Liver cell transplantation holds potential for the treatment of liver failure, end-stage liver disease, and liver-based inborn errors of metabolism; however, the field is in search of a suitable cell therapeutic. Recent progress with tissue-derived stem/progenitor cells and pluripotent stem cell-derived cells may contribute to the generation of a well-characterized source of engraftable and functional liver cells.
Summary
Despite available medical therapy and organ transplantation, a significant unmet medical need remains for the treatment of liver failure, end-stage liver disease, and liver-based inborn errors of metabolism. Liver cell transplantation has the potential to address this need; however, the field is in search of a suitable cell therapeutic. The ability to reproducibly generate a well-characterized source of engraftable and functional liver cells has continued to be a challenge. Recent progress with tissue-derived stem/progenitor cells and pluripotent stem cell-derived cells now offers the field the opportunity to address this challenge.
Introduction
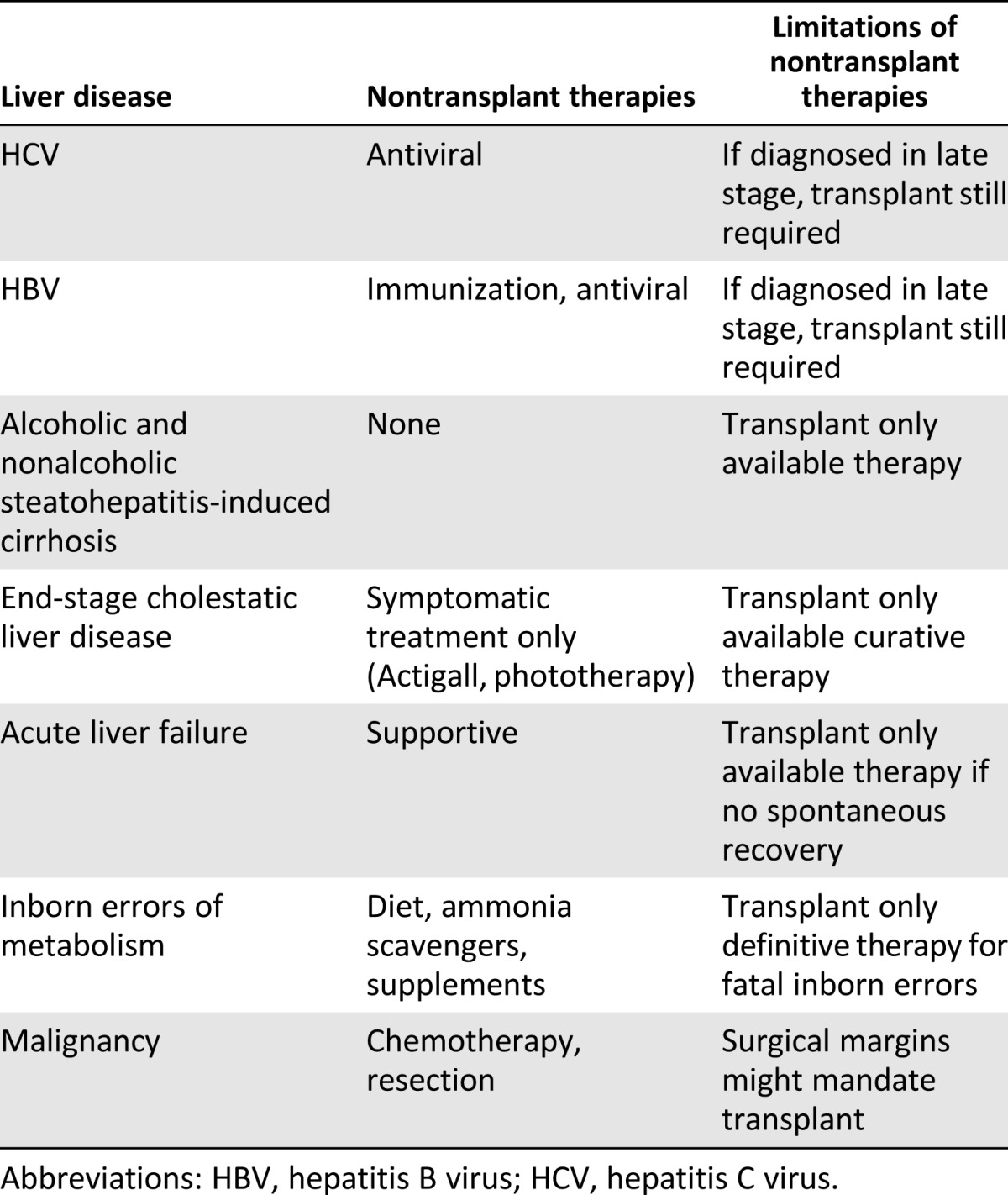
Liver disease remains among the top 12 leading causes of death globally. In the United States and Europe, 110,000 persons die annually of liver disease. It has been estimated that in the United States, the burden of disease results in an annual direct cost of $2.5 billion and an indirect cost of $10.6 billion [1]. Although nontransplant therapies are available for the early stages of some liver diseases (Table 1), organ transplantation is the only definitive treatment of end-stage liver disease and inborn errors of metabolism. However, the invasiveness of the transplantation procedure has limited the candidacy of patients who are “too sick” or “too well” to undergo such surgery. In addition, because of a shortage of donor livers, only a minority of eligible patients will eventually