Abstract
Early human placental and embryonic development occurs in a physiologically low oxygen environment supported by histiotrophic secretions from endometrial glands. In this study, we compare the placental metabolomic profile in the first, second and third trimesters to determine whether the energy demands are adequately met in the first trimester. We investigated whether hypoxia-inducible factors, HIF-1α and/or HIF-2α, might regulate transcription during the first trimester. First and second trimester tissue was collected using a chorionic villus sampling-like (CVS) technique. Part of each villus sample was frozen immediately and the remainder cultured under 2 or 21% O2 ± 1 mM H2O2, and ±the p38 MAPK pathway inhibitor, PD169316. Levels of HIF-1α were assessed by western blotting and VEGFA, PlGF and GLUT3 transcripts were quantified by RT–PCR. Term samples were collected from normal elective Caesarean deliveries. There were no significant differences in concentrations of ADP, NAD+, lactate, and glucose, and in the ATP/ADP ratio, across gestational age. Neither HIF-1α nor HIF-2α could be detected in time-zero CVS samples. However, culture under any condition (2 or 21% O2 ± 1 mM H2O2) increased HIF-1α and HIF-2α. HIF-1α and HIF-2α were additionally detected in specimens retrieved after curettage. HIF-1α stabilization was accompanied by significant increases in VEGFA and GLUT3 and a decrease in PlGF mRNAs. These effects were suppressed by PD169316. In conclusion, our data suggest that first trimester placental tissues are not energetically compromised, and that HIF-1α is unlikely to play an appreciable role in regulating transcriptional activity under steady-state conditions in vivo. However, the pathway may be activated by stress conditions.
Keywords: placenta, HIF-1α, first trimester, metabolomics, energy status
Introduction
The human placenta differentiates in a physiologically low oxygen environment, supported by histiotrophic secretions from the endometrial glands (Hempstock et al., 2004). A major change takes place at the end of the first trimester when the maternal arterial circulation commences (Burton et al., 1999), and the oxygen tension within the intervillous space increases from ∼18 mmHg (equivalent to 2.5% O2) at 8 weeks to ∼60 mmHg (8.5%) at 12 weeks (Jauniaux et al., 2000, 2003). This rise is associated with a burst of oxidative stress in the placental tissues, and with a robust increase in antioxidant defences (Burton and Jauniaux, 2004).
The low oxygen environment is essential for normal embryonic and placental development, and premature onset of blood flow can contribute to pregnancy failure (Hustin et al., 1990; Jauniaux et al., 2003). Metabolism appears adapted to these conditions, for we have shown that polyol pathways are highly active in first trimester placental tissues (Jauniaux et al., 2005). These pathways provide an important mechanism for the reoxidation of pyridine nucleotides under conditions of low oxygenation, enabling glycolysis to continue without an excessive rise in acidity. We speculate that the metabolic needs of the first trimester conceptus are adequately met under 2.5% O2 through these pathways. To test this hypothesis, we here compare the metabolomic profile of first trimester (7–8 weeks), second trimester (14–16 weeks) and term placental samples.
A variety of genes are regulated by oxygen, including vascular growth factor (VEGFA), glycolytic enzymes, erythropoietin (EPO), haemoxygenase-1 (HO1) and inducible nitric oxide synthetase (iNOS). Under hypoxic conditions, activation of these genes maintains the oxygen supply and energy balance by inducing vasodilation, erythropoiesis, angiogenesis and energy production. The hypoxia-inducible factor (HIF) family of basic Helix–Loop–Helix transcription factors plays a key role in inducing these adaptive responses in other systems (Semenza et al., 1994; Forsythe et al., 1996; Semenza, 1999, 2001; Giaccia et al., 2003; Pugh and Ratcliffe, 2003). HIF-1α and HIF-2α exhibit high sequence homology, and activate common as well as unique target genes. HIF-3α lacks a transcriptional activation domain and represses O2-regulated gene expression (Pouyssegur and Mechta-Grigoriou, 2006). HIF-1α heterodimerises with the constitutively expressed HIF-1β (ARNT) and binds to a short DNA motif identified in the 5′-flanking regions of many hypoxia-induced genes (Wang et al., 1995). HIF-1α is rapidly degraded under normoxic conditions through interaction with von Hippel–Lindau protein (VHL) (Maxwell et al., 1999; Bruick and McKnight, 2001; Ivan et al., 2001). HIF-1α stability is increased by hypoxia, but can also be enhanced under non-hypoxic conditions by inflammatory cytokines or microtubule-depolymerizing agents involving the NF-κB pathway (Jung et al., 2003a, b, c). Thus, HIFs might not only regulate the effects of changing O2 supply on placental development, but also the effects of hormones, growth factors and cytokines (Pringle et al., 2010; Kuschel et al., 2011).
Both HIF-1α and HIF-2α proteins are constitutively expressed in the human placenta. HIF-2α mRNA increases significantly with gestational age, whereas HIF-1α mRNA is expressed at a constant level in all placentas but with greater variability in early pregnancy (Rajakumar and Conrad, 2000). In contrast, the abundance of both HIF-1α and HIF-2α protein has been reported to decrease significantly with gestational age (Caniggia et al., 2000; Rajakumar and Conrad, 2000). Two peaks in HIF-1α mRNA and protein seem to occur during early development at 7–10 and 14–18 weeks (Ietta et al., 2006). As the intervillous pO2 rises at 12–14 weeks, the second peak in HIF-1 expression is likely to be induced by factors other than O2, possibly oxidative stress-induced pro-inflammatory cytokines (Ietta et al., 2006). The role of oxygen in regulating the first peak is also questionable, since HIF signalling typically shows maximal activity between 0.5 and 2% O2 (Gorr et al., 2010), whereas the tension in the first trimester corresponds to ∼2.5% O2. Conflicting results have been reported regarding the placental response to hypoxia in vitro. Rajakumar and Conrad (2000) cultured first trimester and term villus explants under 2 or 21% O2 for 3–24 h and reported increases in HIF-1α and HIF-2α protein and HIF-1α DNA binding activity in the 2% O2 samples, but no changes in their mRNA levels. In contrast, Caniggia et al. (2000) cultured first trimester explants at 3 or 21% O2 for 24 h and reported an increase in both HIF-1α protein and mRNA under reduced oxygen. Furthermore, Genbacev et al. (2001) reported an increase only in HIF-2α in first trimester explants following 48 h incubation under 2% O2. Given the more recent data that HIFs can be stabilized by oxygen-independent pathways, we speculate that these changes in HIF abundance might have been influenced by the tissue collection procedure. Early placental samples are usually obtained by curettage, when they are inevitably exposed artifactually to maternal blood. For samples of <10 weeks gestational age, this represents a potential stressor. Hence, in this study, we studied HIF-1α and HIF-2α in samples collected using a chorionic villus sampling-like (CVS) technique under ultrasound guidance; these samples were free of maternal blood contamination. We compared data from these samples with those obtained using material collected by curettage. We also studied HIF-1α protein abundance in short-term-cultured first trimester CVS explants under 2% O2 and 21% O2, in order to assess the functional significance of changes in HIF-1α in terms of its downstream gene targets.
