The therapeutic effects of PDA-001 in mice with chronic heart failure (CHF) were tested. Three weeks after transaortic constriction surgery to induce CHF, the mice underwent direct injection of PDA-001. Intramyocardial injection of PDA-001 significantly improved left ventricular systolic and diastolic function and decreased cardiac fibrosis, demonstrating the cardiac therapeutic potential of PDA-001.
Keywords: Placental adherent cell, Mesenchymal stromal cell, Chronic heart failure, Cell therapy
Abstract
Human placenta-derived adherent cells (PDACs) are a culture-expanded, undifferentiated mesenchymal-like population derived from full-term placental tissue, with immunomodulatory, anti-inflammatory, angiogenic, and neuroprotective properties. PDA-001 (cenplacel-L), an intravenous formulation of PDAC cells, is in clinical development for the treatment of autoimmune and inflammatory diseases. We tested the therapeutic effects of PDA-001 in mice with chronic heart failure (CHF). Three weeks after transaortic constriction surgery to induce CHF, the mice underwent direct intramyocardial (IM) or i.v. injection of PDA-001 at a high (0.5 × 106 cells per mouse), medium (0.5 × 105 cells per mouse), or low (0.5 × 104 cells per mouse) dose. The mice were sacrificed 4 weeks after treatment. Echocardiography and ventricular catheterization showed that IM injection of PDA-001 significantly improved left ventricular systolic and diastolic function compared with injection of vehicle or i.v. injection of PDA-001. IM injection of PDA-001 also decreased cardiac fibrosis, shown by trichrome staining in the vicinity of the injection sites. Low-dose treatment showed the best improvement in cardiac performance compared with the medium- and high-dose groups. In another independent study to determine the mechanism of action with bromodeoxyuridine labeling, the proliferation rates of endothelial cells and cardiomyocytes were significantly increased by low or medium IM dose PDA-001. However, no surviving PDA-001 cells were detected in the heart 1 month after injection. In vivo real-time imaging consistently revealed that the PDA-001 cells were detectable only within 2 days after IM injection of luciferase-expressing PDA-001. Together, these results have demonstrated the cardiac therapeutic potential of PDA-001, likely through a paracrine effect.
Introduction
Heart failure is the leading cause of morbidity and mortality in the world [1]. Currently, ∼6 million patients have heart failure in the United States. Despite intensive medical care, ∼80% of patients will die within 8 years of the diagnosis [2]; thus, the search for new cardiac therapeutic agents is crucial and urgent. Recently, cell therapy for cardiac repair has generated considerable interest, and earlier studies using autologous bone marrow transplantation have shown improved cardiac function after myocardial infarction (MI) [3]. However, more recent studies showed no benefits from bone marrow cell therapy at early stages after MI (3 or 7 days), by either intracoronary or transendocardial injection [4–6]. Although mesenchymal stem cells might provide better cardiac benefits than bone marrow mononuclear cells [7], major factors to consider include the isolation, purification, dosage, and delivery of the cells, the control of cell fate (i.e., cell survival, proliferation, differentiation), possible aberrance in the body after transplantation (e.g., toxicity, immunogenicity, and tumorigenesis), and the determination of the underlying mechanism of action [8, 9].
Human placenta-derived adherent cells (PDACs) are a culture expanded, undifferentiated mesenchymal-like population derived from full-term placental tissue and display immunomodulatory, anti-inflammatory, pro-regenerative, angiogenic, and neuroprotective properties [10–13]. The cells are anchorage-dependent in culture and will adhere to the plastic surface during expansion culture in vitro. PDAC-mediated activities have been studied in both in vitro and animal models, and the results have suggested that the cells might have potential utility across a variety of disease indications. PDA-001 (cenplacel-L), an intravenous formulation of PDAC cells composed of CD44+, CD73+, CD90+, CD105+, CD200+, and CD45− cells, is in clinical development for the treatment of autoimmune and inflammatory diseases [13, 14].
The primary objective of the present study was to provide proof of concept of the utility of PDA-001 in chronic heart failure (CHF) using a mouse model of CHF. The secondary objective was to provide insight into the potential mechanisms mediating the observed therapeutic activity. After performing transaortic constriction to induce CHF, we tested the effect of PDA-001 treatment. The results showed that intramyocardial (IM), but not i.v. injection, of PDA-001 improved cardiac performance and reduced interstitial fibrosis. To investigate the mechanism of action, we quantified cardiac cell proliferation and found that PDA-001 treatment significantly increased the proliferation of endothelial cells and cardiomyocytes. We also found that the in vivo retention of PDA-001 was as short as ∼2 days after IM injection, pointing to a paracrine-mediated mechanism of action rather than engraftment of the cells. Our studies support the continued investigation of PDA-001 as a cell therapy for CHF.
Materials and Methods
Cells
The PDA-001 cells were prepared and cryopreserved at passage 6 at Celgene Cellular Therapeutics (Warren, NJ, https://www.celgene.com) in a Good Manufacturing Practices-compliant facility [13]. These cells were isolated by mechanical and enzymatic digestion of postpartum human placenta, were karyotypically normal, and had the genotype of the newborn and the nominal phenotype CD34−, CD10+, CD200+, and CD105+. The cells were cryopreserved at −190°C before use.
