The utility and advantage of a microfluidic chip culture system to enhance the development of personalized, on-demand, treatment modules using embryoid bodies (EBs) is demonstrated. Under microfluidic conditions, differentiated steroidogenic EBs continued to secrete estradiol and progesterone at physiologically relevant concentrations. These results present a platform for the development of a new therapeutic system for personalized medicine.
Keywords: Stem cells, Cryopreservation, Microenvironment, Microphysiological systems, Hormone secretion, Reproductive medicine
Abstract
Hormone replacement therapies have become important for treating diseases such as premature ovarian failure or menopausal complications. The clinical use of bioidentical hormones might significantly reduce some of the potential risks reportedly associated with the use of synthetic hormones. In the present study, we demonstrate the utility and advantage of a microfluidic chip culture system to enhance the development of personalized, on-demand, treatment modules using embryoid bodies (EBs). Functional EBs cultured on microfluidic chips represent a platform for personalized, patient-specific treatment cassettes that can be cryopreserved until required for treatment. We assessed the viability, differentiation, and functionality of EBs cultured and cryopreserved in this system. During extended microfluidic culture, estradiol, progesterone, testosterone, and anti-müllerian hormone levels were measured, and the expression of differentiated steroidogenic cells was confirmed by immunocytochemistry assay for the ovarian tissue markers anti-müllerian hormone receptor type II, follicle-stimulating hormone receptor, and inhibin β-A and the estrogen biosynthesis enzyme aromatase. Our studies showed that under microfluidic conditions, differentiated steroidogenic EBs continued to secrete estradiol and progesterone at physiologically relevant concentrations (30–120 pg/ml and 150–450 pg/ml, respectively) for up to 21 days. Collectively, we have demonstrated for the first time the feasibility of using a microfluidic chip system with continuous flow for the differentiation and extended culture of functional steroidogenic stem cell-derived EBs, the differentiation of EBs into cells expressing ovarian antigens in a microfluidic system, and the ability to cryopreserve this system with restoration of growth and functionality on thawing. These results present a platform for the development of a new therapeutic system for personalized medicine.
Introduction
Ovaries have two distinct functions that are critical to a woman’s reproductive health: hormone synthesis and gametogenesis. A significant population of reproductive-age patients experience premature ovarian failure (POF) and lose regular hormone synthesis owing to either iatrogenic causes, such as chemotherapy, or idiopathic, presumably genetic, causes. The number of female cancers diagnosed in reproductive-age women has approached 9% of all diagnoses [1], and survival will continue to increase as treatment options and novel biotechnological advances emerge [2]. The loss of ovarian function has physiologic and considerable psychosocial repercussions on patients that negatively affect their quality of life. Currently, gonadal failure and the associated loss of hormone synthesis in patients with POF, or menopausal women, is treated by hormone replacement therapy (HRT) using synthetically produced steroids [3]. However, the Women’s Health Initiative raised several outcome concerns related to this approach for two specific types of conjugated estrogens of hormones, Premarin and Prempro, which increase the risk of stroke, blood clot, myocardial infarction, and neoplasia [4–11]. These reported observations have since been clinically expanded by healthcare providers to include all synthetically generated hormones used in HRT. In contrast, recent reports have suggested that bioidentical hormones could be a safer alternative for HRT [10]. The presumed risks associated with the current HRT regimen necessitate improved therapeutic options. In the present study, we propose a novel approach for HRT using stem cells in a cell-based therapy. On a larger scope, we present evidence supporting the use of a microfluidic system as an exciting new opportunity for developing novel personalized medicine applications [12].
The pluripotent nature of embryonic stem cells (ESCs) presents a unique opportunity for both researchers and clinicians to be able to generate any cell or tissue type through directed differentiation protocols. Nondirected differentiation of ESCs seeded on nonadhesive plates in suspension can lead to formation of an embryoid body (EB), a densely packed spheroid of embryonic stem cells that differentiate into cell types from all three developmental germ layers: endoderm, ectoderm, and mesoderm. More recent studies in our laboratory have suggested that EBs derived from G4 mouse ESCs can differentiate under specific culture conditions into ovarian tissue, a primary steroidogenic organ of the female reproductive system [13] and that these differentiated G4 EBs synthesize physiologically relevant levels of estradiol [14]. Estradiol is the primary female hormone, important for women’s health and development, and is used in a wide range of medical treatments, in particular, in postmenopausal women and infertility patients.
The limitations of long-term in vitro culture of EBs for therapeutic purposes using the current standard tissue culture approaches include the high cost, risk of contamination, dependency on the operator, labor intensity, and necessity for large volumes of reagents. For example, during the interval between culture media changes, toxins and waste accumulation and the depletion of nutrients can interfere with the metabolism of the EBs. Moreover, with the increasing size of the cultured EBs, we have encountered concerns regarding insufficient gas and nutrient exchange at the core regions of the EB, which, in turn, can result in cell death within the EB inner mass [15]. By developing a microfluidic system with a continuous flow of fresh media, this limitation has been partially addressed. Using a dynamic continuous flow system of microfluidic chips, not only will the accumulation of toxins and waste be decreased, but it will also allow improved control of the culture parameters, enabling standardized microenvironments and a sustainable supply of fresh nutrients within a closed system in experiments [16–20]. In the present study, we propose a method in which we immobilize EBs in a closed microfluidic system that provides fresh media and simultaneously collects the steroid hormone from the supernatant from the terminal port. Using this approach, the cells can be kept in a contained system and survive prolonged culture durations without requiring exposure to air or other sources of contamination. Furthermore, the differentiated EBs in individual chips can be cryopreserved and thawed on demand at a later time.