Materials and Methods
Sample collection
First trimester placental samples were collected with written informed consent and approval from the University College London Hospitals Committee on the Ethics of Human Research or the Cambridge Local Research Ethics Committee from patients undergoing surgical termination of normal pregnancies. The method of sample collection varied depending on the purpose of study, i.e. first trimester explant culture, metabolomics study or comparison of different collection methods. Our first and second trimester tissues are routinely collected using a CVS technique under ultrasound guidance from the central region of the placenta. Archival paraffin blocks were used for additional immunostaining, and frozen samples were used for the metabolomics study. For the first trimester explant cultures, we obtained tissue from 15 cases ranging from 5 to 12 weeks gestational age. Gestational age was estimated from the crown rump length of the fetus. Part of the sample was frozen immediately (<2 min) in liquid nitrogen (time zero, T0), part was fixed in 4% paraformaldehyde and embedded in paraffin wax for immunohistochemistry, and part was collected into medium equilibrated with 2% O2/5% CO2 and transported on ice.
To assess the effect of stress introduced as a collection artefact, we also obtained 4 first trimester frozen and 10 paraffin wax-embedded samples retrieved from suction curettage bags. All samples were anonymized, and were from normal pregnancies.
For the metabolomics study, we collected five first trimester samples (7–8 weeks), five second trimester samples (14–16 weeks) under ultrasound guidance by CVS as described above, and four term samples within 10 min of delivery by Caesarean section from healthy pregnancies (Serkova et al., 2003).
Tissue culture
First trimester villus explants were cultured under 2 or 21% O2 for 6 h ± 1 mM H2O2. In each combination, the p38 MAPK pathway inhibitor PD169316 was used at 10 µM, as previously optimised (Cindrova-Davies et al., 2007a). Following culture, the samples were snap-frozen for western blotting and RNA extraction. Transcript levels for the target genes VEGFA, PlGF and GLUT3 were quantified by quantitative real-time RT–PCR.
Western blots
Tissue homogenization and SDS–PAGE electrophoresis and immunoblotting were carried out as previously described (Tjoa et al., 2006). Primary antibodies against phospho-p38, phospho-AKT, TNF-α, Hsp27 and IκB were from Cell Signaling (all used at 1:1000; New England Biolabs, Hitchin, UK); anti-HIF-1α was from Novus Biologicals (used at 1:500; Cambridge, UK). Proteins were revealed and quantified using Image J software (National Institutes of Health, http://rsb.info.nih.gov/ij/). Protein loading was normalized against β-actin or Poncaeu S staining. Values are expressed as a percentage of the control lysate (100%) for each experiment. Western blot measurements were analysed using ANOVA, and the Protected Least Significant Differences post hoc test (Fisher's post hoc test). Differences between two groups were evaluated using a Fisher's post hoc test. Results were considered significant at P < 0.05.
Immunohistochemistry
Immunohistochemistry with DAB detection was performed according to a protocol described previously (Tjoa et al., 2006). Anti-HIF-1α was from Novus Biologicals (used at 1:250; Cambridge, UK) and anti-HIF-2α was from Abcam (used at 1:250; Cambridge, UK). Both antibodies required heat-induced antigen retrieval (Tris-EDTA buffer, pH 9).
Immunoprecipitation
To isolate phosphorylated proteins, villous protein lysate (250 µg) was pre-cleared using A/G PLUS-Agarose Immunoprecipitation Reagent (Santa Cruz, CA, USA) at 4°C for 30 min. Anti-phospho-Ser/Thr/Tyr antibody (Santa Cruz) was added (1 µg) and incubated at 4°C overnight. Protein A/G PLUS-Agarose beads were added, incubated at 4°C for 6 h, and recovered and washed four times. After the final wash, pellets were resuspended in 15–20 µl 3× SDS–PAGE buffer, boiled for 3–5 min and analysed by western blotting.
Sample preparation for 1H- and 31P-NMR spectroscopy
Placental tissues were extracted using 8% perchloric acid (Sigma-Aldrich Co., St Louis, MO, USA) as previously described (Tissot van Patot et al., 2010). Briefly, perchloric acid was added to powdered tissues and centrifuged (20 min, 1300g, 4°C). Hydrophilic metabolites (supernatant) were collected and the procedure repeated on the pellet. The supernatants were combined, neutralized (KOH), centrifuged to remove potassium perchlorate, lyophilized and dissolved in deuterium oxide (D2O) for NMR analysis. Lipophilic metabolites (pellet) were neutralized, lyophilized and dissolved in 0.6 ml of deuterated chloroform/methanol mixture (2:1, vol/vol) for 1H-NMR Analysis.
Quantitative 1H-NMR analysis
Hydrophilic extracts were analysed by high-resolution 1H- and 31P-NMR using a 500 MHz high-resolution Bruker DRX system equipped with Bruker TopSpin software (Bruker Biospin Inc., Fremont, CA, USA) (Serkova et al., 2007). An inverse TXI 5-mm probe was used for all 1H-NMR experiments. An external reference, trimethylsilyl propionic-2,2,3,3,-d4 acid, was used for metabolite quantification of fully relaxed 1H-NMR spectra and as a 1H chemical shift reference (0 ppm). A two-dimensional (2D)-1H, 13C-HSQC (heteronuclear single quantum correlation) NMR sequence was used for metabolite identification. The 1H-NMR peaks for single metabolites were identified and referred to a metabolite chemical shift library. After performing Fourier transformation and making phase and baseline corrections, each 1H peak was integrated using 1D WINNMR (Bruker Biospin Inc.). The absolute concentrations of single metabolites were then referred to the TMSP integral.
The water-soluble (hydrophilic) placental extracts were additionally analysed by 31P-NMR spectroscopy immediately after 1H-NMR (EDTA was to chelate divalent ions bound to ATP) (Serkova et al., 2007). Phosphorous spectra were obtained on a Bruker 300 MHz Avance spectrometer (31P-NMR frequency: 121.5 MHz) equipped with a 5-mm QNP 31P/13C/19F/1H probe using a composite pulse-decoupling (CPD) program. An external standard in a thin capillary, methyl diphosphoric acid (MDP, 2.3 mmol/l D2O, Sigma-Aldrich), was placed into the NMR tube to serve as a reference for both chemical shift (18.6 ppm) and phosphor metabolite quantification.
Data were tested using a non-parametric Kruskal–Wallis test, with P < 0.05 being considered significant.
Creatine kinase activity assay
Creatine kinase (CK) activity was measured in five first trimester (6–7 weeks), five second trimester (14 weeks) and five term samples (39 weeks), according to the manufacturer's instructions (Abcam, Cambridge, UK).