Animals
The Institutional Animal Care and Use Committee of National Cheng Kung University approved all the animal studies, which were performed in compliance with the guidelines established by the National Council of Agriculture, Executive Yuan, Taiwan. Female C57BL/6 mice (weight ∼25 g) were purchased from LASCO, Taiwan (Taipei, Taiwan, http://www.biolasco.com.tw/index.php/tw/). The mice were inspected on arrival to ensure they were fit for the study and were checked daily for any signs of morbidity or mortality throughout the study. After a minimal acclimation period of 5 days, the mice were identified by ear tags and cage cards (total, n = 83). The mice were handled in accordance with the guidelines from the NIH and the Association for Assessment and Accreditation of Laboratory Animal Care. All the mice found in a moribund condition and those showing severe pain and signs of severe distress were humanely euthanized. Mice showing a >20% decrease in body weight from the initial body weight determination were also humanely euthanized. For anesthesia, the mice received Zoletil 50 (tiletamine/zolazepam hydrochloride, 50 mg/kg; Virbac, Carros, France, http://www.virbac.com) and Rompun 20 (xylazine hydrochloride, 4 mg/kg; Bayer HealthCare, Leverkusen, Germany, http://www.bayer.com) before surgery and isoflurane inhalation for maintenance and the in vivo measurements of echocardiography and catheterization. All the animal procedures were performed in a blinded and randomized manner.
CHF and Injection of PDA-001 in Mice
The mouse aorta was exposed through a supraclavicular approach, and left ventricular pressure overload was created by transaortic constriction using a 26-gauge needle, as previously described [15]. Echocardiography was performed 3 weeks later to confirm the success of CHF creation, indicated by an average 20%–25% increase in wall thickness and a 15%–20% decrease in the left ventricular ejection fraction (LVEF). A total of 69 surviving mice were divided into 7 groups: sham and CHF with IM injection of vehicle or PDA-001 at a high (0.5 × 106), medium (0.5 × 105), or low (0.5 × 104) dose, or i.v. injection of vehicle or PDA-001 at a high (0.5 × 106) dose (n ≥9 mice per group). In another study, to determine the mechanism of action of PDA-001 treatment in CHF, we inserted osmotic minipumps (Alzet 1007D; 0.5 µl/hour, 1 week; Durect Corp., Cupertino, CA, http://www.durect.com) subcutaneously in mice for continuous bromodeoxyuridine (BrdU) (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) (40 mg/ml, 100 microliters per mouse) infusion with the PDA-001 treatment. A total of 39 surviving mice were divided into 4 groups: sham and CHF with IM injection of vehicle or PDA-001 at a medium (0.5 × 105) or low (0.5 × 104) dose (n ≥9 mice per group). The mice then underwent echocardiography again and direct ventricular catheterization on day 28 after PDA-001 treatment and were then sacrificed.
Echocardiography
Echocardiography was used to assess the cardiac function in the CHF mice using a 30-MHz probe (Vevo770; VisualSonics, Toronto, ON, Canada, http://www.visualsonics.com). The mice were placed in the left lateral decubitus position. Parasternal long-axis views were obtained with both M-mode and 2-dimensional echocardiographic images. The left ventricular end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) were measured perpendicular to the long axis of the ventricle at the location of the papillary muscle insertion site. The LVEF was calculated automatically by the echocardiography system as (LVEDV − LVESV)/LVEDV × 100%, where LVEDV is the left ventricular end-diastolic volume, calculated as 7.0 × LVEDD3/(2.4 + LVEDD), and LVESV is the left ventricle end-systolic volume, calculated as 7.0 × LVESD3/(2.4 + LVESD) [15, 16].
Ventricular Catheterization
The mice were lightly anesthetized, and the ventricular hemodynamics were measured using a 1.4F pressure-volume sensing catheter (Millar, Inc., Houston, TX, http://www.millar.com). In brief, the catheter was inserted into the right carotid artery and advanced to the left ventricle. After stabilization, the baseline left ventricular (LV) pressure-volume loops were recorded. To change the preload, the inferior vena cava was transiently compressed through an incision in the upper abdomen. The volume calibration and the hemodynamic data were analyzed with software developed by the manufacturer [17].
Luciferase Assay for PDA-001 Retention
PDA-001 cells that constitutively express luciferase were prepared at Celgene Cellular Therapeutics using the Cignal Lenti Positive Control (luc) system (SABiosciences, Qiagen, Venlo, Limburg, http://www.sabiosciences.com), as recommended by the manufacturer. After coronary ligation in the C57BL/6 mice, 3 × 105 cells (in 25 μl) were injected into both the infarcted myocardial region and a normal hindlimb. The luciferin substrate (150 mg/kg; Caliper Life Sciences, PerkinElmer, Hopkinton, MA, http://www.perkinelmer.com) was then administered intravenously, and the luciferin intensity was measured in the whole mouse using an in vivo real-time imaging system (IVIS Spectrum 200, Caliper Life Sciences) at different time points after injection. The lower limit of detection was ∼1,000 cells (∼1% of injected cells) when concentrated in an area of approximately 0.1 ml.
Cardiac Fibrosis Measurement
After all in vivo measurements, the mice were humanely sacrificed. The hearts were harvested, fixed in 4% paraformaldehyde, dehydrated, and paraffinized. Masson’s trichrome staining was used to stain the collagen content. Images were then collected, and the fibrotic tissue was quantified using manual tracing and software calculation (ImageJ; NIH, Bethesda, MD, http://www.nih.gov). The ratios of fibrotic tissue thickness, length, and area were determined as percentages of the total value [17, 18].
