Abstract
Aims and objectives
To examine the prevalence of cancer-related fatigue in women treated for various types of gynaecological cancers and, for these cancers, to assess fatigue in relation to distress, health-related quality of life, demography and treatment characteristics.
Background
Advances in treatment of cancer have improved the likelihood of survival. Consequently, there are a growing number of patients who become survivors after cancer and who face side effects even years after treatment. One of the most frequently reported side effects across all types and stages of the disease is cancer-related fatigue.
Design
A descriptive cross-sectional study.
Methods
One hundred and twenty women treated for gynaecological cancers who were participants in an intervention study were included. Fatigue, psychological distress, health-related QoL and demographics were assessed by questionnaires. Disease and treatment characteristics were extracted from medical records.
Results
Cancer-related fatigue was reported in 53% of the women treated for gynaecological cancers, with a higher proportion in the group of cervical cancer, followed by ovarian cancer. Younger participants reported fatigue more frequently than older participants. When adjusting for age, the type of cancer a woman experiences was shown to have little impact on her risk of experiencing fatigue. The participants with fatigue reported higher levels of anxiety and depression than participants without fatigue. There was a relationship between fatigue and quality of life as measured by SF-36 domains.
Conclusion
The findings underscore the importance of screening for fatigue, patient education and symptom management. This should be included in a standard procedure during treatment and follow-up. Both somatic and psychological aspects of fatigue should be emphasised.
Relevance to clinical practice
The findings imply the need for health personnel to have focus on fatigue during the entire cancer trajectory of women after gynaecological cancers, as well as the need for screening, information, guidance and symptom management.
Keywords: anxiety, depression, fatigue, gynaecological cancers, quality of life
What does this study contribute to the wider global clinical community?
Fatigue is highly prevalent among all gynaecological cancer types and treatment modalities.
Younger women report fatigue more frequently than older women.
Fatigued women report more anxiety and depression and poorer QoL.
Introduction
Advances in treatment of cancer have improved the likelihood of survival. Consequently, there are a growing number of patients who become survivors after cancer and who face side effects even years after treatment. One of the most frequently reported side effects across all types and stages of the disease is cancer-related fatigue (Ahlberg et al. 2003, Hofman et al. 2007, Stone & Minton 2008). Fatigue is often explained as an experience of tiredness or exhaustion, and approximately one-third of the patients continue to experience cancer-related fatigue months and years after the completion of treatment (Hofman et al. 2007).
Background
Cancer-related fatigue is a common symptom in women treated for gynaecological cancers (Ferrell et al. 2003, Liavaag et al. 2007, Vistad et al. 2007, Steele & Fitch 2008, Arriba et al. 2010, Harrington et al. 2010). Steele and Fitch (2008) identified supportive care needs of women diagnosed for various types of gynaecological cancers postdiagnosis. The findings showed that fatigue was the fourth most experienced issue (n = 103). This is also in line with a descriptive study by Beesley et al. (2008), who found fatigue as a fourth ranged unmet support need in 802 women following gynaecological cancer. Furthermore, in a review by Harrington et al. (2010), the symptom burden most commonly reported following primary treatment for cancer (in survivors of breast, gynaecological, prostate and colorectal cancer) was fatigue. Fatigue was found in 17–33% of gynaecological cancer survivors three to eight years after diagnosis.
Fatigue highly affects health-related QoL, as patients with cancer may become too tired to fully participate in daily life and activities, and to fill the roles they previously had. Liavaag et al. (2007) conducted a controlled cross-sectional study to explore fatigue, quality of life and somatic and mental morbidity in ovarian cancer survivors. In the study, 22% reported chronic fatigue, compared with 12% of the controls from the general population. The fatigued reported significantly more somatic disease and complaints, higher scores on anxiety and lower levels of QoL compared with norm samples. The study found minimal differences between women with and without relapse, long or short follow-up time and prognostic index status. Vistad et al. (2007) found in a cross-sectional study of cervical cancer survivors (n = 79) treated with radiotherapy that 30% of the women reported cancer-related fatigue (mean follow-up time 7·9 years). Holzner et al. (2003) found in a cross-sectional study that among 98 patients with ovarian cancer (mean follow-up time 5·7 years), 33% reported fatigue. This group of patients had a significantly lower QoL and higher scores on anxiety and depression.