RNA isolation and quantitative real-time RT–PCR analysis
Total RNA was isolated from snap-frozen placental tissue using RNAeasy kit (Qiagen, Crawley, UK). RNA was quantified by spectrophotometry (Nanodrop Technologies, DE, USA) and integrity was assessed using an Agilent 2100 bioanalyser (Agilent Technologies UK Limited, UK). In brief, 20 µg of total RNA from each sample was reverse transcribed using a master mix containing SuperScript II Reverse Transcriptase in the First Strand Buffer with 0.1 M DTT (Invitrogen, Paisley, UK) and 50 ng/ml random hexamers (Sigma). The ABI PRISM 7700 Sequence Detection System (TaqMan) was used to perform real-time PCR according to the manufacturer's protocols. Ct values for each transcript were compared with those for 18S rRNA (dCt obtained), and these values were compared with T0 samples (ddCt values are reported). Primers for VEGFA (Hs00173626-m1), PlGF (Hs00182176-m1), GLUT3 (Hs00359840-m1) and 18S (Hs99999901-s1) were obtained from Applied Biosystems (ABI, Warrington, UK).
Results
Energy status of first trimester, second trimester and term placental tissue
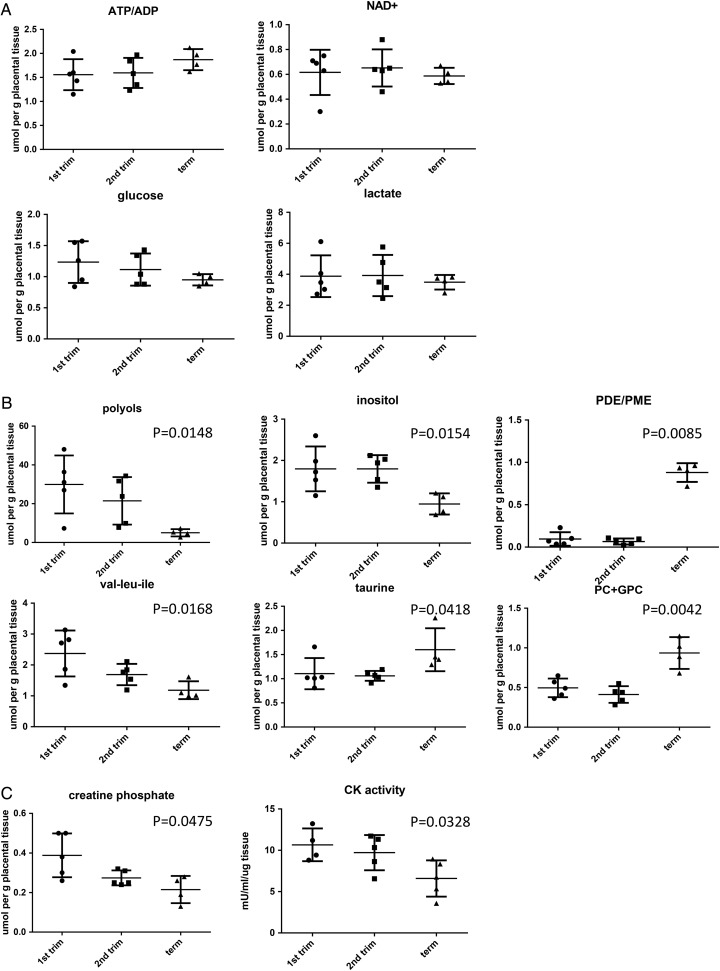
There were no significant differences in the concentrations of the main energy metabolites, such as ADP, NAD+, lactate and glucose, between first and second trimester samples (Table I and Fig. 1A). Importantly, there was no difference in the ATP/ADP ratio, confirming that the first trimester placenta has sufficient ATP and energy reserves to fulfil its energy needs and support the growing conceptus (Fig. 1A).
Table I.
Metabolite concentrations in placental tissue samples.
| Parameter | 7–8 weeks (n = 5) | 14–16 weeks (n = 5) | Term (n = 4) |
|---|---|---|---|
| Acetate | 0.118 (0.07) | 0.134 (0.077) | 0.065 (0.042) |
| ADP | 0.466 (0.063) | 0.6 (0.096) | 0.325 (0.058) |
| Alanine | 0.622 (0.047) | 0.596 (0.116) | 0.448 (0.023) |
| AMP | 0.404 (0.064) | 0.316 (0.064) | 0.2 (0.081) |
| Arginine | 0.336 (0.05) | 0.266 (0.039) | 0.208 (0.036) |
| Aspartate | 0.178 (0.028) | 0.224 (0.026) | 0.295 (0.034) |
| ATP | 0.742 (0.155) | 0.938 (0.168) | 0.59 (0.078) |
| ATP/ADP | 1.56 (0.144) | 1.6 (0.14) | 1.87 (0.11) |
| Cholesterol | 1.61 (0.287) | 1.79 (0.164) | 2.37 (0.057) |
| Choline (H2O soluble fraction) | 0.312 (0.055) | 0.320 (0.059) | 0.178 (0.045) |
| Cholines (lipid fraction) | 3.0 (0.493) | 3.79 (0.109) | 3.39 (0.186) |
| Creatine phosphate | 0.388 (0.05) | 0.274 (0.017) | 0.215 (0.034)a |
| Glucose | 1.234 (0.15) | 1.114 (0.115) | 0.95 (0.046) |
| Glutamate | 1.202 (0.143) | 1.336 (0.151) | 1.01 (0.044) |
| Glutamine | 0.546 (0.074) | 0.59 (0.089) | 0.345 (0.043) |
| Glycerol phosphate | 1.696 (0.27) | 1.848 (0.121) | 1.7 (0.133) |
| Glutathione | 0.308 (0.108) | 0.36 (0.118) | 0.43 (0.088) |
| Hydroxybuterate | 0.062 (0.012) | 0.072 (0.024) | 0.12 (0.036) |
| Inositol | 1.796 (0.242) | 1.796 (0.149) | 0.95 (0.128)a |
| Lactate | 3.878 (0.602) | 3.92 (0.594) | 3.49 (0.235) |
| MUFA | 6.516 (1.748) | 6.718 (1.462) | 5.45 (0.519) |
| NAD+ | 0.616 (0.081) | 0.652 (0.067) | 0.59 (0.033) |
| Pcho + GPC | 0.496 (0.052) | 0.412 (0.047) | 0.935 (0.1)a |
| Phophatidyl choline | 0.588 (0.085) | 0.724 (0.012) | 0.628 (0.038) |
| PDE | 0.550 (0.186) | 0.380 (0.141) | 2.8 (0.337)a |
| PME | 6.102 (0.679) | 5.658 (1.161) | 3.17 (0.274) |
| Phosphatidylethanolamine | 0.158 (0.037) | 0.248 (0.011) | 0.165 (0.015) |
| PDE/PME | 0.095 (0.032) | 0.066 (0.014) | 0.88 (0.048)a |
| Polyols | 29.936 (6.680) | 21.478 (5.469) | 5.0 (0.94)a |
| PUFA | 10.42 (1.722) | 13.326 (0.578) | 10.42 (0.756) |
| PUFA/MUFA | 1.942 (0.343) | 2.340 (0.447) | 1.95 (0.187) |
| Succinate | 0.350 (0.082) | 0.346 (0.029) | 0.29 (0.033) |
| TAG | 0.868 (0.087) | 1.042 (0.113) | 0.705 (0.0378) |
| Taurine | 1.106 (0.144) | 1.06 (0.046) | 1.6 (0.223)a |
| Total FA | 34.58 (5.83) | 40.32 (2.27) | 44.78 (1.217) |
| UDPG | 0.272 (0.037) | 0.338 (0.026) | 0.242 (0.054) |
| Val, leu, Ile | 2.372 (0.333) | 1.69 (0.155) | 1.18 (0.144)a |
aSignificantly different P < 0.05 (non-parametric one-way ANOVA plus Kruskal–Wallis test). Numbers in bold are significantly different between the groups.