Immunohistochemistry and Immunofluorescent Staining
The tissues from the hearts were frozen or fixed in 4% paraformaldehyde, dehydrated, and paraffinized until use. Fixed samples were then deparaffinized, rehydrated, and boiled in 10 mM sodium citrate (pH 7.4) for 10 minutes, followed by incubation with antibodies against isolectin IB4 Alexa Fluor 488 (Molecular Probes, Invitrogen, Carlsbad, CA, http://www.invitrogen.com), SM22α (Abcam, Cambridge, U.K., http://www.abcam.com), BrdU (Roche, Indianapolis, IN, http://www.roche.com), overlapped with anti-cardiac troponin-I (Developmental Studies Hybridoma Bank, Iowa City, IA, http://dshb.biology.uiowa.edu/) for cardiomyocyte staining at 4°C overnight, and then incubated with fluorescein isothiocyanate or Alexa Fluor-conjugated secondary antibodies (Molecular Probes, Invitrogen). Anti-human CD200 (AbD Serotec, Raleigh, NC, http://www.abdserotec.com/) and anti-human vimentin (Dako, Glostrup, Denmark, http://www.dako.com) were also stained to recognize the transplanted PDA-001. After counterstaining with 4′6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), the sections were mounted and observed under a fluorescence microscope. In addition, the capillary and arteriole densities at the border zone were measured, and blinded quantification was performed by manually counting the vessel number within each section.
Enzyme-Linked Immunosorbent Assay
EDTA-treated blood was centrifuged for collection of supernatant plasma and stored at −80°C until studied. Enzyme-linked immunosorbent assay kits were used to quantify the plasma concentrations of C-reactive protein (CRP; Immunology Consultants Laboratory, Portland, OR, http://www.icllab.com) [19].
Statistical Analysis
All data are presented as the mean ± SEM. Statistical analysis was performed using analysis of variance with Bonferroni’s adjustment. A probability value of < .05 was considered statistically significant.
Results
PDA-001 Treatment Improves Cardiac Performance in Mice With CHF
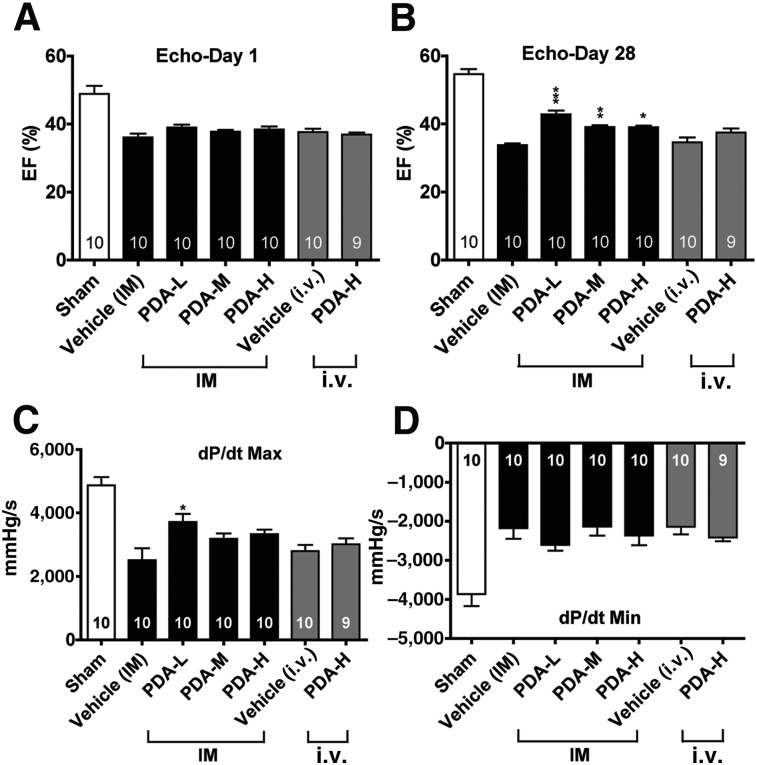
We used a mouse model of CHF induced by transaortic constriction as a model of progressive cardiac injury. The successful induction of CHF in mice was confirmed by echocardiography, with a 23% decrease in the LVEF observed at 3 weeks (Fig. 1A). The mice then underwent injection of a low, medium, or high dose of PDA-001 through IM or i.v. administration. With IM injection, a significant improvement in LVEF was observed in the mice receiving a low, medium, or high dose of PDA-001 at 28 days compared with the vehicle group (Fig. 1B). However, no obvious improvement was seen in the LVEF in mice receiving PDA-001 via i.v. injection (Fig. 1B). A modest improvement was seen in the LVEDD and LVESD by IM injection of low dose PDA-001 but not in the other groups receiving PDA-001 injection via IM or i.v. (supplemental online Fig. 1). Furthermore, hemodynamic measurement showed that the maximum dP/dt, an indicator of LV global systolic function, was also significantly ameliorated in the low-dose IM injection group and was marginally increased in the medium- and high-dose IM injection groups (Fig. 1C). In contrast, no change occurred in the minimum dP/dt, an indicator of LV global diastolic function, in mice receiving IM injection of a low, medium, or high dose or i.v. injection of a high dose of cells (Fig. 1D). Taken together, these results suggest that IM PDA-001 injection in CHF exerts cardiac benefits by increasing regional myocardial contractions, rather than preventing global ventricular dilatation.