receive a transplant [2]. Finally, the 5-year survival with transplantation has only been 70%–80%, and repeat transplantation carries a high mortality risk [3]. Therefore, despite current medical and surgical therapies, a significant unmet medical need exists in the liver field that might be addressed by the emerging area of liver cell transplantation.
Table 1.
List of liver disease categories, available nontransplant therapies and limitations thereof
Liver cell transplantation could be a useful treatment for congenital liver diseases, acute or chronic liver failure, or after large hepatic resection and has several potential advantages compared with whole liver transplantation. Liver cell transplantation can be offered as a minimally invasive “closed” procedure involving the infusion of cells through a catheter. Such a procedure is more easily timed than liver transplantation, which depends on donor availability. In addition, liver cell transplantation could be offered to more patients and could provide a less risky option for retreatment. In contrast to organ transplantation, which requires an operating room, intensive care unit stay, and prolonged hospitalization, liver cell transplantation could offer tremendous cost savings and benefit to patients in the form of a shorter hospital stay, reduced discomfort, and faster recovery time. In addition to benefiting patients in need, clear economic advantages exist to developing cell therapies for liver disease.
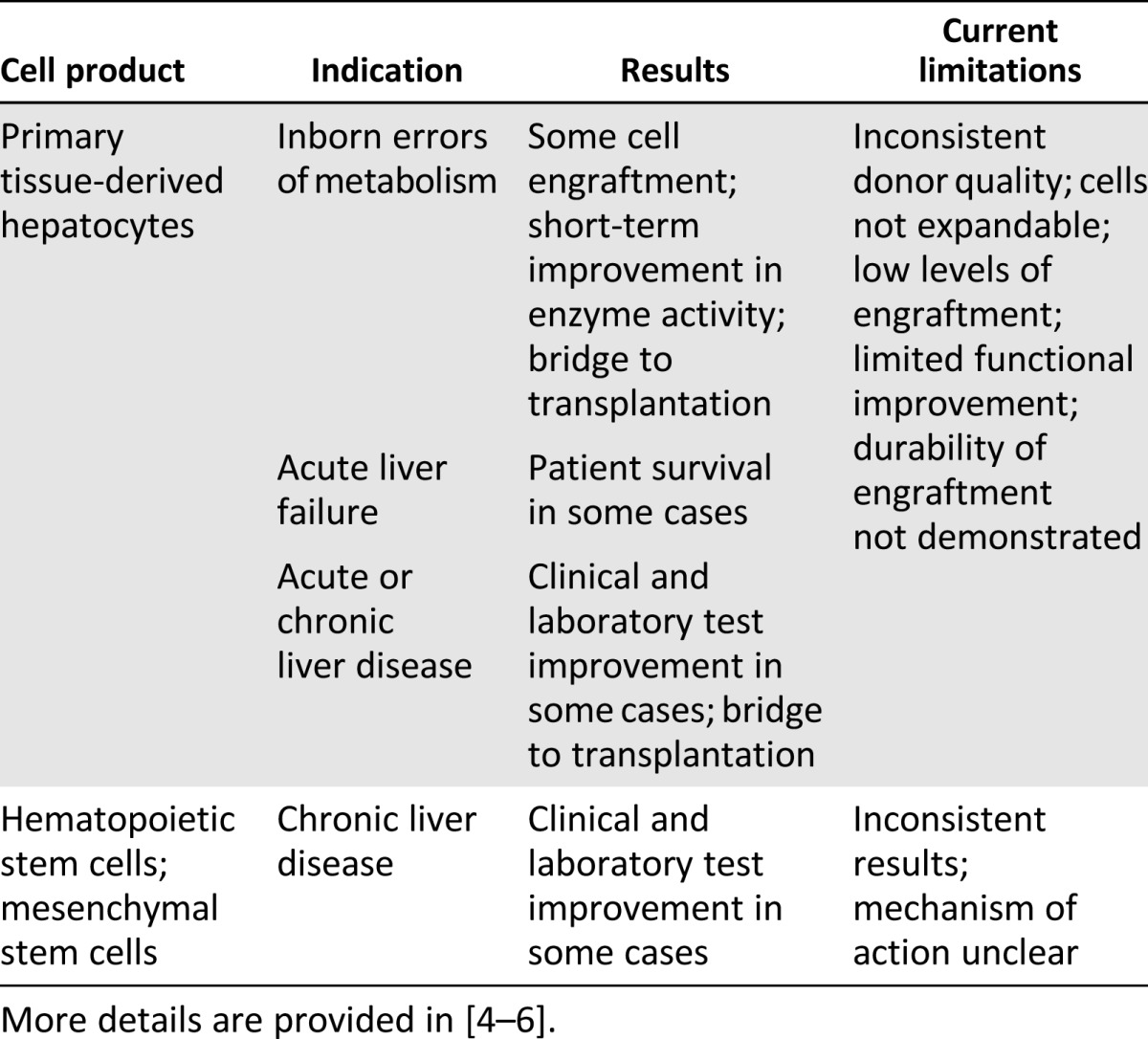
Preliminary clinical proof-of-concept for cell transplantation for treatment of liver disease already exists (Table 2). Using primary cells derived from donor livers and enriched for the hepatocyte population, several studies have demonstrated that it is possible to achieve some donor cell engraftment and functional improvement in various clinical parameters, sufficient to serve