Figure 1.
The metabolomic profile of first (n = 5) and second trimester (n = 5) and term placental tissue (n = 4). Tissue samples were subjected to quantitative 1H-NMR analysis to measure the expression of individual metabolites (expressed as µmol per g placental tissue). (A) There were no significant differences in ATP/ADP ratio, or expression of NAD+, lactate or glucose among the groups. (B) The concentrations of taurine increased significantly with increased gestation, whilst concentrations of polyols, inositol and hydrophobic amino acids (val/leu/ile) decreased with advanced gestation. (C) Creatine phosphate concentration (measured by NMR) was significantly decreased with increased gestational age and was confirmed by reduced CK activity measured placental homogenates by ELISA. All results were analysed by a non-parametric Kruskal–Wallis test.
The data also indicate that the concentrations of phosphodiesters (PDE), phosphocholine (Pcho) and glycerolphosphocholine (GPC), and taurine increase during gestation, whilst those of polyols, inositol, creatine phosphate and hydrophobic amino acids (val/leu/ile) decrease (Table I, Fig. 1B). We validated the decrease in creatine phosphate by measuring the enzymatic activity of CK, and found the CK activity to be significantly reduced in term samples compared with the first or second trimester placentas (Fig. 1C).
HIF-1α and HIF-2 α in first trimester placenta
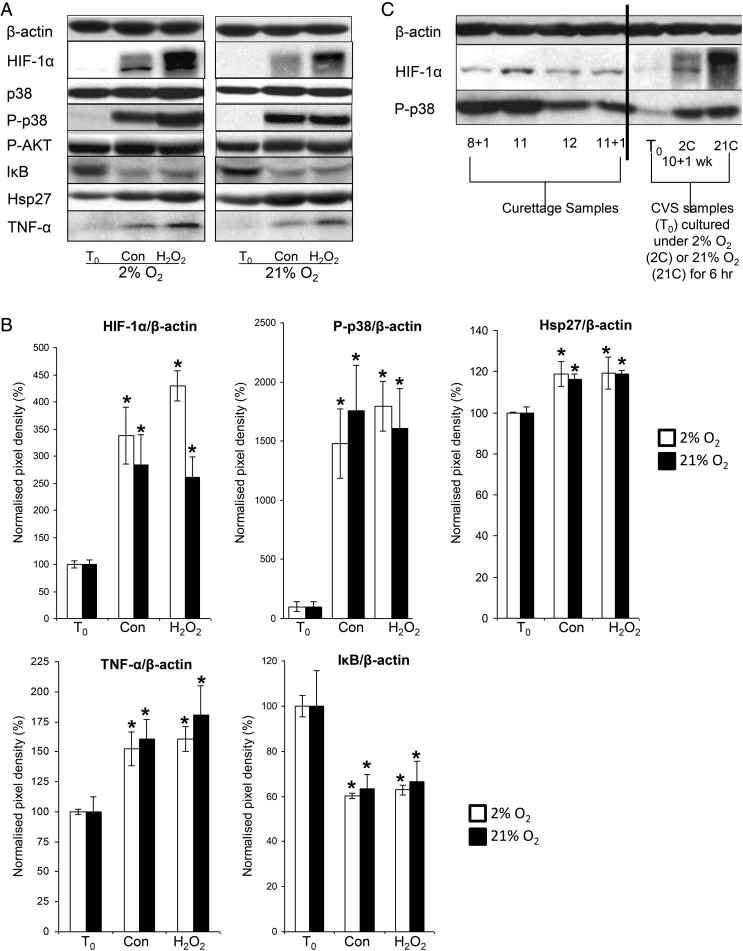
HIF1-α was virtually undetectable by western blotting in first trimester placental tissue collected by CVS. Culture of this tissue under 2 or 21% oxygen induced a marked increase in HIF-1α compared with T0 samples, and administration of the pro-oxidant H2O2 had no additional significant effect at either oxygen level (Fig. 2A). Culture at 2 or 21% O2 induced an increase in phosphorylation of the stress-induced mitogen-activated protein kinase p38 compared with T0 levels, but had no effect on the total level of p38 or AKT phosphorylation (Fig. 2A and B). Additionally, there was evidence of increased inflammatory markers in cultured tissue, as demonstrated by increased TNF-α, reduced IκB and increased Hsp27. Addition of 1 mM H2O2 had no additional effect on any of these markers (Fig. 2A and B).
Figure 2.
HIF-1α, p-p38, p38, Hsp27, TNF-α, IκB and P-Akt in first trimester samples (n = 6) cultured under 2% (white bars) or 21% O2 (black bars) in the presence or absence of 1 mM H2O2 for 6 h. (A) Lysates from first trimester explants were immunoblotted with antibodies against HIF-1α, p-p38, p38, Hsp27, TNF-α, IκB and P-AKT and (B) quantified by densitometry. (C) Lysates from representative first trimester curettage samples and a representative CVS sample cultured under 2% O2 (2C) or 21% O2 (21C) were immunoblotted with antibodies against HIF-1α, or phospho-p38. β-Actin staining served to normalize gel loading. Normalized results (±SEM) are plotted, expressing T0 samples as 100%. Significant differences (P < 0.05) are: * versus T0 samples (one-way ANOVA + Student–Newman–Keuls test). Con—denotes samples cultured under a given oxygen concentration for 6 h.
In contrast, high levels of HIF-1α were apparent in first trimester samples obtained by curettage (Fig. 2C). Multiple HIF-1α bands were apparent in some samples, indicative of phosphorylation (Fig. 2A and C). In addition to increased HIF-1α, there was a marked increase in the phosphorylation of the stress kinase p38 detectable in curettage samples, as in the cultured samples (Fig. 2C).
