions of the samples were treated with 0.5 U/ml keratanase from Pseudomonas (Sigma-Aldrich) in 0.1 M ammonium acetate overnight at 37°C to remove keratan sulfate glycosaminoglycan. The proteoglycans were then separated on 4%–20% denaturing acrylamide gels (BioRad Laboratories, Inc.) and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, http://www.emdmillipore.com). Keratan sulfate was detected by monoclonal antibody J19 (provided by Dr. Nirmala Sundaraj, University of Pittsburgh, Pittsburgh, PA) [7], using IRDye-labeled secondary antibody and imaged using a LI-COR Odyssey Imager (LI-COR Biotechnology, Lincoln, NE, http://www.licor.com).
Histology
DPC pellets after 4 weeks of culture, or mouse eyes 2 weeks after DPC injection, were immersed in frozen tissue-embedding medium (Tissue-Tek O.C.T. Compound; Sakura Finetek, USA, Inc.), flash frozen in isopentane chilled in liquid nitrogen, and sectioned at a thickness of 8 µm. Before staining, the sections were fixed in either 2% paraformaldehyde for 10 minutes or chilled acetone for 5 minutes. Cells cultured on aligned nanofibers were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, http://emsdiasum.com) for 20 minutes and permeabilized in 0.25% Triton X (Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com) for 10 minutes before staining. Immunostaining was performed using antibodies against type I collagen (catalog no. MAB3391; EMD Millipore), keratocan (Ker-1, provided by Dr. Bruce Caterson, Cardiff University, Cardiff, United Kingdom [29] or Sigma-Aldrich; catalog no. HPA039321), human keratocan (provided by Dr. Chia-Yang Liu, University of Cincinnati, Cincinnati, OH) [9], or human type I collagen (catalog no. C2456; Sigma-Aldrich). Fluorescently tagged secondary antibodies Alexa Fluor 488 anti-mouse IgG, Alexa Fluor 546 anti-mouse IgG, and Alexa Fluor 546 anti-rabbit IgG (Life Technologies) were used. Staining was performed in parallel without the primary antibody as a negative control; 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) was used to stain nuclei. Immunostaining was visualized using an Olympus FluoView FV1000 confocal microscope (Olympus Corp., Tokyo, Japan, http://www.olympus-global.com).
Two-Photon Microscopy
Collagenous constructs generated by DPCs cultured on aligned nanofibers were fixed in 4% paraformaldehyde for 20 minutes and permeabilized using 0.25% Triton X (Fisher Scientific) solution for 10 minutes. Nuclei were stained with 1 μM SYTOX Orange (Invitrogen) for 10 minutes. Two-photon microscopy was performed using an Olympus FV1000 multiphoton microscope in backscatter mode at a wavelength of 830 nm to facilitate the generation of second harmonic signal from aligned collagen. The samples were imaged through the depth of the sample at a step size of 1 µm.
Transmission Electron Microscopy
Cells cultured on aligned nanofibers were fixed in 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, http://www.emsdiasum.com) for 1 hour, postfixed in 1% osmium tetroxide (Electron Microscopy Sciences), and dehydrated in graded alcohol washes. The samples were then embedded in Epon (Energy Beam Sciences, East Granby, CT, http://www.ebsciences.com) and sectioned at 70 nm thickness. The sections were stained with 1% phosphotungstic acid (Sigma-Aldrich), pH 3.2, for 10 minutes and visualized with a Jeol 1011 transmission electron microscope (JEOL Ltd, Tokyo, Japan, http://www.jeol.co) at 80 kV.
Statistical Analysis
qPCR analysis was performed using StepOne software, version 2.3 (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com), and the results are presented as mean values, with error bars representing the 95% confidence intervals. Corneal stromal light scatter and volume quantifications from the OCT analyses are presented as averages, with error bars representing the standard deviation. Independent sample, 2-tailed t tests were used to compare the mean values using GraphPad Prism, version 4, software (GraphPad Software, Inc., La Jolla, CA, http://www.graphpad.com), and significance was considered at p < .05.
Results
Differentiation of Human Dental Pulp Cells Into Keratocytes In Vitro
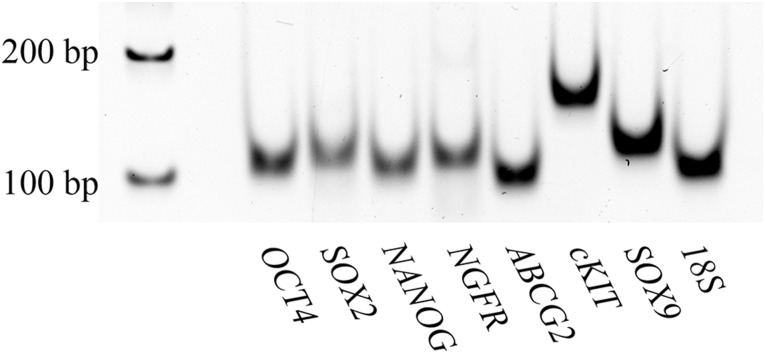
The goal of these studies was to assess the capacity of cells from the dental pulp to differentiate into a keratocyte phenotype for potential use in regenerative corneal therapy. Dental pulp tissue was isolated from extracted human third molars. After tissue digestion, the quality of the cells was assessed by detecting the expression of genes characteristic of neural crest-derived dental pulp stem cells [30–32]. The isolated dental pulp cells expressed pluripotency genes (SOX2, OCT4, NANOG, cKIT), neural crest genes (NGFR, SOX9), and a stem cell gene associated with corneal stromal progenitors (ABCG2) [7], indicating their potential as multipotent stem/progenitor cells for keratocyte differentiation (Fig. 1).