Materials and Methods
Generation of EBs
Mouse embryonic fibroblast (MEF) medium was prepared using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% l-glutamine 200 mM (100×) (Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com). The 5 × 105 MEF feeder cells were mitotically inactivated using mitomycin C (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and seeded on a 100-mm tissue culture plate coated with 0.1% gelatin (Sigma-Aldrich) in MEF medium. The cell culture plates were washed with phosphate-buffered saline (PBS) (Life Technologies) solution, and the media were changed every 2–3 days until the cells were 75%–80% confluent.
Mouse embryonic stem cell media (ES medium) was prepared using DMEM supplemented with 10% stem cell-grade FBS, 1% l-glutamine 200 mM (100×), 105 U/L ESGRO mLIF (Millipore, Temecula, CA, http://www.emdmillipore.com) and 0.2 mM 2-mercaptoethanol (Sigma-Aldrich). Approximately 2–4 hours before plating the G4 mouse embryonic stem cells (mESCs; Samuel Lunenfeld Research Institute, Toronto, ON, Canada, http://www.lunenfeld.ca) onto the layer of MEF feeder cells, MEF medium was replaced with ES medium. Next, 1 × 106 mESCs were seeded on top of the feeder layer using ES medium. The media were changed every day for 5 days to obtain a satisfactory amount of proliferating mESC colonies.
Mouse EB medium was prepared using DMEM/F12 (1:1) (1×) (Life Technologies) supplemented with 15% FBS, 15% Knock Out Serum (Life Technologies), 1% minimal essential medium nonessential amino acids (100×) (Life Technologies), 1% of l-glutamine 200 mM (100×), 0.2 mM 2-mercaptoethanol, and 5 ng/ml of basic fibroblast growth factor (R&D Systems, Minneapolis, MN, http://www.rndsystems.com). Next, 2 × 106 mESCs were seeded on a 100-mm Petri dish or 96-well plate coated with 1.5% agarose to generate EBs in a low-adhesion environment. Using a simple decantation method, at least 50% of the medium was replaced with fresh EB medium every day. The same culture conditions were used for static culture of EBs.
Microfluidic Chip Fabrication
The microfluidic devices were designed and fabricated using 1.5-mm-thick poly(methyl methacrylate) (PMMA; McMaster-Carr, Elmhurst, IL, http://www.mcmaster.com) and 80-µm-thick, double-sided adhesive film (DSA) (iTapestore, Scotch Plains, NJ, http://www.itapestore.com), as described in previous studies [16]. In brief, three 4-mm × 28-mm parallel channels separated by a gap of 3 mm were cut onto a 24-mm × 40-mm DSA film and PMMA plate using a laser cutter (VersaLaser, Scottsdale, AZ, http://www.versalaser.com). The surface of a 24-mm × 40-mm glass coverslip (150 µm thick) or polystyrene plate (1 mm thick) was plasma treated for 90 seconds and adhered to the DSA film, forming the base and middle layer of the microfluidic device, respectively. The channels were covered with 24 mm×40 mm PMMA with 3 inlet and 3 outlet openings of 0.78 mm in diameter, serving as the top layer of the microfluidic device. The openings in this layer were aligned to the end point of the DSA channels to be used as inlets and outlets during the fluid flow. Finally, PMMA channels with the inlet and outlet openings were assembled into the DSA-polystyrene plate combination to make a three-layer microfluidic device with microchannels of 4 mm × 28 mm × 1.5 mm in dimension. All components used in assembly were cleaned with detergent and ethanol and UV sterilized for 15 minutes under a laminar flow hood before assembly.
Dynamic Culture of EBs in Microfluidic Chip
Approximately 5 × 106 EB cells per milliliter were mixed uniformly with ice cold Matrigel (growth factor reduced; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). Next, 70–100 µl of this EB-Matrigel mixture was carefully pipetted into each 4-mm × 28-mm × 1.5-mm channel of the microfluidic chip. The assembled, cell-laden, microfluidic chip was then transferred to a 37°C incubator for 15 minutes to produce a uniform layer of hydrogel on gelation. After gelation of Matrigel, the third layer of the microchip (PMMA layer with the inlet and outlet openings) was carefully aligned and assembled onto the body of the chip. Silicon tubes (inner diameter 0.25 mm; catalog no. EW-06419-00; Cole-Parmer, Vernon Hills, IL, http://www.coleparmer.com) were inserted into the inlet and outlet openings for unidirectional flow through the microchannels. The microchip with encapsulated EB cells was transferred into the cell culture incubator providing continuous flow of fresh EB media at the rate of 2 µL/min using 10-mL syringes (BD Biosciences) and a syringe pump (NE-1600; New Era Pump Systems, Farmingdale, NY, http://www.syringepump.com) (supplemental online Fig. 1). The terminal ends of the channels were connected to 15-ml tubes to collect the drained conditioned medium of 24 hours at days 1, 5, 11, 15, and 21 for detection and quantification of the secreted steroid hormones using enzyme-linked immunosorbent assay (ELISA).