Figure 1.
Intramyocardial injection of PDA-001 cells improved cardiac function in mice with chronic heart failure (CHF). (A–D): Three weeks after transaortic constriction surgery to induce CHF, the mice underwent direct IM or i.v. injection of PDA-001 at a high, medium, or low dose. The mice were sacrificed 4 weeks after treatment. Echocardiography was used to determine the left ventricular ejection fraction at 1 day (A) and 4 weeks (B) after cell injection. Direct ventricular catheterization was also used to measure the cardiac systolic (dP/dt Max) (C) and diastolic (dP/dt Min) (D). The data are presented as the mean ± SEM. The sample size is indicated in the bar chart. ∗, p < .05; ∗∗, p < .01; ∗∗∗, p < .001 compared with vehicle. Abbreviations: Echo, echocardiography; EF, ejection fraction; H, high; IM, intramyocardial; L, low; M, medium; Max, maximum; Min, minimum.
PDA-001 Treatment Reduces Cardiac Fibrosis in Mice With CHF
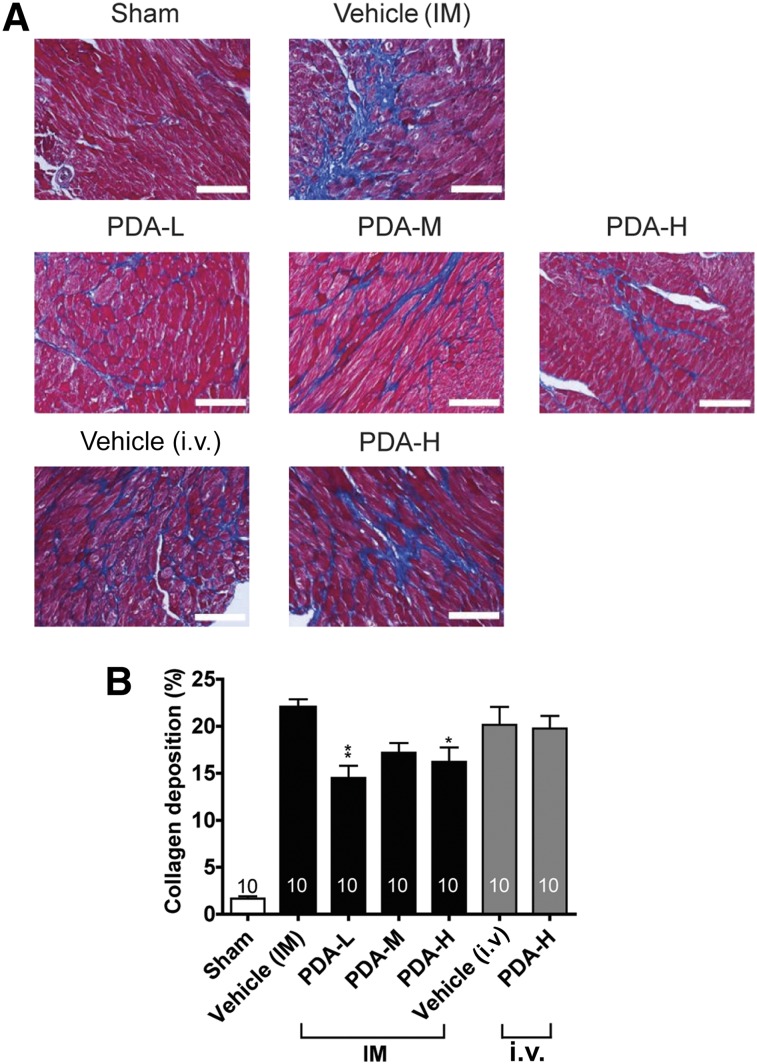
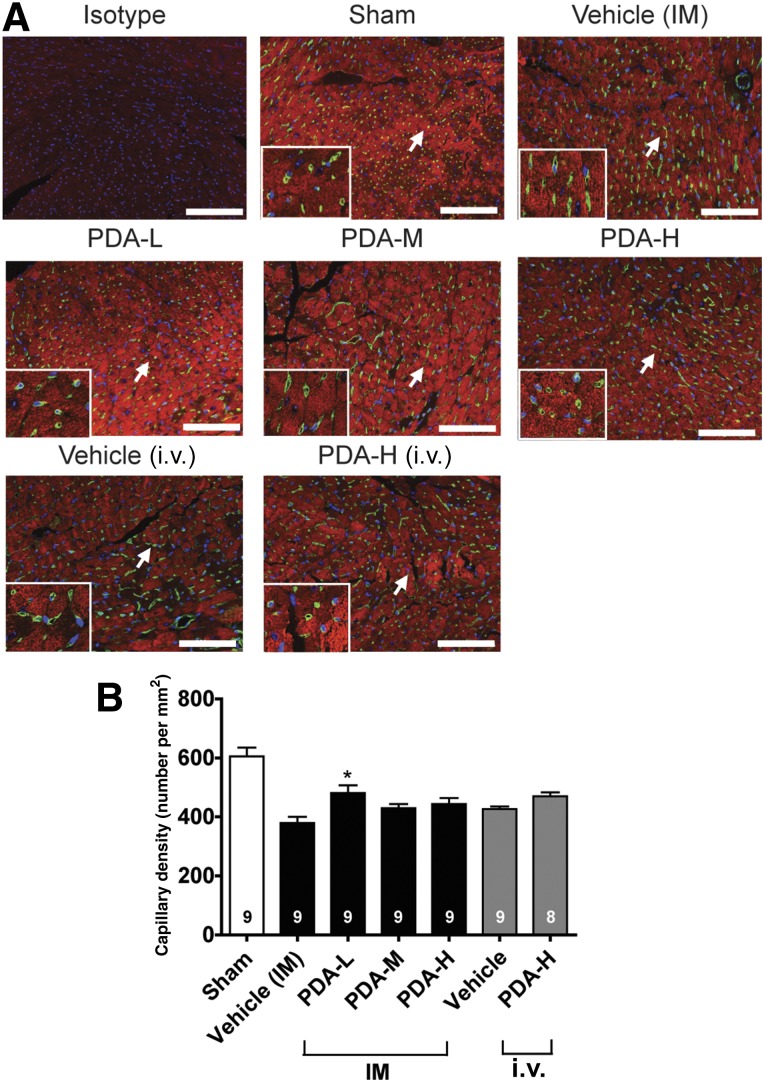
We then used trichrome staining to examine the collagen deposition and interstitial fibrosis of CHF at the cell injection sites. We found that IM injection of PDA-001 significantly reduced cardiac fibrosis, with the low dose having the greatest beneficial effects (Fig. 2). In contrast, i.v. injection of PDA-001 did not affect collagen deposition (Fig. 2). We also detected a significant increase in