Figure 1.
Dental pulp cells express a broad spectrum of stem cell genes. RNA was isolated from adult human dental pulp cells. Pluripotency genes (OCT4, SOX2, NANOG, cKIT), neural crest genes (NGFR, SOX9), and a corneal stromal progenitor gene (ABCG2) were amplified using reverse-transcription polymerase chain reaction, as described in Materials and Methods, and the amplified products were separated and visualized on 5% TBE acrylamide gel.
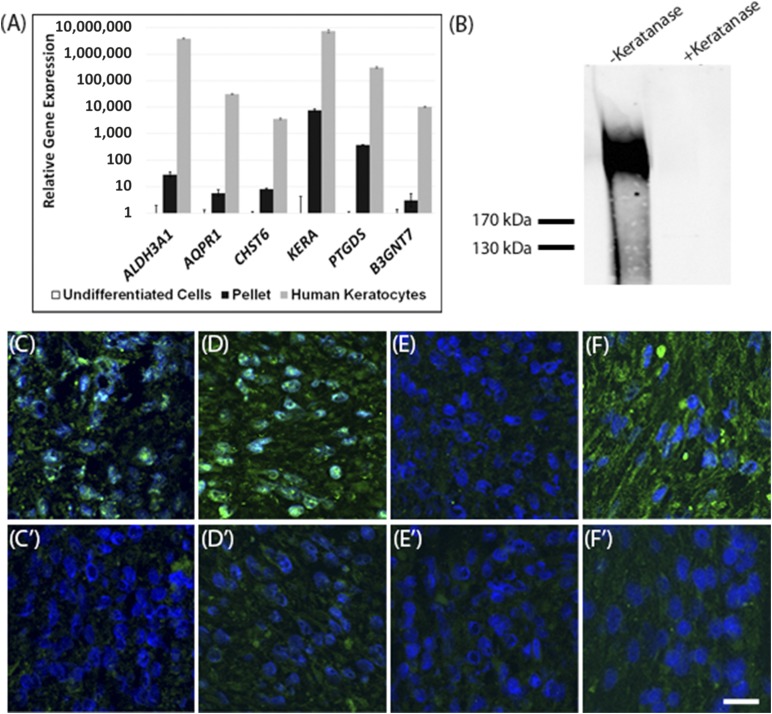
As previously described, adult stem cells can be induced to differentiate toward a keratocyte phenotype in pellet culture with a keratocyte differentiation medium [23, 24]. In the present study, similar methods were used to assess the keratocyte differentiation potential of DPCs. After inducing differentiation, DPCs had upregulated expression of the genes characteristic of keratocytes (ALDH3A1, AQPR1, CHST6, KERA, PTGDS, B3NGT7) [23, 33–36] (Fig. 2A). Particularly significant was the 10,000-fold increase in keratocan expression (KERA), because keratocan is a unique component of the stromal matrix and is widely considered a highly specific marker for keratocytes. The expression levels of the keratocyte genes were drastically increased relative to undifferentiated DPCs, indicating that DPCs are differentiating toward a keratocyte phenotype; however, potentially longer culture periods or the in vivo environment might be required to facilitate the maturation of differentiated DPCs to the gene expression levels of native keratocytes. The expression levels of native keratocytes for these genes have been provided for comparison (Fig. 2A).
Figure 2.
Dental pulp cells (DPCs) differentiate into keratocytes in vitro. DPCs were cultured in keratocyte differentiation medium as pellets for 2 weeks, as described in Materials and Methods. (A): Results of quantitative real-time polymerase chain reaction comparing expression of keratocyte genes in the DPCs after pellet culture or in primary human keratocytes. The expression level in undifferentiated DPCs was set to 1. (B): Immunoblot for keratan sulfate in proteoglycans isolated from conditioned pellet culture media, before and after keratanase treatment. (C): Immunostaining of sections of DPC pellets after 2 days of culture in growth medium for type I collagen (green), with a no-primary antibody control (C′). (D): Immunostaining of sections of DPC pellets after 2 weeks of culture in keratocyte differentiation medium for type I collagen (green), with a corresponding negative control (D′). (E): Immunostaining for keratocan (green) after 2 days of culture in growth medium, with a corresponding negative control (E′). (F): Immunostaining for keratocan (green) after 2 weeks of culture in keratocyte differentiation medium, with a corresponding negative control (F′). Blue represents nuclei stained with 4′,6-diamidino-2-phenylindole. Scale bars = 20 µm. Error bars represent the 95% confidence interval of data.