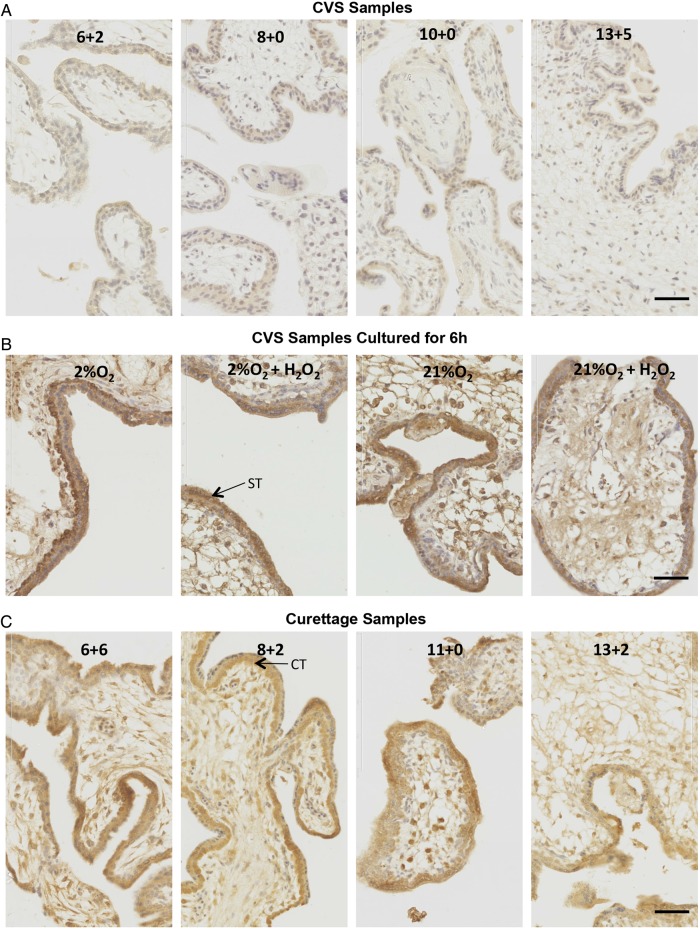
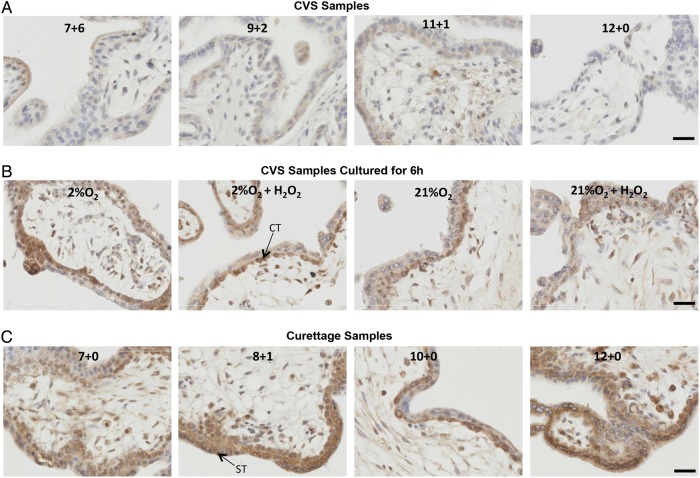
We confirmed the changes in HIF-1α by immunostaining. Whilst first trimester samples of various gestational ages collected by the CVS-like method showed minimal staining, and virtually no nuclear staining suggestive of transcriptional activity (Fig. 3A), there was marked nuclear localization of HIF-1α in all first trimester explants cultured for 6 h under 2 or 21% O2 in the presence or absence of H2O2 (Fig. 3B). Gestationally matched first trimester samples obtained by curettage (Fig. 3C) showed a similar staining pattern. The nuclear staining of explant and curettage samples localized primarily in syncytiotrophoblast and cytotrophoblast nuclei (Fig. 3B and C). There was no change in the pattern or intensity of staining with respect to gestational ages between 6 and 13 weeks. These results suggest that HIF-1α can be induced by stress. Similarly, we also examined HIF-2α in these groups of samples and found a strikingly similar pattern of staining. There was minimal nuclear staining in first trimester samples of various gestational ages collected by the CVS-like method (Fig. 4A), whilst there was marked nuclear localization of HIF-2α in all first trimester explants cultured for 6 h under 2 or 21% O2 ± H2O2 (Fig. 4B). There was also marked nuclear localization in gestationally matched first trimester samples obtained by curettage (Fig. 4C), suggesting that HIF-2α can also be induced by stress. Since HIF-2α is not involved in the regulation of VEGF in bone marrow, playing only an indirect role in haematopoiesis through small changes in the microenvironment (Scortegagna et al., 2003), we decided to concentrate on the regulation and functional activity of HIF-1α in subsequent experiments.
Figure 3.
HIF-1α localization in first trimester placentas collected by a CVS-like technique (A), collected by CVS and then cultured for 6 h (B) or collected by curettage (C). Representative images of HIF-1α staining are shown. Gestational age is indicated in each representative image in (A and B), and culture conditions are indicated in (C). Brown colour signifies positive staining. HIF-1α was almost undetectable in the CVS tissue (A) whilst a prominent cytotrophoblast and syncytiotrophoblast nuclear staining was detected in all curettage specimens (B) and in cultured explants (C). Scale bar = 50 µm. ST, arrow points to a syncytiotrophoblast nucleus; CT, arrow points to a cytotrophoblast nucleus.
Figure 4.
HIF-2α localization in first trimester placentas collected by a CVS-like technique (A), collected by CVS and then cultured for 6 h (B), or collected by curettage (C). Representative images of HIF-2α staining are shown. Gestational age is indicated in each representative image in A and B, and culture conditions are indicated in C. Brown colour signifies positive staining. HIF-2α was almost undetectable in the CVS tissue (A) whilst a prominent cytotrophoblast and syncytiotrophoblast nuclear staining was detected in all curettage specimens (B) and in cultured explants (C). Scale bar = 50 µm. ST, arrow points to a syncytiotrophoblast nucleus; CT, arrow points to a cytotrophoblast nucleus.
Increased VEGFA and decreased PlGF mRNA in cultured first trimester explants
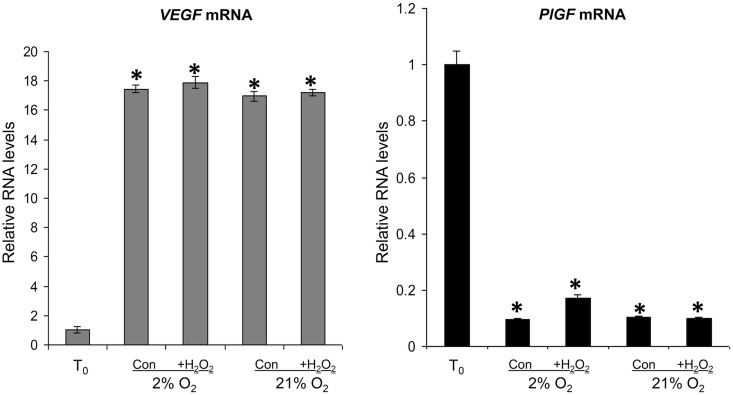
To investigate whether the increase in HIF-1α was of functional significance, we measured the mRNA levels of VEGFA and PlGF by quantitative real-time RT–PCR in the T0 samples, and after culture in 2% O2 or 21% O2 ± 1 mM H2O2 for 6 h. All cultured samples showed a significant increase in VEGFA mRNA and significant decrease in PlGF mRNA, compared with T0 values (Fig. 5). Administration of H2O2 had no additional effect.