Cryopreservation of EB Immobilized Microfluidic Chips
After 24 hours of dynamic culture, the EB immobilized microfluidic chips were washed with PBS, and the channels were filled with cryoprotecting solution (80% FBS, 20% dimethyl sulfoxide). After blocking the inlets and outlets, the microfluidic chips were sealed, immersed in isopropanol (Sigma-Aldrich), frozen at −80°C overnight, and then transferred into liquid nitrogen. After 48 hours, the cryopreserved chips were thawed in a 37°C water bath and rinsed 3 times with fresh culture media.
Viability and Proliferation Assays
The viability of the cells within the EB was assessed after 21 days of microfluidic chip culture and after thawing with calcein-AM/ethidium homodimer-1, LIVE/DEAD assay (Life Technologies). The assay was performed directly within the microfluidic chip without harvesting the EBs by incorporating the LIVE/DEAD kit reagents and subsequent washing steps. The samples were imaged using the Zeiss Axio fluorescence microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com). The proliferation of cells was determined using the bromodeoxyuridine (BrdU) proliferation assay kit (Sigma-Aldrich) according to the manufacturer’s instructions.
Immunocytochemical Analysis
Mouse ESC colonies, EBs in suspension, and EBs in the microfluidic chip were harvested and fixed with 1% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, http://www.emsdiasum.com). The samples were blocked with 1% bovine serum albumin (Sigma-Aldrich), permeabilized with 0.3% TritonX 100 (Sigma-Aldrich), and stained for stem cell markers Oct4 (ab18976; Abcam, Cambridge, U.K., http://www.abcam.com), SSEA-4 (330410; BioLegend, San Diego, CA, http://www.biolegend.com), and Nanog (ab80892; Abcam), germ layer markers α-fetoprotein (sc-8108, Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com), α-smooth muscle actin (ab5694; Abcam), and neurofilament (ab7794; Abcam) and ovarian tissue markers anti-müllerian hormone receptor (AMHR) type II (ab64762; Abcam), inhibin β-A (sc-166503; Santa Cruz Biotechnology), follicle-stimulating hormone receptor (FSHR; sc-7798; Santa Cruz Biotechnology), and anti-aromatase (CYP19A1) (ab35604; Abcam) primary antibodies overnight at 4°C. Alexa Fluor 488 and Alexa Fluor 568 were used as secondary antibodies, and the cell nuclei were stained with 4′6-diamidino-2-phenylindole (Life Technologies). The stained samples were analyzed using a Zeiss LSM 510 META confocal microscope.
Enzyme-Linked Immunosorbent Assay
Conditioned medium from EBs cultured in a 96-well plate under static conditions and conditioned medium collected from the terminal end of the EB-immobilized microfluidic channels before and after cryopreservation were collected for a 24-hour period and analyzed for the presence of the sex hormones estradiol, progesterone, and testosterone. The levels of secreted steroid hormones were detected using ELISA with a specific kit for estradiol, progesterone, testosterone, and anti-müllerian hormone (AMH) according to protocols of the Wisconsin National Primate Research center, University of Wisconsin, Madison (Madison, WI). The antibodies for progesterone and testosterone were provided by Coralie Munro from University of California, Davis (Davis, CA); the antibody for estradiol was supplied by Holly Hill Biologicals (Hillsboro, OR). ELISA analysis for the basal levels of the sex hormones in EB media was done in-house according to manufacturer’s instructions (estradiol and progesterone from Calbiotech, Spring Valley, CA, http://www.calbiotech.com; testosterone from Enzo Life Sciences, Farmingdale, NY, http://enzolifesciences.com).
Statistical Analysis
The experimental results were analyzed using analysis of variance with Tukey’s post hoc test for multiple comparisons and Student’s two-tailed t test for single comparisons, with statistical significance set at p < .05. Unless otherwise stated, the mean values represent three experiments with two or three channels per experiment and the error bars represent the SEM. Statistical analyses were performed using GraphPad Prism, version 5 (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com).
Results
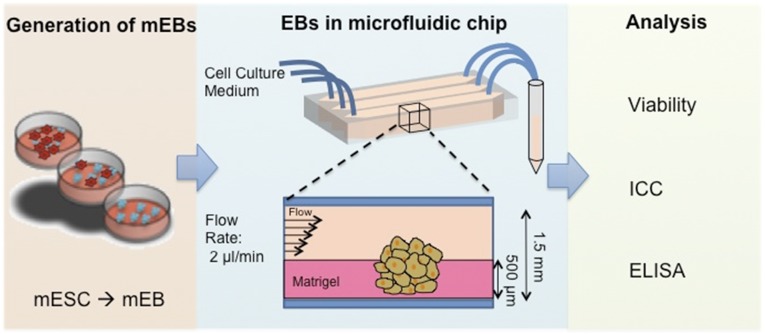
We fabricated a simple microfluidic device to physically stimulate the generated EBs with continuous laminar flow and shear stress. Dynamic culture introduces mechanical stimulation on cells in their native environment [16]. The bottom of the device is designed as a 150-μm-thick glass coverslip, enabling a sufficient penetration depth to monitor the EBs using confocal microscopy. To immobilize the EBs within a microfluidic channel and provide ECM-like support, we plated the EBs within a Matrigel depth of 500 μm, avoiding total encapsulation. After immobilization of the EBs, the microfluidic channel allowed 1.5 mm of depth for the flow of the media (Fig. 1). The cell culture media were perfused with a syringe pump with flow rate of 2 μl/min. We used silicon tubing that allows gas exchange for oxygenation of the media. The contained microfluidic system developed in the present study provides advantages over classic two-dimensional culture because it uses smaller amounts of reagents and multiplying the test conditions for high-throughput analyses. The designed chip also allows an in situ tracking and staining platform without the removal of the EBs from the channels (supplemental online Fig. 1).