DPC differentiation was also characterized by the expression of keratocyte markers at the protein level. Secreted high-molecular-weight corneal keratan sulfate proteoglycans were detected in pellet-conditioned culture media by immunoblotting (Fig. 2B). Immunostaining was performed on sections prepared from pellets before differentiation, while the pellets were still in growth medium after the first 2 days of culture, and on sections of pellets cultured for 2 weeks in keratocyte differentiation medium. Weak expression of type I collagen was found after 2 days of culture, and the expression had become much stronger after 2 weeks of pellet culture relative to negative controls (Fig. 2C, 2D). Keratocan was not seen in the pellet matrix after 2 days of culture (Fig. 2E) but was found after 2 weeks of culture (Fig. 2F). Type I collagen is abundantly found in both corneal stromal tissue and dental pulp tissue; therefore, it is expected that DPCs would express this molecule at both predifferentiated states and late stages of in vitro differentiation. Keratocan, however, is unique to the corneal stroma; therefore, the lack of keratocan expression in the undifferentiated pellets followed by the expression of this molecule after 2 weeks of differentiation culture indicates that the DPCs were differentiating toward a keratocyte phenotype.
In Vitro Formation of Corneal Stromal Constructs by Human Dental Pulp Cells
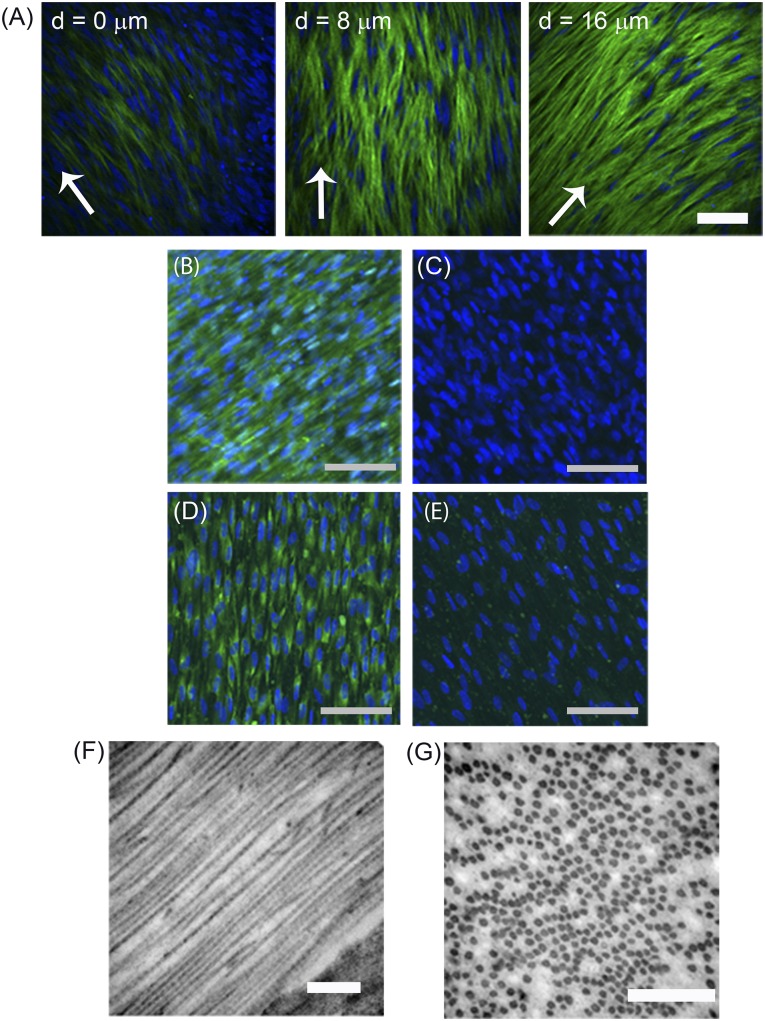
The matrix of the corneal stroma is highly organized in a lamellar structure in which each lamella comprises long, parallel oriented, and uniformly spaced collagen fibrils; this structure contributes to the transparency of the tissue. Tissues engineered to replace damaged corneal stromal tissue must recapitulate this highly organized structure. In the present study, the ability of DPCs to generate engineered tissues with an organized matrix similar to that of native corneal tissue was assessed. DPCs were cultured on substrates with aligned nanofibers to direct cell orientation and matrix deposition. After culture on aligned nanofibers for 4 weeks, DPCs generated an approximately 30-µm-thick corneal stromal-like construct. Two-photon microscopy showed the collagen within the engineered tissue to be aligned in parallel, and the direction of the collagen changed at different depths, indicative of the formation of multiple lamellae (Fig. 3A). Immunostaining verified that the matrix contained type I collagen (Fig. 3B), relative to negative control (Fig. 3C), and keratocan (Fig. 3D), relative to negative control (Fig. 3E). Transmission electron microscopy also showed the presence of long fibrils of parallel aligned, banded collagen (Fig. 3F, 3G), with a diameter of 40 ± 5.6 nm (n = 50) and a predominately uniform distribution. These results have demonstrated that DPCs have the capacity to generate engineered corneal stromal-like constructs for potential use in regenerative therapies.