capillary density at the injected site in the mice receiving low-dose IM injection of PDA-001 but not in the other treatment groups (Fig. 3, supplemental online Fig. 4), suggesting a local angiogenic effect at the low dose. Small (but not statistically significant) decreases in the systemic CRP level were consistently observed with IM or i.v. administration of PDA-001 (supplemental online Fig. 2), suggesting that PDA-001 injection might have modest anti-inflammatory effects in the CHF model.
Figure 2.
PDA-001 cells prevented cardiac fibrosis in mice with chronic heart failure. (A): Representative images of the collagen content at the injected area of the heart from each group. (B): Statistical analysis of the collagen content (n ≥10 in each group). ∗, p < .05; ∗∗, p < .01 compared with vehicle. Scale bar = 100 μm. Abbreviations: H, high; IM, intramyocardial; L, low; M, medium.
Figure 3.
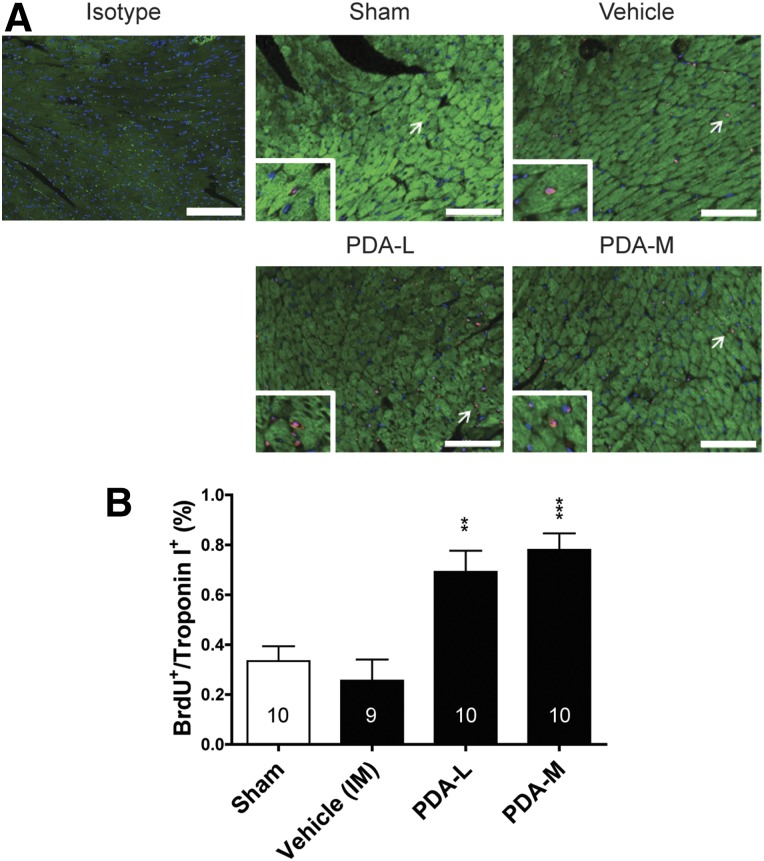
IM injection of PDA-001 cells increased the capillary density at the injected sites. (A): Representative images of isolectin (green) and cardiac troponin-I (red) at the injected area of the heart from each group. Nuclei were stained using 4′6-diamidino-2-phenylindole. (B): Statistical analysis of capillary density. The sample size is indicated in the bar chart. ∗, p < .05 compared with vehicle. Scale bar = 100 μm. Abbreviations: H, high; IM, intramyocardial; L, low; M, medium.