as a bridge to transplantation. However, the levels of engraftment and function in these studies were low and inconsistent, and the primary cells used were limited by poor expansion capacity in culture and donor availability. Studies have also been done infusing patients with hematopoietic stem cells or mesenchymal stem cells, also with inconsistent results. In this case, the mechanism by which the cells might improve liver function is not clear and might primarily be a short-term trophic effect. Hence, results to date have indicated that cell therapies can improve clinical and laboratory test results and metabolic function and, in some instances, might serve as a bridge to transplant. Key challenges to the further development of these therapies include developing a consistent source of expandable, bankable, engraftable, and functional liver cells (i.e., hepatocytes or a progenitor population) and determining the best method to prepare the recipient liver and deliver the donor cells.
Table 2.
Primary cells transplanted into patients for treatment of liver diseases
Development Landscape of Liver Cell Therapies
Because of the early indications of clinical improvement after primary cell transplantations, industry has been interested in performing these studies in a rigorous clinical trial setting. One of the key questions being addressed is which cell type will be the most suitable source for a cell-based therapy. In addition, for each indication, it will be necessary to establish cell dose, delivery mode, and level of engraftment needed to achieve therapeutic benefit. Ongoing industry-sponsored clinical trials are addressing these questions. These companies have chosen to focus on primary cells or specific cell lines as a starting cell source for developing a therapeutic agent. A summary of selected cell-based liver therapies in clinical trials is provided in Table 3.
Table 3.