Figure 3.
Dental pulp cells (DPCs) generate an engineered corneal stromal construct. DPCs were cultured for 4 weeks on aligned nanofiber substrata in keratocyte differentiation medium, as described in Materials and Methods. (A): Optical sections obtained by two-photon microscopy showed second harmonic signal emitted by fibrillar collagen (green) and DPC nuclei (blue) at three different depths of the tissue-like construct formed on the substratum. The orientation of the collagenous matrix is shown by arrows and the depth of the section indicated on the images. Collagen type I (B), with no primary antibody control (C), and keratocan (D), with no primary antibody control (E), were detected by immunostaining, as described in Materials and Methods. 4′,6-Diamidino-2-phenylindole was used to localize cell nuclei (blue). (F): Transmission electron microscopy of the constructs revealed long fibrils of banded collagen and, in cross section (G), showed uniformly packed fibrils of regular diameter. Scale bars = 100 μm (A–E) and 500 nm (F, G).
In Vivo Injection of Human Dental Pulp Cells Into Mouse Corneal Stroma
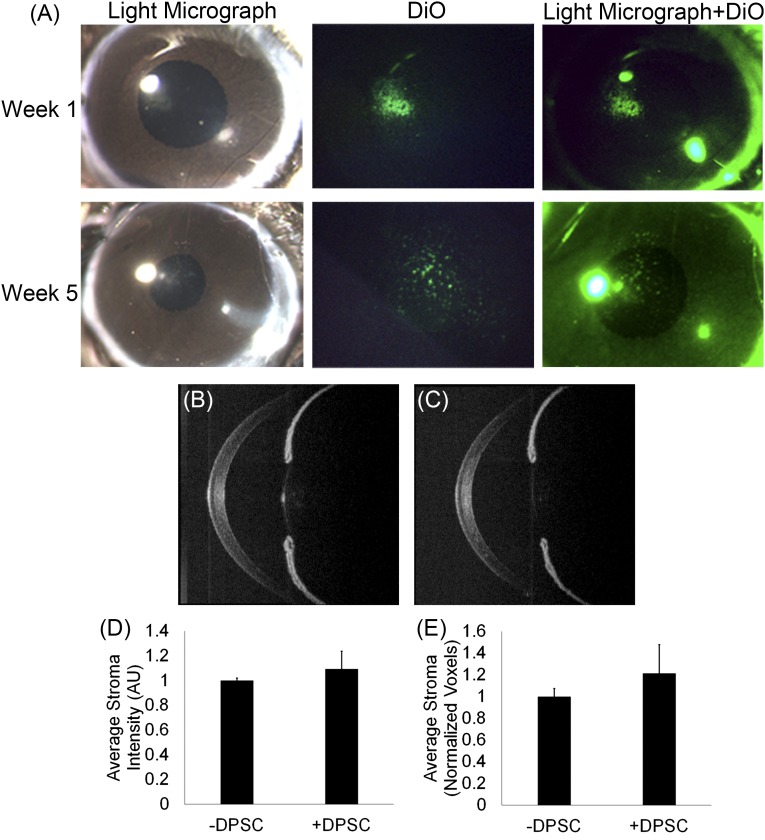
The ability of DPCs to maintain their keratocyte phenotype after in vivo implantation was assessed. After differentiation in vitro, the DPCs were fluorescently labeled and injected into the corneal stomas of C57Bl/6 mice to evaluate DPCs in the in vivo microenvironment. At 1 and 5 weeks after injection, the corneas appeared clear, and the DiO-labeled DPCs could still be visualized (Fig. 4A). OCT was used to assess light scattering in the murine corneas. OCT images of the mouse eyes with and without injected DPCs appeared similar (Fig. 4B, 4C). Quantitatively, no difference in the intensity of light scatter between the DPC-injected mouse corneas and the control corneas was detected (p = .32), indicating that transparency was not significantly altered by the long-term presence of human DPCs in the mouse corneal stroma (Fig. 4D). In addition, the OCT image reconstructions did not indicate a significant difference in stromal volume between the DPC-injected corneas and the control corneas (p = .14), demonstrating that DPCs did not elicit stromal edema (Fig. 4E).
Figure 4.
Dental pulp cells (DPCs) did not alter corneal transparency or volume after injection into mouse corneal stroma. (A): Light micrograph images showing that mouse corneas remained clear after injection and the fluorescently labeled (green) DPCs remained in the cornea at 1 and 5 weeks after injection. Representative in vivo images of mouse corneas obtained with optical coherence tomography (OCT) of control mouse cornea without DPCs (B) or mouse cornea with injected DPCs (C). Quantification of the three-dimensional OCT data of central corneal stromal region show statistically similar values of light scatter (D) and stromal volume (E) between control corneas and corneas with injected DPCs. Abbreviation: DPSC, dental pulp stem cell.