PDA-001 Increases Cardiac Cell Proliferation in Mice With CHF
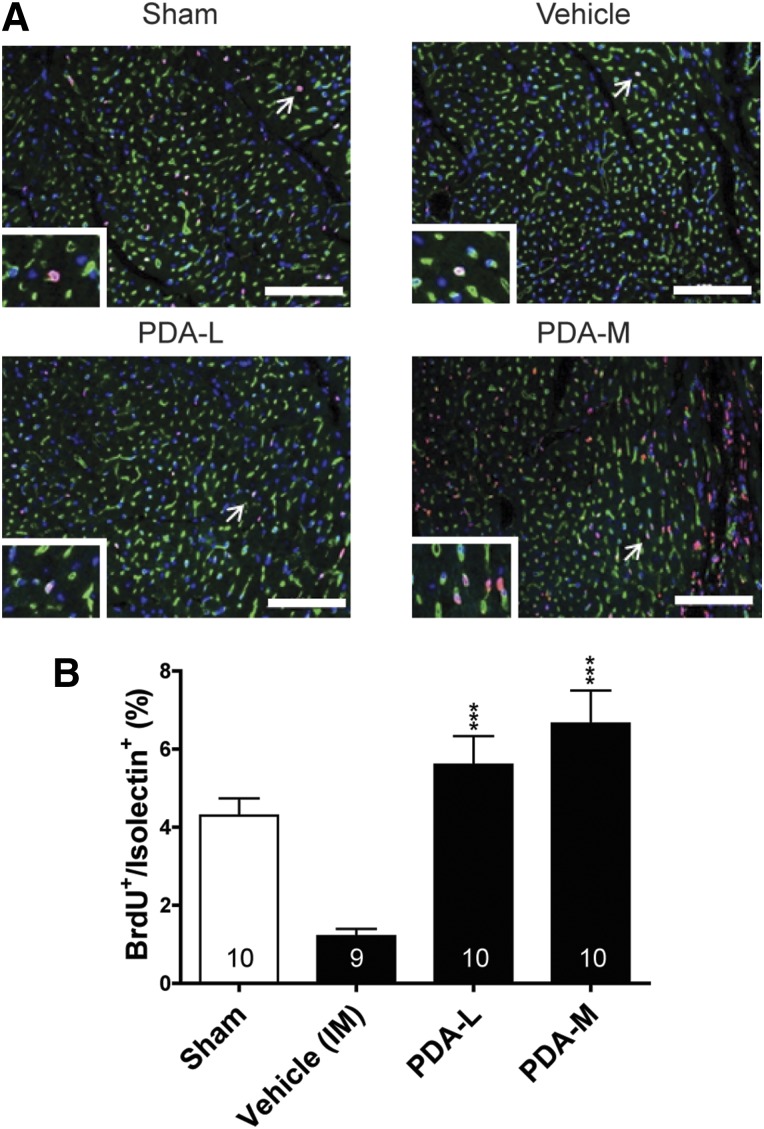
The increase in regional cardiac systolic function and decrease in interstitial fibrosis in CHF with PDA-001 treatment prompted us to conduct a mechanism of action study to determine whether the benefit of IM injection of PDA-001 to the heart would be accompanied by a proliferation of cardiac cells. We used the same CHF mouse model with IM injection of low and medium doses of PDA-001, plus a continuous infusion of BrdU for up to 2 weeks, to measure the proliferation of cardiac cells. The mechanism of action study confirmed the increase in the LVEF with a low or medium dose of PDA-001. Statistically significant improvement in the LVESD and modest (although not significant) improvement in the LVEDD were also observed in mice receiving the low-dose PDA-001 injection (supplemental online Fig. 3). We then found that injection of PDA-001 significantly increased endothelial cell proliferation at the injection sites at a low or medium dose compared with the vehicle treatment (Fig. 4B, supplemental online Fig. 5). We also observed an approximate threefold increase in cardiomyocyte proliferation with low- or medium-dose PDA-001 treatment (Fig. 5B), suggesting promotion of cardiomyocyte regeneration. Thus, PDA-001 treatment appears to increase the proliferation of both endothelial cells and cardiomyocytes.
Figure 4.
IM injection of PDA-001 cells increased the capillary density at the injected sites. (A): Representative images of CD31 (brown) at the injected area of the heart from each group. (B): Statistical analysis of capillary density. Sample size is indicated in the bar chart. ∗, p < .05 compared with vehicle. Scale bar = 50 μm. Abbreviations: BrdU, bromodeoxyuridine; H, high; IM, intramyocardial; L, low; M, medium.
Figure 5.
IM injection of PDA-001 cells promoted cardiomyocyte proliferation in mice with chronic heart failure. (A): Representative images of cardiac troponin-I and BrdU double-positive cells at the injected area of the heart from each group. The nuclei were stained using 4′6-diamidino-2-phenylindole. (B): Statistical analysis of cardiomyocyte proliferation. Sample size is indicated in the bar chart. ∗∗, p < .01; ∗∗∗, p < .001 compared with vehicle. Scale bar = 100 μm. Abbreviations: BrdU, bromodeoxyuridine; H, high; IM, intramyocardial; L, low; M, medium.
PDA-001 Cells Do Not Remain Within the Heart for More Than 2 Days After Intramyocardial Injection
We then tested whether the injected PDA-001 cells remain in the myocardium after IM or i.v. injection. Using immunohistochemistry with antibodies against human CD200 and human vimentin, we were not able to detect any surviving human cells in the mouse myocardium at 28 days after CHF (supplemental online Fig. 6), suggesting that PDA-001 cells did not engraft.