Selected clinical trials using cell therapies for liver disease
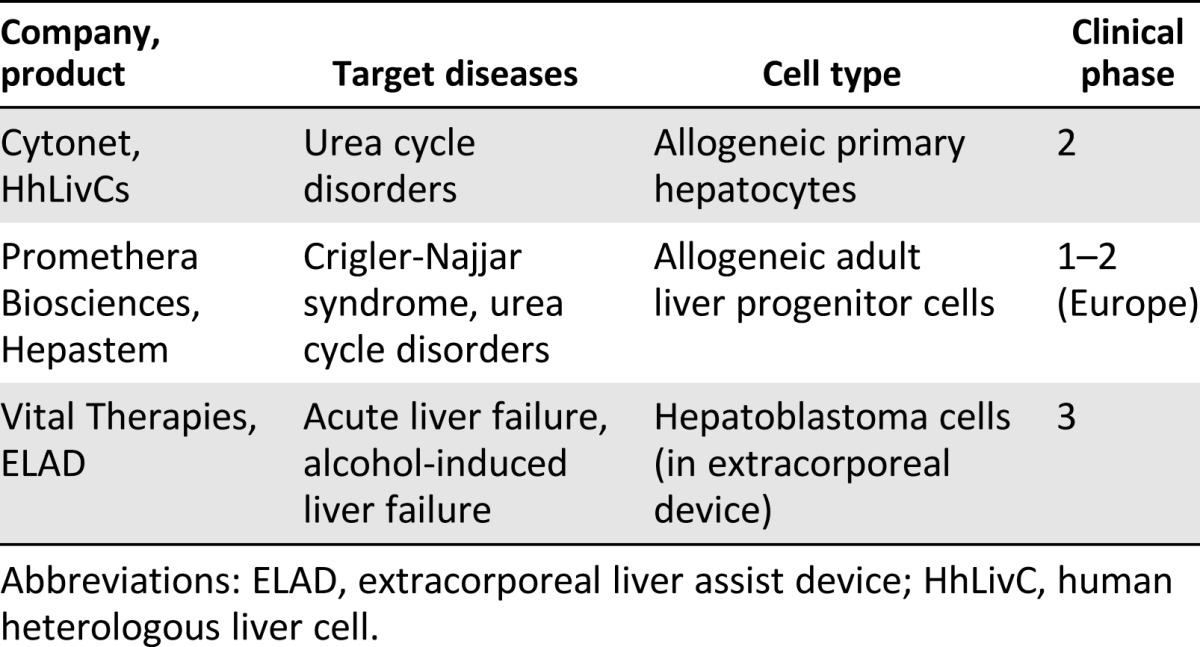
Extracorporeal devices such as bioartificial livers are primarily intended to be used for acute or acute-on-chronic liver failure, either to provide a bridge to a more permanent solution such as whole organ transplant or to treat acute poison-induced toxicity. One such device being tested in the clinic is the extracorporeal liver assist device (ELAD) system developed by Vital Therapies (San Diego, CA, http://www.vitaltherapies.com). The use of this device requires vascular access, because blood is drawn from the patient, passed through the device, in which the liver metabolic functions take place, and then returned to the patient. The ELAD incorporates the C3A tumor cell (subclone of the HepG2 hepatoblastoma cell line) to perform the liver functions [7]. Extracorporeal devices are expected to be safe, which has so far been supported by the safety data from ELAD clinical trials including more than 150 patients [7]. The key question for bioartificial livers that incorporate cell lines (whether primary or immortalized hepatocytes) is the underlying function of the cells and their synthetic and metabolic capacity.
Other clinical approaches to treating liver disease use primary cells. Promethera Biosciences (Mont-Saint-Guibert, Belgium, http://www.promethera.com) is using liver-derived progenitor cells that are expanded in vitro, washed, and packaged into a final product that is then injected into the portal vein of the patient. Promethera Biosciences is initially targeting metabolic disorders in newborns and young children. Ongoing studies are evaluating the ability of the cells to differentiate into functional hepatocytes in vivo and engraft and incorporate into the parenchyma.
Cytonet (Weinheim, Germany, http://www.cytonetllc.com) is developing a cell suspension of primary hepatocytes extracted from nontransplantable donor livers for the treatment of congenital metabolic disorders of the liver and acute liver failure in adult patients. In the case of newborns, the goal of therapy is to maintain liver function until the infants are old enough for liver transplantation. In cases of acute hepatic failure in adult patients, the goal is for the transplanted liver cells to perform the function of normal hepatocytes until the patient’s liver has regenerated sufficiently [8]. In both cases, the cells are injected into the liver through a catheter, a minimally invasive procedure.