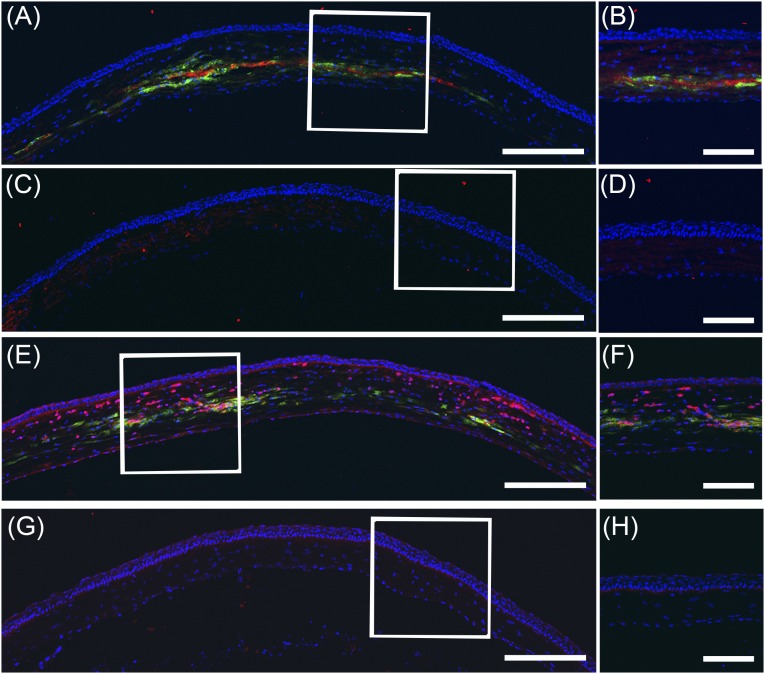
Human DPCs maintained the keratocyte phenotype after being injected into the mouse corneas. Immunostaining of the mouse cornea sections using antibodies nonreactive to mouse antigens showed the expression of human type I collagen (Fig. 5A–5D) and human keratocan (Fig. 5E–5H) localized around the injected human DPCs. These data indicate that the DPCs secreted the appropriate matrix in the in vivo corneal stromal microenvironment.
Figure 5.
Dental pulp cells (DPCs) produce human corneal stromal matrix in vivo. Cryosections of mouse corneas, 2 weeks after injection of DPCs, were immunostained with human-specific antibodies and imaged using confocal microscopy, as described in Materials and Methods. (A, B): Collagen type I (red) localized to regions containing DPCs (green) in the injected cornea. (C, D): In contrast, no human collagen was seen in the corneas without injected DPCs. (E, F): Human keratocan (red) was localized near DiO-labeled DPCs (green) but was not detected in uninjected control corneas (G, H). In each case, higher magnification of the boxed region is shown in the right-hand image. Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Scale bars = 250 μm (A, C, E, G) and 100 μm (B, D, F, H).
Discussion
Dental pulp contains a population of neural crest-derived, mesenchymal stem cells with the potential to differentiate into several phenotypes. This tissue can be easily isolated from adult teeth via minimally invasive endodontic methods for therapeutic use. Additionally, DPCs can be banked from exfoliated deciduous teeth or adult third molars after routine extraction for potential future use [37]. Because of this, DPCs are being investigated as a powerful autologous stem cell source for multiple types of therapies. Pathologic features of the cornea, also a neural crest-derived tissue, could possibly be corrected with stem cell therapies. Owing to their similar embryonic origins, the potential of DPCs as an autologous stem cell source for treating corneal blindness was investigated. Through gene and protein expression, DPCs were shown to differentiate effectively into keratocytes in vitro, to generate a tissue-engineered corneal stroma-like tissue construct, and to function as keratocytes in vivo without eliciting overt rejection. These studies have provided promising data on the potential translation of DPCs as an autologous cell source for regenerative corneal therapies.
The differentiation of DPCs into keratocytes was detected by the expression of keratocyte-specific molecules at both mRNA and protein levels. This expression of keratocan and keratan sulfate is of particular significance. The uniform spacing of collagen fibrils in the corneal stromal extracellular matrix is regulated by proteoglycans [38–43]. Keratan sulfate (KS), a sulfated glycosaminoglycan constituting 60% of the corneal stromal proteoglycans, is more abundant by at least an order of magnitude in the cornea than in other tissues of the body [23]. The DPC-secreted keratan sulfate proteoglycan (KSPG) is >200 kDa (Fig. 1B), indicating that the 50-kDa KSPG core proteins are modified with high-molecular-weight keratan sulfate chains. This DPC-generated KSPG is, in fact, larger than that produced by human corneal stromal stem cells in vitro [44] and in the same size range reported for KSPG synthesized in intact human corneal tissue [45]. The immunoblot in Figure 1 was developed with a monoclonal antibody specific for sulfated epitopes on the KS chains [46], and the keratanase enzyme that removed immunoreactivity from KSPG requires sulfation of the KS chain for glycolytic cleavage of the polysaccharide chain [47]. Together, these data provide inescapable evidence that the DPCs are producing sulfated KS chains of a molecular size consistent with that in normal human cornea. This conclusion has been further supported by our observation of the upregulation of mRNA for CHST6 and B3GNT7, sulfotransferase enzymes that add sulfate to corneal KS. From the expression of these molecules and the additional matrix molecules characteristic of keratocytes (type I collagen, ALDH3A1, AQPR1, PTGDS), it is clear that DPCs differentiated into keratocytes and produced an extracellular matrix containing components typical of corneal stromal tissue.