Figure 5.
VEGFA and PlGF mRNA in T0 controls (T0), and in explants cultured under 2 or 21% O2 ± 1 mM H2O2 for 6 h. RNA was isolated and relative levels of VEGFA and PlGF mRNA were detected using quantitative real-time RT–PCR. VEGFA and PlGF mRNA levels were normalized to the 18S RNA levels. Significant differences (P < 0.05) are: * versus T0 controls (one-way ANOVA + Student–Newman–Keuls test).
Changes in HIF-1α protein and VEGFA and GLUT3 mRNA in first trimester explants with p38 inhibition
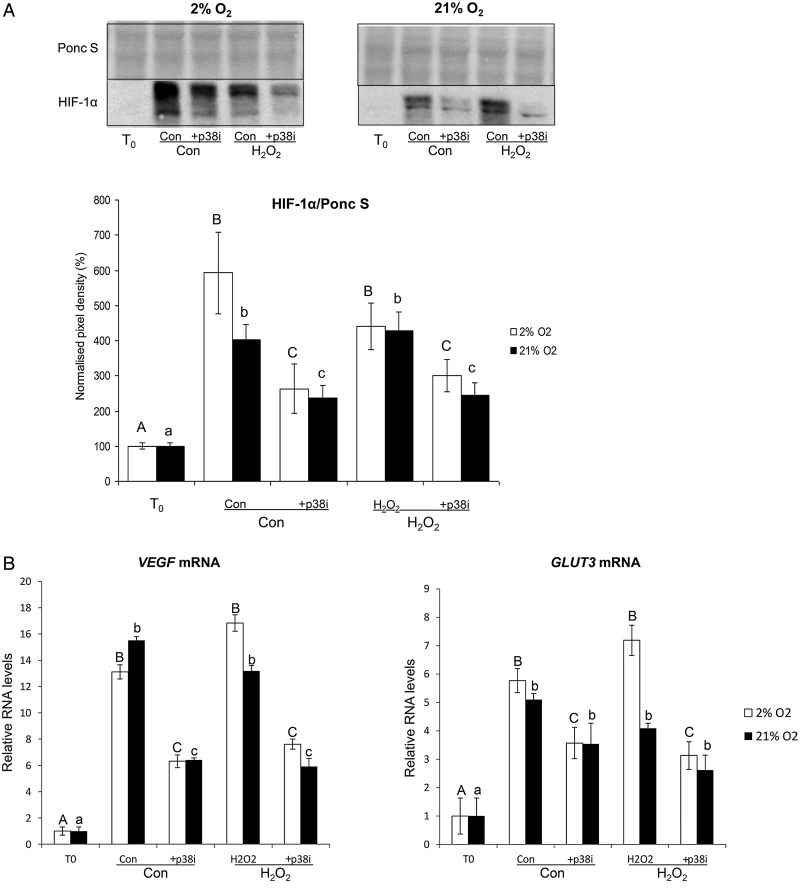
Because of the increased p38 phosphorylation and multiple anti-HIF-1α immunoreactive bands in cultured samples, we hypothesized that p38 may regulate HIF-1α in vitro. Explants were pre-treated with a p38 inhibitor, PD169316 (10 µM), and subsequently cultured under 2 or 21% O2 ± 1 mM H2O2 (Fig. 6). Inhibition of the p38 pathway resulted in a significant reduction in HIF-1α in all samples (Fig. 6A), and suppression of VEGFA mRNA (Fig. 6B). PD169316 also reduced GLUT3 mRNA, which is regulated by HIF-1α, but the suppression was only statistically significant in samples cultured under 2% O2 (Fig. 6B).
Figure 6.
The effect of p38 inhibition on HIF-1α protein (A) and VEGFA and GLUT3 mRNA (B) in first trimester placental explants subjected to 2 or 21% O2 ± 1 mM H2O2 for 6 h. (A) Lysates from first trimester explants cultured with or without the p38 inhibitor PD169316 (p38i; 10 µM) were immunoblotted with HIF-1α antibody. Poncaeu S staining served to normalize gel loading. Normalized results (±SEM) are plotted, expressing T0 samples as 100%. (B) RNA was isolated and relative levels of VEGFA and GLUT3 mRNA were detected using quantitative real-time RT–PCR. VEGFA and GLUT3 mRNA levels were normalized to the 18S RNA levels. Different letters indicate groups that are significantly different (P < 0.05; one-way ANOVA + Student–Newman–Keuls test).
Immunoprecipitation to evaluate the phosphorylation of HIF-1α
Immunoprecipitation with an antibody against phospho-Ser/Thr/Tyr was performed on two samples from the same placenta (10 + 3 weeks) that were either snap frozen (T0) or cultured under 2% O2 in the presence of 1 mM H2O2 for 6 h. A western blot was probed with anti-HIF-1α and re-probed with anti-phospho-Ser/Thr/Tyr. Phosphorylated HIF-1α was only detected in the cultured placental samples, suggesting that phosphorylation could be involved in the up-regulation of its activity during culture (Fig. 7).
Figure 7.
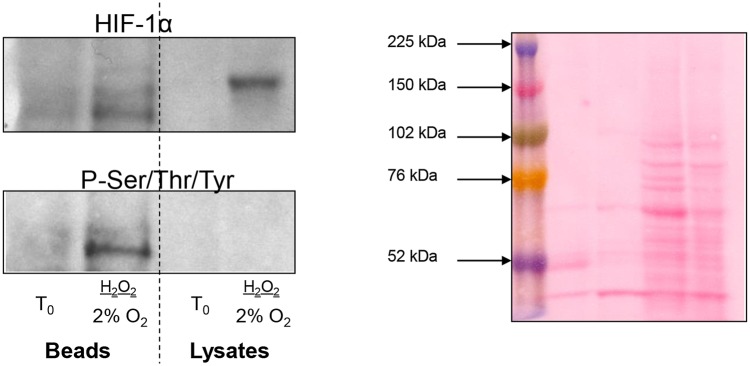
Immunoprecipitation experiment to evaluate the role of phosphorylation in HIF-1α regulation. Two samples from the same placenta (10 + 3 weeks) were snap frozen (T0) or cultured under 2% O2 in the presence of 1 mM H2O2 for 6 h. Lysates were immunoprecipitated with an antibody against phospho-Ser/Thr/Tyr, western blots were run, probed with anti-HIF-1α and re-probed with anti-phospho-Ser/Thr/Tyr. HIF-1α was only detected on the P-Ser/Thr/Tyr-coated beads from the cultured placenta.