Figure 1.
Schematic diagram of the experimental setup. mESCs are suspended in agarose-coated tissue culture dishes to generate EBs. The generated EBs are partially embedded in Matrigel within a microfluidic channel. Constant and continuous EB medium was flowed at 2 μl/minute through the channels for 21 days. The conditioned media were collected daily for ELISA analysis of hormone production. Abbreviations: EBs, embryoid bodies; ELISA, enzyme-linked immunosorbent assay; mEB, mouse embryoid body; ICC, immunocytochemistry; mESCs, mouse embryonic stem cells.
Microfluidic System Supported Long-Term Culture of Mouse ESC-Derived Embryoid Bodies
In the present study, we generated EBs from mouse embryonic stem cells and incorporated them into microfluidic channels with culture under continuous flow (supplemental online Fig. 2). The EBs used in the present study ranged from 70 to 200 μm in diameter. After culture under continuous laminar flow in the microfluidic channels for 21 days, we observed that the EBs were highly viable (Fig. 2A) comparable to that observed in standard tissue culture plates. Minimal cell death was detected within the EBs (Fig. 2A), demonstrating that the microenvironment and the physiological conditions supported the viability of the cells. We also demonstrated the preservation of the metabolic activity of the cells within the microfluidic culture. We investigated the proliferation of long-term cultured EBs using the BrdU assay. The newly formed cells within the EBs were detected using an anti-BrdU assay (Fig. 2C) showing that the cells were metabolically active and pursuing proliferation.
Figure 2.
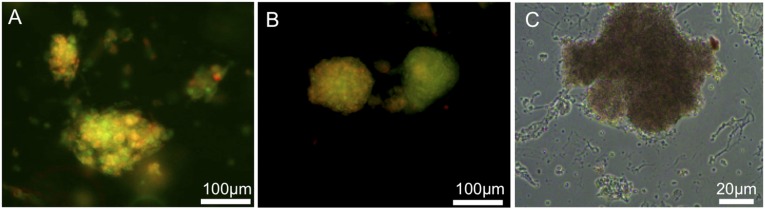
Embryoid bodies (EBs) showed high viability after long-term culture (21 days) and cryopreservation in a microfluidic chip. (A): Live/dead viability staining of EBs cultured for 21 day on chip. (B): Live/dead viability staining of EBs after cryopreservation. Green indicates live cells; red, dead cells. (C): Bromodeoxyuridine staining showing EBs proliferating after 21 days of culture in a microfluidic chip.
mESCs and EBs Cultured in Microfluidic Chips Continued to Grow and Differentiate
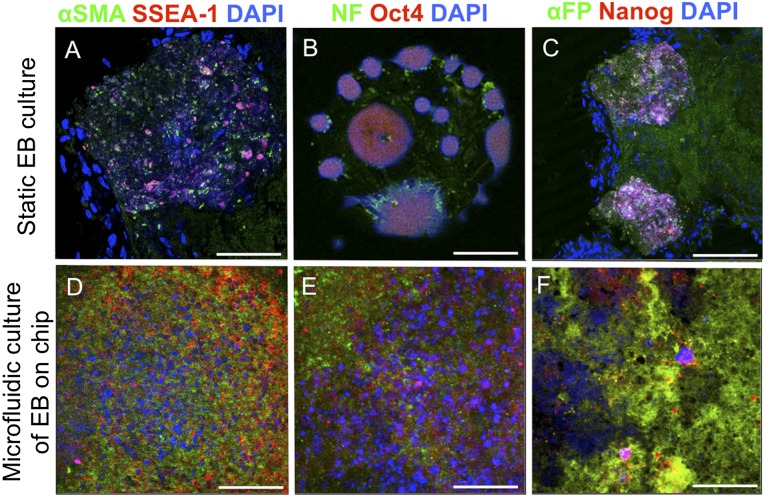
The characterization of the germ layers within the EBs after static and microfluidic culture was assessed using immunocytochemistry for stem cell markers and germ layer-specific cell surface markers. The stemness properties of the mESC colonies, EBs grown in static conditions, and EBs in the microfluidic chips were assessed by staining for anti-Nanog, anti-Oct4, and anti-SSEA-1 markers. The mESC colonies and EBs in microfluidic channels expressed these ESC antigens after 21 days comparable to that of the mESCs and EBs grown in tissue culture plates (Fig. 3). The EBs also demonstrated cell differentiation into the three major germ layers mesoderm (α-smooth muscle actin), ectoderm (neurofilament), and endoderm (α-fetoprotein). This immunocytochemistry assay showed that the EBs under laminar flow conditions were able continue to differentiate into the three germ layers.