DPCs were able to respond to topographical cues to generate an engineered corneal stromal-like construct recapitulating the highly organized nature of native tissue. These constructs contained lamellae with long, parallel, aligned collagen fibrils that were approximately uniform in spacing. The collagen diameter in these constructs was approximately 40 nm. Although the collagen diameter in the natural human corneal stroma is approximately 31 nm [48], the collagen produced by the DPCs was comparable to that produced by corneal stromal stem cells in similar engineered tissues [8] and was typical of stromal collagen of other mammalian corneas [48]. In the human corneal stroma, the collagen fibril diameter is thought to be regulated by the copolymerization of type V collagen and type I collagen with a type I/type V ratio of 4:1 [49, 50]. The difference in collagen fibril diameter between the engineered tissues and the native stroma could be due to a greater ratio between types I and V collagen [8]. Because the collagen fibril diameter and spacing are approximately uniform, it is anticipated that these constructs would become transparent after in vivo implantation. In addition, the in vivo environment might facilitate the maturation and remodeling of engineered tissue to acquire the proper collagen diameter. These engineered corneal stroma-like tissues generated from DPCs are thus a potential alternative to allogeneic graft tissue for surgical replacement of corneal scar tissue.
DPCs demonstrated keratocyte functionality in vivo in the mouse corneal stroma microenvironment by remaining in the stromal tissue and producing human type I collagen and human keratocan. Furthermore, the DPCs did not elicit any signs of frank rejection (Figs. 4, 5). We have previously shown that injection of human fibroblasts into the mouse stroma elicited T-cell infiltration within 14 days, leading to degradation in stromal transparency [9]. The ocular response to the injection of DPCs in vivo, however, had no such effect but produced results similar to those we observed after injection of stem cells from human corneal stroma [9]. Neither DPCs nor CSSCs elicited the vascularization or haze indicative of rejection [9]. Immunostaining found no evidence of CD45+ inflammatory cells in the cornea 2 weeks after injection (data not shown). The lack of an immune response to xenogeneic transplantation of human corneal stromal stem cells or DPCs likely resulted from the well-known immunosuppressive characteristics of mesenchymal stem cells [51, 52]. Our data, therefore, have provided in vivo evidence in a murine model that DPCs can be noninflammatory in an allogeneic or xenogeneic transplant in the corneal stromal microenvironment, producing the proper extracellular matrix and maintaining corneal transparency.
Multiple cell types have been investigated for potential use in regenerative corneal therapies. Primary human keratocyte isolation and expansion has proved challenging, because these cells have limited proliferative capacity when cultured in serum-free conditions, and the expansion of these cells in the presence of serum results in cell differentiation toward a fibroblastic phenotype [28, 53]. Corneal fibroblasts produced by culturing keratocytes in serum-containing medium have been assessed for potential use for regenerative corneal therapy; however, the use of these cells would not bypass the limitation of insufficient availability of donor tissue associated with current treatment [24, 54, 55]. Fibroblasts could also be limited in utility because they do not produce keratan sulfate proteoglycans, a unique molecular component of the stroma, that have been linked to corneal transparency [55]. Keratocyte differentiation from embryonic stem cells has been established [26]; however, ethical issues related to the isolation of these cells might hinder their translation to clinical application. Bone marrow- and adipose-derived mesenchymal stem cells have also been shown to differentiate toward a keratocyte phenotype and, similar to DPCs, can be easily isolated for autologous therapies [56–58]. DPCs were selected for investigation for corneal regeneration, because, unlike other adult stem cell populations studied for potential corneal stromal regeneration, the dental pulp shares developmental origins with the corneal stroma, which potentially could be advantageous for regenerating corneal therapy.
Several stem cell populations have been discovered in the craniofacial tissues, and many are being investigated for potential use in regenerative ocular therapy. Two studies have shown promising results using stem cells from exfoliated deciduous teeth (SHED) for corneal epithelium regeneration [14, 19]. These studies reported that SHED express markers similar to those of corneal limbal stem cells, and the delivery of cell sheets composed of SHED with and without the addition of amniotic membrane resulted in the regeneration of the corneal epithelium in total limbal stem cell deficiency rabbit models. Recently, a report showed that the inhibition of Wnt and bone morphogenetic protein signaling induced retinal cell differentiation from stem cells isolated from the periodontal ligament in vitro [59]. These studies, in addition to the present work, present promising data on the use of craniofacial stem cells for ocular therapies.