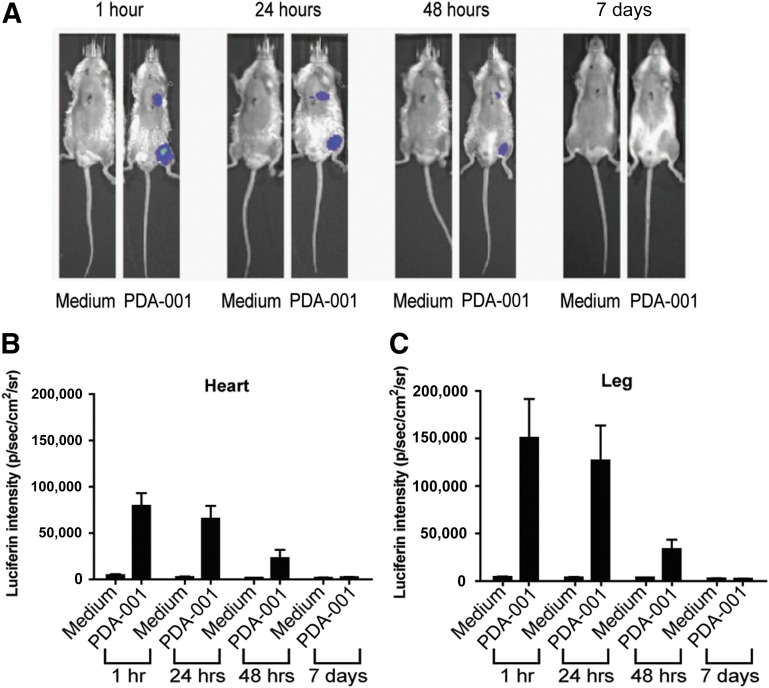
To trace the in vivo location and retention time of PDA-001 cells after injection into the mouse heart, we used PDA-001 cells containing a luciferase reporter gene. Mice were injected in the heart and in the leg as a control. The limit of detection is ∼1,000 cells (∼1% of injected cells). After in vivo administration of the luciferin substrate of the reporter gene, the PDA-001 signal was clearly detectable at 1, 24, and 48 hours after injection at both the heart and the leg injection sites (Fig. 6). At 7 days, however, no luciferin signal was detected in either the heart or the leg (Fig. 6), suggesting short-term persistence of PDA-001 in the mouse heart, with >99% of the injected cells absent by that time.
Figure 6.
PDA-001 cells survived for only 2 days after injection into the mouse heart and leg. (A): Representative in vivo real-time images of mice injected with medium or luciferase-expressing PDA-001 (heart: 0.5 × 105; thigh: 1.5 × 105 cells) taken at different time points. (B): Luciferin intensity over the heart area. (C): Luciferin intensity over the leg area. Injection of medium served as control; n = 3 in both groups. Abbreviations: p, photon; sr, steradian.
Discussion
Although cell therapy has shown some promising results in cardiac repair [8, 9], to date, the selection of cell type, dosage, delivery route and timing, and indication for different heart diseases remain largely undetermined. In the present series of studies to test the therapeutic effects of PDA-001 in heart injury, we showed that IM injection of PDA-001 improves systolic function and reduces interstitial fibrosis in a mouse model of CHF. BrdU staining and in vivo real-time imaging experiments suggest that the main mechanism of action for the proliferation of endothelial cells and cardiomyocytes might be through a paracrine effect. Together, these blinded and randomized animal trials consistently showed cardiac benefits after PDA-001 injection, demonstrating the true potential of using PDA-001 for the treatment of heart failure.
Previous stem cell therapy studies have shown that the use of human bone marrow cells has mixed effects on cardiac repair after infarction, in both animals and humans [20–25]. More recently, three independent clinical trials in the United States concluded that human bone marrow cell therapy for heart injury had no cardiac benefits [4–6]. PDA-001 (cenplacel-L) is a culture-expanded, mesenchymal-like cell population that is isolated from normal, full-term placenta for off-the-shelf use. Our results suggest that PDA-001 warrants additional study as a promising cell therapy candidate for cardiac repair.
In the present study, we repeatedly observed improvement in cardiac function at the injected sites after IM PDA-001 treatment of CHF. Because the injected cells did not cover the whole left ventricle, we only observed improvement in systolic function, without prevention of cardiac dilatation, an indicator of global structural change. It is therefore necessary to design a more efficient approach to deliver the cells to the whole ventricular region to ameliorate cardiac function in future studies.
The timing and dosing regimen of PDA-001 in CHF is likely to play an important role in the achievement of optimal therapeutic effects and needs to be determined. In particular, the expression of factors controlling the post-CHF myocardial microenvironment could affect PDA-001 persistence and function. This is because the myocardial microenvironment undergoes a chronological change from inflammation and fibrosis to ventricular remodeling [1] when the heart is under pressure overload. In our studies, PDA-001 was administered 3 weeks after transaortic constriction in mice with confirmed CHF (at least a 20% decrease in LVEF), and significant improvements in cardiac function and fibrosis were observed. Because we could not detect any human cells in the mouse heart at 28 days after IM injection, we suspect that very few, if any, PDA-001 cells survived at the injected area during the study period. Moreover, previous studies in different animal disease models have suggested that an appropriate cell dose, rather than a high cell dose, can benefit the injured tissue for tissue repair [26–29]. Given the complexity of the myocardial microenvironment after CHF, it is crucial to pursue additional research to determine the optimal therapeutic time window for PDA-001 administration to exert an effect. It is also important to know whether earlier PDA-001 treatment after transaortic constriction could completely reverse CHF.