Figure 3.
Germ layer differentiation and stemness properties of EBs. Differentiation and stemness of EBs were analyzed and compared with static conditions after 21 days of culture in a microfluidic chip. Mesoderm was stained for α-SMA, ectoderm for NFs, and endoderm for α-FP. Stem cell markers SSEA-1, Oct4, and Nanog were confirmed in EBs cultured in both static conditions and microfluidic chips. Scale bars = 100 μm (A–C) and 50 μm (D–F). Abbreviations: α-FP, α-fetoprotein; α-SMA, α-smooth muscle actin; DAPI, 4′6-diamidino-2-phenylindole; EBs, embryoid bodies; NFs, neurofilaments.
EB-Microfluidic Chips Can Be Cryopreserved With Recovery of Function
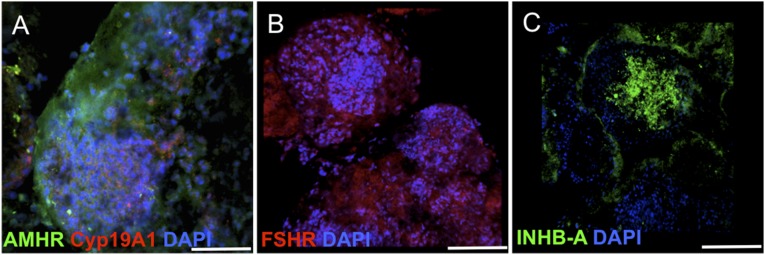
The fabricated microfluidic cassettes are designed to resist the low temperatures (−196°C) of cryopreservation by replacing the glass coverslip with a 1-mm-thick polystyrene plate. EB-loaded microfluidic chips were cultured for 24 hours under continuous laminar flow and later cryopreserved according to the adopted cryopreservation technique by slow freezing of the samples in isopropanol and storage in liquid nitrogen. After cryopreservation, the viability of the EBs was assessed using the LIVE/DEAD assay directly on the microfluidic chip (Fig. 2B). Moreover, the cryopreserved and differentiated EBs were also positive for ovarian lineage markers, such as AMHR, expressed by granulosa cells (Fig. 4A); FSHR, expressed by gonadotropic cells in immature ovarian follicles (Fig. 4B); and inhibin β-A (Fig. 4C). The functional activity of the differentiated tissue was confirmed further with the expression of CYP19A1, showing enzymatic activity for the secretion of estradiol (Fig. 4A).
Figure 4.
Embryoid bodies cryopreserved in a microfluidic channel showing differentiation to ovarian tissue markers: AMHR type II and estrogen biosynthesis enzyme aromatase Cyp19A1 (A), FSHR (B), and INHB-A (C) markers. Scale bars = 200μm. Abbreviations: AMHR, anti-müllerian hormone receptor; DAPI 4′6-diamidino-2-phenylindole; FSHR, follicle-stimulating hormone receptor; INHB-A, inhibin β-A.
Cryopreserved EB-Microfluidic Chips Recovered Steroidogenic Function When Thawed and Cultured
The presence of the steroid hormones estradiol, testosterone, and progesterone within the conditioned media collected from the dynamic culture of EBs before and after cryopreservation was detected using ELISA analysis. The samples for 24-hour period were collected at days 1, 5, 11, 15, and 21 and stored frozen until analysis. The estradiol level present in the collected samples from the noncryopreserved samples was stable at 64–79 pg/ml for the 21-day period (Fig. 5). After cryopreservation of the EB-containing microfluidic chip, the estradiol levels had decreased but not to a significant amount (54–62 pg/ml) compared with the 21-day culture period. Secretion of progesterone in the 21-day period showed a similar trend for the samples with and without cryopreservation. The range of progesterone for the noncryopreserved sample was 144–423 pg/ml for the 21-day period. The level of secreted testosterone fluctuated from 76 to 141 pg/ml for the noncryopreserved sample and from 47 to 107 pg/ml for the cryopreserved sample. The estradiol and progesterone analysis for the EBs cultured under static conditions was performed for 9 days (supplemental online Fig. 3). The estradiol levels secreted from the EBs range from 23.4 to 28.77 pg/ml and for progesterone from 6.546 to 4.55 ng/ml. The basal levels of the steroid hormones in blank EB media were as follows: estradiol, 12.61 pg/ml; progesterone, 58.5 pg/ml; and testosterone, 54.33 pg/ml. The AMH levels for the noncryopreserved channels were 17–41 pg/ml over 20 days of culture. After cryopreservation, the AMH levels were similar at 19–45 pg/ml (supplemental online Table 1). The AMH results support the presence of estrogen-secreting cells and confirm the immunostaining results for AMHR. Moreover, AMH secretion shows the existence of ovarian cells that differentiate from embryonic stem cells and survive and remain functional after cryopreservation.
Figure 5.
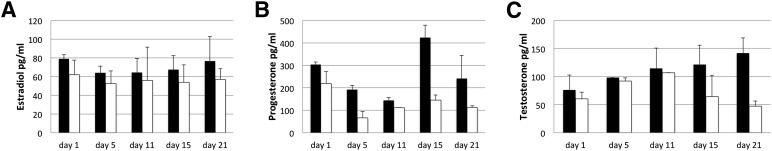
Steroid hormones secreted by mouse embryoid bodies in a microfluidic chip detected by enzyme-linked immunosorbent assay analysis after 21 days of culture. Noncryopreserved samples (black bars) and cryopreserved samples (white bars). Hormones estradiol (A), progesterone (B), and testosterone (C).