California Institute for Regenerative Medicine Liver Portfolio
Although the current clinical approaches to liver cell therapy use primary or immortalized cells, the California Institute for Regenerative Medicine (CIRM) is funding several “next generation” approaches still in the research stage (Table 4). One approach is to use fully mature hepatocytes derived from pluripotent stem cells expanded in culture. This approach requires investigators to address the safety hurdles of using pluripotent cells and to develop reliable processes to differentiate stem cells into mature, functional, and engraftable hepatocytes but has the potential advantages of a renewable supply of hepatocytes and the ability to genetically correct patient cells for autologous transplantation. A modification of this approach is to reprogram somatic cells to the liver fate without going through a pluripotent intermediate stage. Yet another line of investigation is examining the use of placental cells that express specific hepatic enzymes to correct certain congenital metabolic diseases. All these CIRM-funded projects are aimed at achieving preclinical proof of concept in animal models of disease or injury.
Table 4.
CIRM preclinical liver cell therapy portfolio
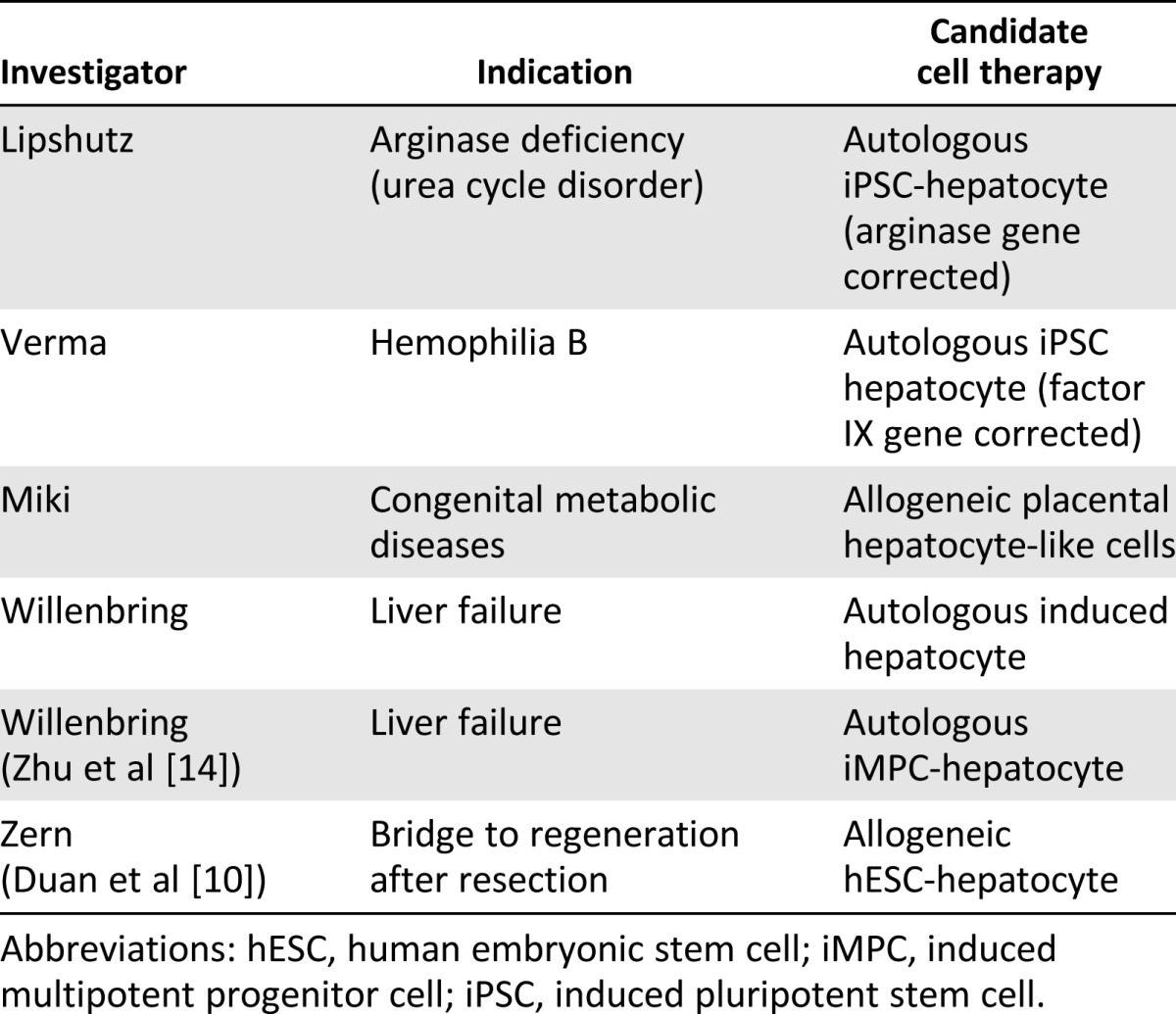
Congenital liver diseases often manifest soon after birth and can range from severely debilitating to lethal. One program funded by CIRM is working to treat arginase deficiency, one of a class of diseases termed “urea cycle disorders,” each caused by a mutation in one of the enzymes responsible for removing ammonia from the bloodstream. Children born with these diseases experience ammonia-mediated neurotoxicity that can cause irreversible brain damage and death. Although liver transplantation is potentially one treatment option, it suffers from the limits of organ availability and that infants can be too small for such an operation to be successful and could require lifelong immune suppression. The only other treatment currently available is to limit nitrogen intake in the diet and provide alternate methods for nitrogen disposal; however, this treatment is not completely effective or easy to maintain.
The CIRM-funded program aims to obtain preclinical proof-of-concept for a therapeutic approach to arginase deficiency consisting of autologous patient-derived induced pluripotent stem cells (iPSC) that have been genetically corrected at the arginase locus and then differentiated into functional and engraftable hepatocytes that can be transplanted into patient livers. If successful, this approach would not be limited by cell availability, would involve a relatively noninvasive route of administration through the portal vein, and would not be expected to require immune suppression. Furthermore, it is estimated that less than 10% of the normal levels of arginase will be sufficient to maintain normal serum ammonia levels, increasing the chances that only partial replacement of the liver with gene-corrected hepatocytes might be curative. If this approach is successful, it will also provide proof of principle that this method could be used to treat other metabolic enzyme deficiencies.