Discussion
Because of the low intraplacental oxygen tensions reported, the first trimester intrauterine environment is frequently described as ‘hypoxic’. However, hypoxia cannot be defined by just the prevailing oxygen tension, but must be related to the metabolic demands of the tissue or cells. The term implies a pathologically low pO2 that is inadequate to meet the energy demands of the tissues, and so it is best evinced by metabolic parameters or transcriptional responses. Our finding that there were no differences in the ATP/ADP ratio, the levels of ADP, NAD+, lactate, or glucose across gestational ages indicate that the first trimester placenta is not energetically compromised. Thus, while the pO2 is low, it is inappropriate to describe this as ‘hypoxia’ as it is in fact the normal physiological state. The data also indicate that placental metabolism alters with gestational age, as the concentrations of PDE, Pcho and GPC, and taurine increased, whilst those of phosphomonoesters, polyols, hydrophobic amino acids (val/leu/ile) and creatine phosphate decreased towards term. We confirmed the decrease in creatine phosphate by showing a significant reduction in CK activity in term samples. Our findings complement the data of Thomure et al. (1996) who found reduced ubiquitous mitochondrial and cytosolic brain CK protein expression in term samples, compared with first and second trimester placentas. We also describe a remarkable increase in the PDE spectral intensity fraction and the PDE/PME spectral intensity ratio towards term. Whilst these parameters are unchanged between the first and second trimesters, there is a 15-fold increase in the PDE/PME ratio at term. Similar changes were reported by Sohlberg et al. (2014) in two groups of women with healthy pregnancies (mean gestational age 29.1 versus 37.9 weeks). The spectral intensity ratio of PDE/PME is regarded as an index of cell membrane turnover (Menon et al., 1995), and the increase with gestational age could be explained by apoptosis (Smith et al., 1997; Ishihara et al., 2000) and a concomitant decrease in trophoblast cell proliferation (Ishihara et al., 2000; Chen et al., 2002).
The polyol metabolic pathway is highly active in the human conceptus during early pregnancy (Jauniaux et al., 2005). This pathway will be advantageous under these conditions since it allows for regeneration of NAD+ to maintain glycolysis, and therefore ATP generation, without the need for oxygen. Although fewer molecules of ATP are produced per molecule of glucose than through oxidative phosphorylation, there is no evidence that these pathways are metabolically limiting if there is a plentiful supply of glucose. During the first trimester, the endometrial secretions are rich in glycogen, and this substrate accumulates in the syncytioplasm (Burton et al., 2002), suggesting that this requirement is met.
The metabolomics study was a small-scale discovery analysis, using only 4–5 samples per group. We did not subject these results to correction for multiple testing because of the small numbers. Equally, the number of observations is too low to use principal component analysis (PCA). Thus, our interpretations of the data are based on statistical significance (P < 0.05) and the fact that concentrations of a variety of metabolites within specific pathways changed according to known biological pathways. Larger studies are therefore necessary to confirm our results.
In this study, neither HIF-1α nor HIF-2α were detectable in first trimester CVS samples, but could be detected in samples removed by curettage, the most common method of obtaining early placental tissue. Detectable HIF-1α and HIF-2α protein in the curettage samples could reflect oxidative stress, as these samples had been in contact with maternal blood. These samples showed increased p38 phosphorylation, which, as we show, likely plays a role in HIF-1α stabilization. Culture of unstressed first trimester samples under either 2 or 21% O2 for 6 h also induced a marked increase in HIF-1α and HIF-2α, oxidative stress and inflammation. Hydrogen peroxide, a pro-oxidant, had no additional effect, suggesting that the culture conditions per se were sufficient to induce the maximal stress responses. Other authors have reported an increase in HIF-1α protein (Caniggia et al., 2000; Rajakumar and Conrad, 2000) and mRNA (Caniggia et al., 2000) in first trimester samples cultured under 2% (Rajakumar and Conrad, 2000) or 3% (Caniggia et al., 2000) O2 but not in samples cultured under what they describe as ‘normoxia’ (21% O2). Similar increase in HIF-2α protein was also reported in first trimester explants following 48 h incubation under 2% O2 (Genbacev et al., 2001). The discrepancies with our results could be due to the starting material. It is not stated how the placental samples were obtained in these studies, whether they were collected immediately or were contaminated with maternal blood.
We previously showed that labour increases placental HIF-1α, VEGFA and sFlt1 mRNA and protein levels, but not PlGF levels (Cindrova-Davies et al., 2007b). During labour, the placenta is exposed to repetitive ischaemia–reperfusion. Similarly, in vitro exposure of term placental villi to hypoxia-reoxygenation increased HIF-1α, sFlt1 and VEGFA protein levels. These effects could be blocked by administration of antioxidant vitamins, and by inhibiting the p38 MAPK and NF-κB pathways. Collectively, these findings suggest the involvement of oxidative stress signalling in HIF-1α and sFlt1 regulation (Cindrova-Davies et al., 2007a; Cindrova-Davies, 2009). In this study, increased HIF-1α protein as well as associated up-regulation of VEGFA and GLUT3 mRNA could also be blocked by inhibition of the p38 pathway. There was a marked mobility shift in the HIF in cultured explants, indicative of a phosphorylation regulation of this protein. Additionally, in our immunoprecipitation experiments, phosphorylated HIF-1α was detected in isolates of cultured placental tissue, but not of fresh tissue. This suggests that phosphorylation could be involved in its regulation and p38 is a likely effector. p38 can act as a pro-inflammatory kinase and the suppressive effects may be partly due to reduced levels of inflammatory markers. HIF-1α stability is increased by hypoxia, but it can also be up-regulated under non-hypoxic conditions by inflammatory cytokines or microtubule-depolymerizing agents involving the NF-κB pathway (Jung et al., 2003a, b,). Our in vitro findings are consistent with reports that cytokine-mediated HIF-1α activation leads to production of VEGFA, and seems to proceed via a pathway involving an up-stream PI3K/AKT/mTOR pathway, NFκB activation and also COX2 expression (Jung et al., 2003a, b).