Discussion
The current clinical approaches to regenerative medicine aim to use pluripotent stem cells in cell- and gene-based therapies and tissue engineering applications. Because the capacity to form trophoblastic clusters and secrete steroidogenic hormones such as estradiol has been demonstrated, the idea has emerged to use pluripotent stem cells as in vitro agents for the secretion of endocrine hormones [14, 21]. Existing tissue culture methods face specific challenges, including elucidating specific differentiation signals, reproducing in vivo-like differentiation conditions, tissue tolerance, and the long-term viability of the differentiated tissues in culture [20, 22].
Designing a Microfluidic Device for Sustainable Long-Term Dynamic Culture of EBs
We have described an innovative culture system (Fig. 1) to grow, differentiate, and cryopreserve EBs in a microfluidic environment that allows the development of functionally specialized cells and tissues, such as ovarian cells and endocrine tissue. EBs are formed from embryonic stem cells or induced pluripotent stem cells (iPSCs) and have the potential to differentiate into any desired cell type, such as cardiac cells [23], osteogenic and chondrogenic cells [24], neurons [25], insulin-secreting β-cells [26], and steroid hormone-secreting cells [21]. EBs are three dimensional; thus, their growth and duration in culture have been restricted by physical limitations such as the penetration of media nutrients to the EB’s core. In the present study, we attempted to address the considerations regarding the survival of EBs using continuous laminar flow within a microfluidic system. We have demonstrated sustained high viability of EBs for up to 21 days, with high proliferation activity within the microfluidic system, indicating favorable culture conditions. We also observed dead cells within the EBs that can be explained by the progress of cell cycle dynamics, in which cells grow and die [27].
Moreover, microfluidic cultures provide advantages such as the ability to mimic native-like environments and investigate biological systems [18, 28, 29]. The physical and chemical properties of cellular microenvironments (e.g., matrix stiffness, gradient of chemokines and growth factors) are known to significantly influence and direct the differentiation process of stem cells [26, 30–33]. Microfluidic systems also enable minimization of the sample size, allowing costly reagents to be used in smaller quantities, and provide a dynamic platform for high-throughput screening of chemicals or drugs [34, 35]. We used these advantages of the microfluidic platforms to differentiate EBs toward ovarian tissue. Steroidogenic cells of the ovary are the primary endocrine tissue of the female reproductive tract, and they are critical to normal female development, reproductive function, and maintenance of health.
Differentiation of EBs Toward Ovarian Tissue in Microfluidic Systems
In the present study, by introducing dynamic microfluidic culture, we have extended our previous work, in which we showed that differentiation of EBs under static culture conditions can develop trophoblastic tissue that secretes estradiol, progesterone, and human chorionic gonadotropin (hCG) [21]. We have demonstrated the presence of granulosa and gonadotropic cells within the EBs differentiated in the designed microfluidic system. Together with the hormone secretion profiles, these findings have confirmed the ovarian tissue characteristics of the differentiated EBs. Similar findings were reported by Lipskind et al. [13], who found expression of ovarian antigens in the differentiating EBs under static culture conditions. In the present study, we have validated the functional performance of differentiated EBs in the microfluidic system. These data show the dynamics of the generated microenvironment on the chip. Taken together, these results suggest that a dynamic flow system using microfluidic chips is a viable option for differentiation of ESC-derived EBs that will successfully differentiate toward functional ovarian tissue.
Cryopreservation of the EB Differentiation Platform
Traditional drug development and therapeutic approaches to cure diseases have been based on mimicking the synthesis of natural molecules or designing biologically active compounds. The activity of a therapeutic agent and the physiological response can vary greatly among patient populations [10]. The current trends in medicine are focusing on personalized approaches specific to the patient, developing customized tools for curing diseases. The microfluidic culture system presented in our study combines stem cell biology with bioengineering to formulate a platform for personalized medicine, in which a dynamic microfluidic system can reflect the natural in vivo environment. To show the potential for future clinical applications, which require “on demand use,” we tested and found that the microfluidic system can be successfully cryopreserved and thawed on demand with viable and functional differentiated EBs. These advances could be translated to useful applications such as in cell-based therapies.
Toward Personalized Medicine
An exciting application of the presented platform in regenerative medicine is the development of patient-specific microfluidic treatment modules. The potential applications of such a system are far-reaching for the treatment of other endocrine or neurohormonal disorders, such as diabetes with insulin replacement, Parkinson’s disease with dopamine replacement, or ovarian failure with estrogen and progesterone replacement. For each of these situations, as the specific signals that direct the differentiation of embryonic and pluripotent stem cells into the desired cell types are unraveled, one could use such microfluidic cassette cultures to harvest secreted bioidentical hormones and to cryopreserve the differentiated EBs within the chip for future use, as needed. Similar to the medication cartridges used today, in the foreseeable future, patients might receive autologous personalized treatment using their own iPSCs that have been differentiated into the desired secretory cell and grown in individual microfluidic chips. For the production of personalized biological agents, in addition to device simplicity and ease-of-use, reproducibility, reliability, and robustness through automation are required by regulatory agencies [12]. To allow the presented system to meet the levels of production at physiologically relevant hormone concentrations, high-throughput, multiplexed systems are needed for hormone therapies.