A second CIRM-funded program is applying these same principles to hemophilia B, which also results from a failure of the liver to produce a needed protein. That team is trying to obtain proof-of-concept in an animal model that gene-corrected autologous iPSC-derived hepatocytes can treat the disease. Hemophilia B is caused by mutations in the factor IX (FIX) gene, causing failure of proper blood clotting and recurrent bleeding that can lead to physical disabilities or death. Just as is the case for arginase, FIX is normally expressed in the liver, and it is expected that significantly less than 100% of the normal levels of FIX expression might be curative.
A different approach to identifying a therapeutic agent for congenital liver metabolic disease has been taken by another CIRM-funded project. Their goal is to obtain proof-of-concept that human placental cells, or amniotic epithelial cells (AECs), can acquire sufficient hepatocyte-like functions to rescue various congenital metabolic diseases after injection into the liver. Preliminary experiments have suggested the feasibility of this approach [9], with refinements that include purifying subsets of AECs expressing the desired hepatic enzymes. This approach is an allogeneic one and will likely require immune suppression or the generation of a cell bank to enable immunotype matching with the patient.
CIRM is also funding projects aimed at identifying optimal methods to generate hepatocytes in vitro. In one project, the goal was to generate human embryonic stem cell (hESC)-derived hepatocytes to transplant into patients with an inadequate liver mass for survival. In this project, it was demonstrated that hESCs can be differentiated to hepatocyte-like cells at very high efficiencies and that the cells perform many of the functions of mature hepatocytes, including drug metabolism and albumin secretion [10, 11]. The in vivo efficacy of cells generated by this protocol in mouse liver failure models remains to be established.
In order to avoid the potential teratoma risks of treating patients with pluripotent stem cell-derived hepatocytes, one CIRM-funded project has focused on generating directly reprogrammed hepatocytes (“induced hepatocytes”) by reprogramming fibroblasts and adipocytes with certain hepatocyte transcription factors. Two groups not funded by CIRM have reported direct reprogramming of fibroblasts to induced hepatocytes using methods that require the expression of oncogenes or knockdown of tumor suppressors. The cells generated using this approach can engraft and rescue a mouse model of liver failure [12,13].