Conclusion
In the present study, neural crest-derived adult dental pulp cells were shown to be able to differentiate into keratocytes in vitro and in vivo and generated a tissue-engineered corneal stromal-like tissue. Adult dental pulp cells can be easily isolated from autologous tissues and therefore have great translational potential for clinical personalized therapies to treat corneal blindness.
Acknowledgments
We thank Dr. Charles Sfeir and Barbara Streba at the University of Pittsburgh School of Dental Medicine for providing the teeth for our study. We also thank Gregory Gibson, Mara Grove, and Jonathan Franks from the University of Pittsburgh, Center for Biological Imaging, for help with 2-photon microscopy and transmission electron microscopy. We also thank Katherine Davoli for helping prepare the histological tissue sections. This research was supported by NIH Grants EY016415 (J.L.F), EY009368 (J.L.F.), and P30-EY008098, Research to Prevent Blindness, and the Eye and Ear Foundation of Pittsburgh. F.N.S. is an OTERO fellow of the Louis J. Fox Center for Vision Restoration.
Author Contributions
F.N.S.-P.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Y.D., M.M.M. and M.L.F.: collection and/or assembly of data, final approval of manuscript; K.L.L.: data analysis and interpretation of data, final approval of manuscript; J.L.F.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.L.F. is a compensated stockholder in Exxon. The other authors indicated no potential conflicts of interest.
References
- 1.Levin LA, Kaufman PL. Adler’s Physiology of the Eye: Clinical Application. Edinburgh. Saunders/Elsevier. 2011;xii:795. [Google Scholar]
- 2.Smolin G, Foster CS, Azar DT et al. Smolin and Thoft’s The Cornea: Scientific Foundations and Clinical Practice. Philadelphia: Lippincott Williams & Wilkins; 2005:xviii, 1323.
- 3.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 4.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 5.Williams KA, Esterman AJ, Bartlett C, et al. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:896–901. doi: 10.1097/01.tp.0000185197.37824.35. [DOI] [PubMed] [Google Scholar]
- 6.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010;18:162–171. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y, Funderburgh ML, Mann MM, et al. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Du Y, Watkins SC, et al. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials. 2012;33:1343–1352. doi: 10.1016/j.biomaterials.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y, Carlson EC, Funderburgh ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Zhang J, Liu CY, et al. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: Lumican null mice. PLoS One. 2010;5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanci A. Ten Cate's Oral Histology: Development, Structure, and Function. St. Louis, MO: Elsevier Mosby; 2013. [Google Scholar]
- 12.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 13.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes JA, Geraldes Monteiro B, Melo GB, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci. 2010;51:1408–1414. doi: 10.1167/iovs.09-4029. [DOI] [PubMed] [Google Scholar]
- 15.Govindasamy V, Ronald VS, Abdullah AN, et al. Human platelet lysate permits scale-up of dental pulp stromal cells for clinical applications. Cytotherapy. 2011;13:1221–1233. doi: 10.3109/14653249.2011.602337. [DOI] [PubMed] [Google Scholar]
- 16.Iohara K, Zheng L, Ito M, et al. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 17.Ishkitiev N, Yaegaki K, Calenic B, et al. Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J Endod. 2010;36:469–474. doi: 10.1016/j.joen.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Mao JJ, Prockop DJ. Stem cells in the face: Tooth regeneration and beyond. Cell Stem Cell. 2012;11:291–301. doi: 10.1016/j.stem.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteiro BG, Serafim RC, Melo GB, et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009;42:587–594. doi: 10.1111/j.1365-2184.2009.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds AJ, Jahoda CA. Cultured human and rat tooth papilla cells induce hair follicle regeneration and fiber growth. Differentiation. 2004;72:566–575. doi: 10.1111/j.1432-0436.2004.07209010.x. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama M, Iohara K, Wakita H, et al. Dental pulp-derived CD31−/CD146− side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2011;17:1303–1311. doi: 10.1089/ten.TEA.2010.0306. [DOI] [PubMed] [Google Scholar]
- 22.Syed-Picard FN, Jayaraman T, Lam RS, et al. Osteoinductivity of calcium phosphate mediated by connexin 43. Biomaterials. 2013;34:3763–3774. doi: 10.1016/j.biomaterials.2013.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du Y, Sundarraj N, Funderburgh ML, et al. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Du Y, Mann MM, et al. Bioengineering organized, multi-lamellar human corneal stromal tissue by growth factor supplementation on highly aligned synthetic substrates. Tissue Eng Part A. 2013;19:2063–2075. doi: 10.1089/ten.tea.2012.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boote C, Du Y, Morgan S, et al. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest Ophthalmol Vis Sci. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AA, Hertsenberg AJ, Funderburgh ML, et al. Differentiation of human embryonic stem cells into cells with corneal keratocyte phenotype. PLoS One. 2013;8:e56831. doi: 10.1371/journal.pone.0056831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: A role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees SG, Waggett AD, Kerr BC, et al. Immunolocalisation and expression of keratocan in tendon. Osteoarthritis Cartilage. 2009;17:276–279. doi: 10.1016/j.joca.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Ferro F, Spelat R, D’Aurizio F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PLoS One. 2012;7:e41774. doi: 10.1371/journal.pone.0041774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda MJ, Nakashima F, Satomura K, et al. Side population cells expressing ABCG2 in human adult dental pulp tissue. Int Endod J. 2007;40:949–958. doi: 10.1111/j.1365-2591.2007.01301.x. [DOI] [PubMed] [Google Scholar]