Previous studies have demonstrated the ability of EBs from human ESCs to produce functional trophoblastic tissue secreting estradiol, progesterone, and hCG [21] and ovarian granulosa-like cells secreting AMH and follicle-stimulating hormone [36]. With the discovery of iPSCs, excitement has been heightened in the field of regenerative medicine, because a primary obstacle to cell-based therapies has been the antigenic matching of tissue. With iPSCs, we have the option of developing autologous patient-specific treatment systems using pluripotent iPSCs autologous to the patient [37]. Regarding hormone replacement therapy, in recent years, concerns have been raised by studies such as the Women’s Health Initiative, regarding the risks associated with the use of synthetically produced hormones [4]. Combining the autologous nature of iPSCs with the potential to differentiate iPSC-derived EBs into steroidogenic cells, it will be possible to produce bioidentical hormones for patient treatment [38]. A potential future direction with the presented microfluidic platform will be to further evaluate human iPSCs for clinically relevant applications.
Conclusion
The present study has demonstrated several innovative advancements in regenerative medicine and microfluidic systems. We showed that microfluidic chips present a viable system for maintaining mouse EB growth and differentiation. Also, estradiol and progesterone were produced at physiologically relevant levels. Finally, functionally established microfluidic chips with EBs can be cryopreserved and thawed with restoration of function for use at a later time point. These findings strongly support the potential use of microfluidic chips for future personalized hormone therapies. This approach could be adapted to broad clinical applications, such as the generation of patient-specific β-islet cells or a drug-screening platform for patient-derived tumorigenic cells.
Supplementary Material
Acknowledgments
U.D. acknowledges that this material was based in part on work supported by the National Science Foundation under NSF CAREER Award Number 1150733 and Grant R01 EB015776. R.M.A. acknowledges research support from Michael Cassidy and Caroline Wang, the Department of OB GYN, BWH (to R.M.A.) and MSys Inc., India (to S.C.). Research reported in this publication was supported in part by the Office of the Director, NIH, under Award Number P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin, Madison. This research was conducted in part at a facility constructed with support from the Research Facilities Improvement Program (Grants RR15459-01 and RR020141-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We thank the Harvard NeuroDiscovery Center, the Enhanced Neuroimaging Core Facility for support on the confocal images. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. B.G.-N. is currently affiliated with the Department of Oral and Maxillofacial Pathology, Oral Medicine, and Craniofacial Pain, Tufts University School of Dental Medicine, Boston, MA. U.A.G. is currently affiliated with the Case Biomanufacturing and Microfabrication Laboratory, Department of Mechanical and Aerospace Engineering, and Department of Orthopaedics, Case Western Reserve University, Advanced Platform Technology Center, Louis Stokes Cleveland Veterans Affairs Medical Center, Cleveland, OH.
Author Contributions
S.G., J.S.L., I.P., S.C., M.D.N., and B.G.-N.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; U.A.G.: conception and design, final approval of manuscript; R.M.A.: conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; U.D.: conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
U.D. is a founder of, and has an equity interest in: (a) DxNow Inc., a company that is developing microfluidic and imaging technologies for point-of-care diagnostic solutions, and (b) Koek Biotech, a company that is developing microfluidic IVF technologies for clinical solutions; his interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policies. The other authors indicated no potential conflicts of interest.
References
- 1.Howlader N, Noone AM, Krapcho M et al., eds. SEER Cancer Statistics Review, 1975–2010, National Cancer Institute. Bethesda, MD. Available at http://seer.cancer.gov/csr/1975_2010/. Updated April 2013. Accessed March 1, 2014.