Another essential issue is the delivery mechanism of PDA-001. The concept of direct IM or i.v. injection for cell therapy for cardiac repair has several fundamental limitations. It is well known that after intracoronary or IM injection of stem cells, only ∼1% of the cells will remain in the heart within the first 24 hours, and most administered cells will be dead within a few days [8, 9]. Thus, future studies should test whether any delivery vector, such as injectable scaffolds using biodegradable hydrogels or hyaluronan, could prolong the persistence of PDA-001 in the myocardium after delivery, improving the therapeutic efficacy [16–18]. It is also important to develop an easily applicable and potentially translatable method for quantitative assessment of cell therapy delivery, such as positron emission tomography.
It is also important to note that although the current mechanism of action study showed evidence of cardiomyocyte regeneration with IM PDA-001 injection, it is not clear whether the newly formed cardiomyocytes were from the pre-existing cardiomyocytes [30] or from an endogenous stem/progenitor cell pool [15], or both [31]. Finally, it is necessary to test the cardiac therapeutic effects of PDA-001 in a large animal model. The minipig model would be particularly attractive, because the pig heart resembles the human heart in morphology, size, and coronary artery distribution [18, 32]. Furthermore, owing to the technical difficulty of injecting cells to cover the whole LV in mice, cell therapy for CHF might not have been optimized. In contrast, direct injection of cells in minipigs can be more easily applied to the whole injured area, increasing the chance of achieving therapeutic effects with cardiac cell therapy.
Conclusion
The current results have demonstrated a therapeutic benefit of PDA-001 treatment in a mouse model of CHF, which should be further investigated to optimize the effect by controlling the dose, timing, and delivery.
Supplementary Material
Acknowledgments
We thank the National Cheng Kung University Animal Center for the in vivo real-time imaging experiments. This study was supported by grants from Celgene Cellular Therapeutics.
Author Contributions
H.-J.C.: conception and design, data analysis and interpretation; C.-H.C., M.-Y.C., and D.-C.T.: conception and design, collection and/or assembly of data, data analysis and interpretation; E.Z.B.: data analysis and interpretation, manuscript writing; R.H.: conception and design; U.H.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; P.C.H.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
H.-J.C. has compensated employment with Celgene Cellular Therapeutics. E.Z.B., R.H., U.H., and P.C.H.H. are compensated Celgene employees and have compensated stock options. The other authors indicated no potential conflicts of interest.
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: Heart disease and stroke statistics—2013 Update: A report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Jeevanantham V, Butler M, Saad A, et al. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: A systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perin EC, Willerson JT, Pepine CJ, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Shehadah A, Pal A, et al. Neuroprotective effect of human placenta-derived cell treatment of stroke in rats. Cell Transplant. 2013;22:871–879. doi: 10.3727/096368911X637380. [DOI] [PubMed] [Google Scholar]
- 11.He S, Khan J, Gleason J, et al. Placenta-derived adherent cells attenuate hyperalgesia and neuroinflammatory response associated with perineural inflammation in rats. Brain Behav Immun. 2013;27:185–192. doi: 10.1016/j.bbi.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Ling W, Pennisi A, et al. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells. 2011;29:263–273. doi: 10.1002/stem.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Morschauser A, Zhang X, et al. Human placenta-derived adherent cells induce tolerogenic immune responses. Clin Transl Immunology. 2014;3:e14. doi: 10.1038/cti.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer L, Pandak WM, Melmed GY, et al. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant Crohn’s disease: A phase 1 study. Inflamm Bowel Dis. 2013;19:754–760. doi: 10.1097/MIB.0b013e31827f27df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh PC, Segers VFM, Davis ME, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CH, Wang SS, Wei EI, et al. Hyaluronan enhances bone marrow cell therapy for myocardial repair after infarction. Mol Ther. 2013;21:670–679. doi: 10.1038/mt.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh PC, Davis ME, Gannon J, et al. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YD, Yeh ML, Yang YJ, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010;122(suppl):S132–S141. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 19.Park DW, Yun SC, Lee JY, et al. C-reactive protein and the risk of stent thrombosis and cardiovascular events after drug-eluting stent implantation. Circulation. 2009;120:1987–1995. doi: 10.1161/CIRCULATIONAHA.109.876763. [DOI] [PubMed] [Google Scholar]
- 20.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: Systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 21.Rosenzweig A. Cardiac cell therapy—Mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 22.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 23.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 24.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 25.Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 26.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Goldschlager T, Rosenfeld JV, Ghosh P, et al. Cervical interbody fusion is enhanced by allogeneic mesenchymal precursor cells in an ovine model. Spine. 2011;36:615–623. doi: 10.1097/BRS.0b013e3181dfcec9. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh P, Moore R, Vernon-Roberts B, et al. Immunoselected STRO-3+ mesenchymal precursor cells and restoration of the extracellular matrix of degenerate intervertebral discs. J Neurosurg Spine. 2012;16:479–488. doi: 10.3171/2012.1.SPINE11852. [DOI] [PubMed] [Google Scholar]
- 29.Shehadah A, Chen J, Pal A, et al. Human placenta-derived adherent cell treatment of experimental stroke promotes functional recovery after stroke in young adult and older rats. PLoS One. 2014;9:e86621. doi: 10.1371/journal.pone.0086621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malliaras K, Zhang Y, Seinfeld J, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YD, Luo CY, Hu YN, et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med. 2012;4:146ra109. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.