- 32.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Iorio E, Barbaro V, Volpi N, et al. Localization and expression of CHST6 and keratan sulfate proteoglycans in the human cornea. Exp Eye Res. 2010;91:293–299. doi: 10.1016/j.exer.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Hamann S, Zeuthen T, La Cour M, et al. Aquaporins in complex tissues: Distribution of aquaporins 1-5 in human and rat eye. Am J Physiol. 1998;274:C1332–C1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- 35.Kitayama K, Hayashida Y, Nishida K, et al. Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J Biol Chem. 2007;282:30085–30096. doi: 10.1074/jbc.M703695200. [DOI] [PubMed] [Google Scholar]
- 36.Pappa A, Estey T, Manzer R, et al. Human aldehyde dehydrogenase 3A1 (ALDH3A1): Biochemical characterization and immunohistochemical localization in the cornea. Biochem J. 2003;376:615–623. doi: 10.1042/BJ20030810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gioventu S, Andriolo G, Bonino F, et al. A novel method for banking dental pulp stem cells. Transfus Apheresis Sci. 2012;47:199–206. doi: 10.1016/j.transci.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Carlson EC, Liu CY, Chikama T, et al. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakravarti S, Magnuson T, Lass JH, et al. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakravarti S, Zhang G, Chervoneva I, et al. Collagen fibril assembly during postnatal development and dysfunctional regulation in the lumican-deficient murine cornea. Dev Dyn. 2006;235:2493–2506. doi: 10.1002/dvdy.20868. [DOI] [PubMed] [Google Scholar]
- 41.Funderburgh JL. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 42.Funderburgh JL, Caterson B, Conrad GW. Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Dev Biol. 1986;116:267–277. doi: 10.1016/0012-1606(86)90130-2. [DOI] [PubMed] [Google Scholar]
- 43.Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconj J. 2002;19:275–285. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Du Y, Mann MM, et al. Corneal stromal stem cells versus corneal fibroblasts in generating structurally appropriate corneal stromal tissue. Exp Eye Res. 2014;120:71–81. doi: 10.1016/j.exer.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Midura RJ, Hascall VC, MacCallum DK, et al. Proteoglycan biosynthesis by human corneas from patients with types 1 and 2 macular corneal dystrophy. J Biol Chem. 1990;265:15947–15955. [PubMed] [Google Scholar]
- 46.SundarRaj N, Willson J, Gregory JD, et al. Monoclonal antibodies to proteokeratan sulfate of rabbit corneal stroma. Curr Eye Res. 1985;4:49–54. doi: 10.3109/02713688508999966. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda MN. Endo-beta-galactosidases and keratanase. In Ausubel FM, ed. Current Protocols in Molecular Biology. Hoboken, NJ: Wiley; 2001:Unit 17, 17B. [DOI] [PubMed]
- 48.Meek KM, Leonard DW. Ultrastructure of the corneal stroma: A comparative study. Biophys J. 1993;64:273–280. doi: 10.1016/S0006-3495(93)81364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birk DE. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32:223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 50.Birk DE, Fitch JM, Babiarz JP, et al. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 52.Bray LJ, Heazlewood CF, Munster DJ, et al. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy. 2014;16:64–73. doi: 10.1016/j.jcyt.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Jester JV, Barry-Lane PA, Cavanagh HD, et al. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 54.Carrier P, Deschambeault A, Audet C, et al. Impact of cell source on human cornea reconstructed by tissue engineering. Invest Ophthalmol Vis Sci. 2009;50:2645–2652. doi: 10.1167/iovs.08-2001. [DOI] [PubMed] [Google Scholar]
- 55.Karamichos D, Funderburgh ML, Hutcheon AE, et al. A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One. 2014;9:e86260. doi: 10.1371/journal.pone.0086260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, et al. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–579. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 57.Du Y, Roh DS, Funderburgh ML, et al. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680–2689. [PMC free article] [PubMed] [Google Scholar]
- 58.Park SH, Kim KW, Chun YS, et al. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp Eye Res. 2012;101:16–26. doi: 10.1016/j.exer.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Huang L, Liang J, Geng Y, et al. Directing adult human periodontal ligament-derived stem cells to retinal fate. Invest Ophthalmol Vis Sci. 2013;54:3965–3974. doi: 10.1167/iovs.13-11910. [DOI] [PubMed] [Google Scholar]