The stability of HIF-1α and HIF-2α is regulated by prolyl hydroxylase domain (PHD) proteins. PHD hydroxylation of HIF-1α and HIF-2α under normoxia leads to HIF binding of VHL and subsequent polyubiquitination and proteosomal degradation (Maxwell et al., 1999; Bruick and McKnight, 2001; Ivan et al., 2001). Ietta et al. (2006) reported an inverse correlation between PHD proteins and HIF-1α in first trimester placentas, and demonstrated that degradation of HIF-1α takes place after 10 weeks of gestation, coincident with the placental O2 rise. Inhibition of PHDs activity increases HIF-1α stability in villus explants and stimulates TGFβ3 expression, which is important for placental development. PHD1, 2 and 3 (both protein and mRNA) are regulated by O2 in placental explants in vitro. In fact, low O2 (3%) induces mRNA of all three PHDs, compared with explants cultured under 20% O2. Similarly, exposure of explants to 3 and 8% O2 increases PHD1 and PHD3, but not PHD2 protein. The reduction in PHDs mRNA and protein under atmospheric O2 conditions could thus explain differences we see in HIF-1α expression between different starting materials.
In conclusion, there were no differences in the placental metabolomic profile across gestational age in terms of ATP/ADP ratio, NAD+, lactate, or glucose levels, indicating that the first trimester placenta is not compromised energetically. In addition, HIF-1α and HIF-2α protein were undetectable in first trimester villous samples collected by chorionic villus sampling. Culture of first trimester explants induced HIF-1α stabilization and up-regulation of the target genes, VEGFA and GLUT3, and down-regulation of PlGF under widely differing oxygen levels. Blocking the p38 MAPK pathway suppressed these changes. These data suggest that HIF-1α is unlikely to play an appreciable role in regulating placental transcriptional activity under steady-state conditions during the first trimester in vivo, but that the pathway may be activated by fluctuations in oxygenation.
Authors’ roles
T.C.-D. planned and carried out most experiments and experimental analyses and wrote the first draft of the manuscript. M.T.P. carried out the metabolomics analysis and reviewed the manuscript. L.G. collected and sorted placental samples. E.J. obtained patient consent, obtained tissue under ultrasound guidance and critically reviewed the manuscript. G.J.B. provided experimental planning, data analysis and discussion and critically reviewed the manuscript. D.S.C.-J. provided experimental planning, data analysis and discussion and critically reviewed the manuscript.
Funding
This work was supported by the Wellcome Trust (084804/2/08/Z). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Conflict of interest
The authors have no conflict of interest.
References
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RJ, Chu CT, Huang SC, Chow SN, Hsieh CY. Telomerase activity in gestational trophoblastic disease and placental tissue from early and late human pregnancies. Hum Reprod. 2002;17:463–468. doi: 10.1093/humrep/17.2.463. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T. Gabor Than Award Lecture 2008: pre-eclampsia—from placental oxidative stress to maternal endothelial dysfunction. Placenta. 2009;30(Suppl. A):S55–S65. doi: 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol. 2007a;170:1511–1520. doi: 10.2353/ajpath.2007.061035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007b;171:1168–1179. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Kaelin W, Fisher SJ. Human cytotrophoblast expression of the von Hippel–Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev Biol. 2001;233:526–536. doi: 10.1006/dbio.2001.0231. [DOI] [PubMed] [Google Scholar]
- Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- Gorr TA, Wichmann D, Hu J, Hermes-Lima M, Welker AF, Terwilliger N, Wren JF, Viney M, Morris S, Nilsson GE, et al. Hypoxia tolerance in animals: biology and application. Physiol Biochem Zool. 2010;83:733–752. doi: 10.1086/648581. [DOI] [PubMed] [Google Scholar]
- Hempstock J, Cindrova-Davies T, Jauniaux E, Burton GJ. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol. 2004;2:58. doi: 10.1186/1477-7827-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11:477–486. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- Ietta F, Wu Y, Winter J, Xu J, Wang J, Post M, Caniggia I. Dynamic HIF1A regulation during human placental development. Biol Reprod. 2006;75:112–121. doi: 10.1095/biolreprod.106.051557. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez J, Samoto T, Maruo T. Changes in proliferative potential, apoptosis and Bcl-2 protein expression in cytotrophoblasts and syncytiotrophoblast in human placenta over the course of pregnancy. Endocr J. 2000;47:317–327. doi: 10.1507/endocrj.47.317. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial bloodflow and placental oxidative stress; a possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab. 2005;90:1171–1175. doi: 10.1210/jc.2004-1513. [DOI] [PubMed] [Google Scholar]
- Jung Y-J, Isaacs JS, Lee S, Trepel J, Liu ZG, Neckers L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappaB activation. Biochem J. 2003a;370:1011–1017. doi: 10.1042/BJ20021279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y-J, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1b mediated up-regulation of HIF-1a via an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003b;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Jung Y-J, Isaacs JS, Lee S, Trepel J, Neckers L. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J Biol Chem. 2003c;278:7445–7452. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- Kuschel A, Simon P, Tug S. Functional regulation of HIF-1alpha under normoxia—is there more than post-translational regulation? J Cell Physiol. 2011;227:514–524. doi: 10.1002/jcp.22798. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Menon DK, Sargentoni J, Taylor-Robinson SD, Bell JD, Cox IJ, Bryant DJ, Coutts GA, Rolles K, Burroughs AK, Morgan MY. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417–427. [PubMed] [Google Scholar]
- Pouyssegur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem. 2006;387:1337–1346. doi: 10.1515/BC.2006.167. [DOI] [PubMed] [Google Scholar]
- Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update. 2010;16:415–431. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis. Semin Cancer Biol. 2003;13:83–89. doi: 10.1016/s1044-579x(02)00103-7. [DOI] [PubMed] [Google Scholar]
- Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod. 2000;63:559–569. doi: 10.1095/biolreprod63.2.559. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1, O2, and the 3PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Serkova N, Bendrick-Peart J, Alexander B, Tissot van Patot MC. Metabolite concentrations in human term placentae and their changes due to delayed collection after delivery. Placenta. 2003;24:227–235. doi: 10.1053/plac.2002.0908. [DOI] [PubMed] [Google Scholar]
- Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics. 2007;31:15–24. doi: 10.1152/physiolgenomics.00028.2007. [DOI] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–65. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- Sohlberg S, Wikstrom AK, Olovsson M, Lindgren P, Axelsson O, Mulic-Lutvica A, Weis J, Wikstrom J. In vivo (3)(1)P-MR spectroscopy in normal pregnancy, early and late preeclampsia: a study of placental metabolism. Placenta. 2014;35:318–323. doi: 10.1016/j.placenta.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Thomure MF, Gast MJ, Srivastava N, Payne RM. Regulation of creatine kinase isoenzymes in human placenta during early, mid-, and late gestation. J Soc Gynecol Investig. 1996;3:322–327. [PubMed] [Google Scholar]
- Tissot van Patot MC, Murray AJ, Beckey V, Cindrova-Davies T, Johns J, Zwerdlinger L, Jauniaux E, Burton GJ, Serkova NJ. Human placental metabolic adaptation to chronic hypoxia, high altitude: hypoxic preconditioning. Am J Physiol Regul Integr Comp Physiol. 2010;298:R166–R172. doi: 10.1152/ajpregu.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoa M-L, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol. 2006;169:400–404. doi: 10.2353/ajpath.2006.060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Nat Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]