One CIRM-funded reprogramming approach obviates the need for modulating cell cycle control. In this method, an “induced multipotent progenitor cell” (iMPC) is generated by the partial reprogramming of fibroblasts. These iMPCs can be expanded, followed by subsequent differentiation to hepatocytes that are able to rescue an animal model of chronic, but not acute, liver failure [14]. Because these iMPCs can be expanded in vitro, they have an advantage for larger scale production compared with more differentiated cells. In addition, these cells appear not to have entered a pluripotent state before differentiation into hepatocytes, thus potentially lowering the safety concerns of using such cells in human patients.
One other approach to hepatocyte therapy, although not funded by CIRM, is of interest for our discussion. In this approach, hepatic progenitor cells derived from iPSCs were able to self-organize into liver buds (LBs) in vitro. These in vitro-grown iPSC-LBs were able to connect with the host vasculature on transplantation and then mature to tissue resembling adult liver in terms of protein expression and drug metabolic capabilities. In addition, transplanted iPSC-LBs were able to rescue an animal model of liver failure, giving preclinical proof-of-concept that this might be a viable clinical approach [15].
Although all these approaches are still in the early stages of research, it is encouraging that multiple approaches to cell therapy for the liver are being tested and showing signs of efficacy in animal models.
Conclusion
The need for cell therapeutic approaches to the treatment of liver disease is great, given the many limitations of whole organ transplantation. “First-generation” cell therapy approaches using primary cells or cell lines are already in the clinic, and the CIRM is funding “second-generation” projects aimed to demonstrate preclinical proof-of-concept that hESC-derived hepatocytes, in vitro-derived autologous hepatocytes, or allogeneic placental-derived primary cells can improve disease in animal models. Most of these “second-generation” cell therapies will be amenable to gene modification (e.g., to correct congenital defects) and to scalability of production. The current challenge in the field is to develop reliable processes to differentiate stem cells into functional and engraftable cells, whether they are mature hepatocytes or progenitor cells capable of differentiation after transplantation into mature hepatocytes. Just as for liver transplantation, the potential for immunogenicity of these cell-based products will need to be addressed. In addition, the products derived from pluripotent cells or by direct reprogramming from other cell types will need to be evaluated for potential toxicity and tumorigenicity. Optimism is great that once these research challenges are solved, new treatments will be available to address some of the critical unmet needs for patients with liver disease.
Author Contributions
L.C.K. and M.T.M.: conception and design, manuscript writing, final approval of manuscript; L.R.C. and N.J.L.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim JA, Petrowsky H, Saab S, et al. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773–1784. doi: 10.1111/j.1600-6143.2011.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scientific Registry of Transplant Recipients. Available at http://www.srtr.org. Accessed November 25, 2014.
- 4.Fisher RA, Strom SC. Human hepatocyte transplantation: Worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- 5.Huebert RC, Rakela J. Cellular therapy for liver disease. Mayo Clin Proc. 2014;89:414–424. doi: 10.1016/j.mayocp.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–347. doi: 10.1097/TP.0b013e31823b72d6. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Fisher JE, Lillegard JB, et al. Cell therapies for liver diseases. Liver Transpl. 2012;18:9–21. doi: 10.1002/lt.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liver cell therapy. Cytonet website. Available at http://www.cytonetllc.com/?page_id=135. Accessed November 25, 2014.
- 9.Marongiu F, Gramignoli R, Dorko K, et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53:1719–1729. doi: 10.1002/hep.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan Y, Ma X, Zou W, et al. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2011;28:674–686. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Duan Y, Tschudy-Seney B, et al. Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells. Stem Cells Translational Medicine. 2013;2:409–419. doi: 10.5966/sctm.2012-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du Y, Wang J, Jia J, et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014;14:394–403. doi: 10.1016/j.stem.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Huang P, Zhang L, Gao Y, et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384. doi: 10.1016/j.stem.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhu S, Rezvani M, Harbell J, et al. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93–97. doi: 10.1038/nature13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]