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Pattimakiel L, Thacker HL. Bioidentical hormone therapy: Clarifying the misconceptions. Cleve Clin J Med. 2011;78:829–836. doi: 10.3949/ccjm.78a.10114. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Billeci AM, Paciaroni M, Caso V, et al. Hormone replacement therapy and stroke. Curr Vasc Pharmacol. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- 7.Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: A meta-analysis. BMJ. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beral V, Million Women Study Collaborators. Bull D, et al. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369:1703–1710. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- 9.Holtorf K. The bioidentical hormone debate: Are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy? Postgrad Med. 2009;121:73–85. doi: 10.3810/pgm.2009.01.1949. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz D. A comprehensive review of the safety and efficacy of bioidentical hormones for the management of menopause and related health risks. Altern Med Rev. 2006;11:208–223. [PubMed] [Google Scholar]
- 11.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363:301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 13.Lipskind S, Lindsey JS, Kiezun A et al. Embryoid bodies as a stem cell model for steroidogenic cell commitment and ovarian development. Reprod Sci 2013;20(suppl 1):188A. [Google Scholar]
- 14.Golos TG, Pollastrini LM, Gerami-Naini B. Human embryonic stem cells as a model for trophoblast differentiation. Semin Reprod Med. 2006;24:314–321. doi: 10.1055/s-2006-952154. [DOI] [PubMed] [Google Scholar]
- 15.Gothard D, Roberts SJ, Shakesheff KM, et al. Controlled embryoid body formation via surface modification and avidin-biotin cross-linking. Cytotechnology. 2009;61:135–144. doi: 10.1007/s10616-010-9255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizvi I, Gurkan UA, Tasoglu S, et al. Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci USA. 2013;110:E1974–E1983. doi: 10.1073/pnas.1216989110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurkan UA, Tasoglu S, Akkaynak D, et al. Smart interface materials integrated with microfluidics for on-demand local capture and release of cells. Adv Healthcare Mater. 2012;1:661–668. doi: 10.1002/adhm.201200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta K, Kim DH, Ellison D, et al. Lab-on-a-chip devices as an emerging platform for stem cell biology. Lab Chip. 2010;10:2019–2031. doi: 10.1039/c004689b. [DOI] [PubMed] [Google Scholar]
- 19.Tasoglu S, Safaee H, Zhang X, et al. Exhaustion of racing sperm in nature-mimicking microfluidic channels during sorting. Small. 2013;9:3374–3384. doi: 10.1002/smll.201300020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane BJ, Zinner MJ, Yarmush ML, et al. Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem. 2006;78:4291–4298. doi: 10.1021/ac051856v. [DOI] [PubMed] [Google Scholar]
- 21.Gerami-Naini B, Dovzhenko OV, Durning M, et al. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- 22.Kossowska-Tomaszczuk K, Pelczar P, Güven S, et al. A novel three-dimensional culture system allows prolonged culture of functional human granulosa cells and mimics the ovarian environment. Tissue Eng Part A. 2010;16:2063–2073. doi: 10.1089/ten.TEA.2009.0684. [DOI] [PubMed] [Google Scholar]
- 23.Pesl M, Acimovic I, Pribyl J, et al. Forced aggregation and defined factors allow highly uniform-sized embryoid bodies and functional cardiomyocytes from human embryonic and induced pluripotent stem cells. Heart Vessels. 2014;29:834–846. doi: 10.1007/s00380-013-0436-9. [DOI] [PubMed] [Google Scholar]
- 24.Lee TJ, Jang J, Kang S, et al. Mesenchymal stem cell-conditioned medium enhances osteogenic and chondrogenic differentiation of human embryonic stem cells and human induced pluripotent stem cells by mesodermal lineage induction. Tissue Eng Part A. 2014;20:1306–1313. doi: 10.1089/ten.tea.2013.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Pak C, Han Y, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanan K, Lim VY, Shen J, et al. Extracellular matrix-mediated differentiation of human embryonic stem cells: Differentiation to insulin-secreting beta cells. Tissue Eng Part A. 2014;20:424–433. doi: 10.1089/ten.TEA.2013.0257. [DOI] [PubMed] [Google Scholar]
- 27.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Primiceri E, Chiriacò MS, Rinaldi R, et al. Cell chips as new tools for cell biology—Results, perspectives and opportunities. Lab Chip. 2013;13:3789–3802. doi: 10.1039/c3lc50550b. [DOI] [PubMed] [Google Scholar]
- 29.Meyvantsson I, Beebe DJ. Cell culture models in microfluidic systems. Ann Rev Anal Chem (Palo Alto Calif) 2008;1:423–449. doi: 10.1146/annurev.anchem.1.031207.113042. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimitsu R, Hattori K, Sugiura S, et al. Microfluidic perfusion culture of human induced pluripotent stem cells under fully defined culture conditions. Biotechnol Bioeng. 2014;111:937–947. doi: 10.1002/bit.25150. [DOI] [PubMed] [Google Scholar]
- 31.Nampe D, Tsutsui H. Engineered micromechanical cues affecting human pluripotent stem cell regulations and fate. J Lab Autom. 2013;18:482–493. doi: 10.1177/2211068213503156. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Zhu F, Hu Q, et al. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials. 2014;35:1176–1184. doi: 10.1016/j.biomaterials.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YG, Moon S, Kuritzkes DR, et al. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens Bioelectron. 2009;25:253–258. doi: 10.1016/j.bios.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tasoglu S, Gurkan UA, Wang S, et al. Manipulating biological agents and cells in micro-scale volumes for applications in medicine. Chem Soc Rev. 2013;42:5788–5808. doi: 10.1039/c3cs60042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson JL, Suri S, Singh A, et al. Single-cell analysis of embryoid body heterogeneity using microfluidic trapping array. Biomed Microdevices. 2014;16:79–90. doi: 10.1007/s10544-013-9807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lan CW, Chen MJ, Jan PS, et al. Differentiation of human embryonic stem cells into functional ovarian granulosa-like cells. J Clin Endocrinol Metab. 2013;98:3713–3723. doi: 10.1210/jc.2012-4302. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Li H, Wu Z, et al. Differentiation of rat iPS cells and ES cells into granulosa cell-like cells in vitro. Acta Biochim Biophys Sin (Shanghai) 2013;45:289–295. doi: 10.1093/abbs/gmt008. [DOI] [PubMed] [Google Scholar]
- 38.Ruggiero RJ, Likis FE. Estrogen: Physiology, pharmacology, and formulations for replacement therapy. J Midwifery Womens Health. 2002;47:130–138. doi: 10.1016/S1526-9523(02)00233-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.