Although specific studies on fatigue and the association between different treatment modalities are lacking, there is evidence of an association between multiple therapy and impaired QoL and fatigue (Carlsson et al. 2000, Ahlberg et al. 2005, Frumovitz et al. 2005, Korfage et al. 2009, Bjelic-Radisic et al. 2012). Bjelic-Radisic et al.'s (2012) study shows that patients with cervical cancer treated with multiple therapy reported more impairments in their QoL than those treated with only one therapy. Korfage et al. (2009) found, among 291 cervical cancer survivors 2–10 years postdiagnosis, that radiotherapy was associated with more treatment-related side effects. Ahlberg et al. (2005) showed in their study that among women with uterine cancer who received radiation therapy, fatigue scores increased significantly during and after radiotherapy, compared with pretreatment scores. Carlsson et al. (2000) showed that patients who had been treated with chemotherapy had lower role and cognitive functioning and more problems with, for example, fatigue. However, Liavaag et al. (2008) found no significant differences regarding fatigue and QoL and treatment modalities.
A few studies have illuminated the relationship between fatigue and psychological distress (Brown & Kroenke 2009, Oh & Seo 2011). The review by Brown and Kroenke (2009) included 59 studies and assessed evidence regarding associations of cancer-related fatigue with depression and anxiety. They confirmed these associations. In addition, a literature review and meta-analysis by Oh and Seo confirmed those results (2011). These findings highlight the importance of dealing with psychological distress and symptom distress in relation to cancer-related fatigue. This is also in line with Vistad et al. (2007), who found that the women treated for cervical cancer who reported cancer-related fatigue had significantly lower QoL, higher levels of anxiety and depression, and more physical impairments. For women diagnosed with different gynaecological cancers and with different treatment modalities, few descriptions of symptom experience like fatigue and QoL are available. As fatigue has a major impact on women's lives and well-being, a clearer understanding of the effect of fatigue on QoL in these gynaecological cancer groups is needed.
The primary aim of this study was to examine the prevalence of cancer-related fatigue in women treated for different gynaecological cancers and, for these cancers, to assess fatigue in relation to anxiety, depression, health-related QoL, demography and treatment characteristics.
Methods
Patients
The women in this study were participants in a randomised controlled study. The purpose of the latter was to measure and compare the effects of two interventions on women's self-reported quality of life and coping, namely an educational and counselling group versus a physical training group. The study was carried out between 2009 and 2012. The participants were recruited from three different hospitals in Norway, using mailed letters. Inclusion criteria were the following: (1) women finished with treatment for curative purpose of gynaecological cancers; (2) age > 18 years; (3) specific physical functioning (able to walk on a treadmill); (4) no significant amnesic symptoms; and (5) agreement to participate, as specified by consent form.
An invitation to participate in this study was sent to all women treated for gynaecological cancer at all three hospitals, thus fulfilling the inclusion criteria 1 and 2. The women who wished to participate needed to return a consent letter. One hospital invited women treated during the period 2009–2011, and two hospitals invited those treated in 2011–2012. Six hundred and twenty invitations were sent.
Measures and questionnaires
The women were assessed using psychometric instruments measuring fatigue, anxiety and depression, health-related QoL, coping, sexuality, socio-demographic characteristics (age, level of education, marital status, household status, employment status, etc.) and treatment characteristics. Workability was explored with the single question ‘Has managing your job become more difficult after you returned from sick leave compared to the time before you got sick’, with the following response categories: ‘Yes’, ‘No’ and ‘Don't know’. Details regarding diagnosis and treatment were retrieved from the patient's medical records. Data related to physical activity were retrieved with a single question: ‘Not including office hours, how many times a week do you exercise to such a degree that you are sweating or need to catch your breath?’ The response categories were the following: ‘Seven times or more’, ‘4–6 times a week’, ‘2–3 times a week’, ‘Once a week’, ‘Once a month or less’ and ‘Never’.
The Fatigue Questionnaire (FQ) (Chalder et al. 1993) is validated and has 11 items concerning the fatigue intensity women have felt during the last month compared with the latest well-being. We used a Norwegian translation of a modified version (Cella & Chalder 2010) of the FQ. This translation has also been used in two Norwegian studies on gynaecological cancer survivors (Liavaag et al. 2007, Vistad et al. 2007) and in a large population survey in a general Norwegian sample (Loge et al. 1998a).
Each item has four choice alternatives on an ordinal response scale (0, 1, 2 or 3). Higher scores imply more fatigue. The wording in the response choices for 0 and 1 for some of the items in the Norwegian version differs slightly from those in the English version, which makes using the Likert scoring described in Cella and Chalder (2010) problematic. We have therefore used the recommended bimodal scoring system, where fatigue is identified according to the procedure described in Cella and Chalder (2010): for each woman, the number of items with the response 2 (‘more than usual’) or 3 (‘much more than usual’) is counted, and a count of four or more such responses is defined as indicating fatigue.
The Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith 1983) is often used to assess symptoms of anxiety and depression in a nonpsychiatric context. It has been widely used in Norwegian studies examining cancer populations, including gynaecological cancer (Liavaag et al. 2009). HADS is a self-report questionnaire comprising four-point ordinal response items (0, 1, 2 or 3): seven items for anxiety (HADS-A) and seven items for depression (HADS-D). Higher scores reflect higher symptom loads on both subscales. Cases of HADS-defined anxiety disorder (HADS-A) or depression (HADS-D) are defined by a score of 8 or greater on the subscales. HADS has shown both good reliability and validity in measuring levels of anxiety and depression in primary care and in clinical populations (Bjelland et al. 2002).
SF-36 is a multidomain self-report generic health measure assessing general health perception (Ware & Sherbourne 1992). It is not disease, age or treatment specific and is widely used to compare study samples from the general population. The Physical health domain comprises physical functioning (10 items), physical role limitations (four items), bodily pain (two items) and general health (five items). The Mental health domain comprises scales for energy/vitality (four items), social functioning (two items), emotional role limitations (three items) and mental health (five items). The psychometric properties are well documented, also in Norwegian populations (Loge et al. 1998a,b). The questionnaire's response options vary from yes/no to answers on a 3-, 5- or 6-point ordinal response scale. The scores of the eight dimensions were transformed into scales from 0 (poorest/worst health) to 100 (best health).
Ethics
The study was approved by the Regional Committee for Medical Research Ethics (2009/895). Each woman gave written informed consent.
Data handling and statistics
The data were analysed using SPSS version 19.0 (Chicago, IL, USA) and R 3.0.0 and 3.0.2 (R Core Team 2013). Categorical data were analysed using cross-tabulation and Fisher's exact test. Continuous data were compared using Welch's two-sample t-test and reported with 95% confidence intervals. Logistic regression analysis was used to explore the association between caseness of chronic fatigue (dependent variable) and various explanatory variables. The results are reported as odds ratios with 95% confidence intervals, along with p-values.
Results
Patient characteristics
A total of 120 women (mean age 56) with different types of gynaecological cancers answered the questionnaires. Distribution of socio-demographic and disease characteristics of the sample is shown in Table 1. Most of the women (78%) were married or cohabiting. Almost all the women (88%) had finished high school or university/college. While 26% of the women were retired, half of the women (52%) were employed, and 5% were disabled.
Table 1.
Distribution of patient characteristics and treatment-related factors in fatigued and nonfatigued gynaecological cancers survivors
| Nonfatigued (n = 56) | Fatigued (n = 64) | p-value | Total sample (n = 120) | |
|---|---|---|---|---|
| Age at survey time mean, SD (95% CI) | 61, 12 (57·4–63·8) | 53, 12 (49·8–56·0) | <0·001 | 56, 13 (54·2–58·8) |
| Civil status, n (%) | ||||
| Paired relation | 44 (80) | 49 (77) | 0·68 | 93 (78) |
| Single | 5 (9) | 5 (8) | 10 (8) | |
| Divorced | 2 (4) | 6 (9) | 8 (7) | |
| Widow | 4 (7) | 4 (6) | 8 (7) | |
| Regular physical activity, n (%) | ||||
| 7 times a week or more | 4 (7) | 2 (3) | 0·03 | 6 (5) |
| 4–6 times a week | 11 (20) | 12 (19) | 23 (20) | |
| 2–3 times a week | 26 (48) | 16 (26) | 42 (36) | |
| Once a week | 8 (15) | 21 (34) | 29 (25) | |
| Less than once a week | 5 (9) | 11 (18) | 16 (14) | |
| Employment status, n (%) | ||||
| Employed | 24 (43) | 38 (59) | 0·09 | 62 (52) |
| Retired | 21 (38) | 10 (16) | 31 (26) | |
| Unemployed | 6 (11) | 8 (12) | 14 (12) | |
| Disability pension | 3 (5) | 3 (5) | 6 (5) | |
| Housewife | 2 (4) | 3 (5) | 5 (4) | |
| Other | 0 (0) | 2 (3) | 2 (2) | |
| Time from diagnosis to survey (months) mean, SD (95% CI) | 17·4, 8·5 (15·1–19·7) | 15·5, 9·6 (13·0–17·6) | 0·25 | 16·3, 9·1 (14·7–18·0) |
| Diagnosis, n (%) | ||||
| Uterine | 32 (58) | 22 (35) | 0·04 | 54 (46) |
| Ovarian | 12 (22) | 20 (32) | 32 (27) | |
| Cervical | 9 (16) | 20 (32) | 29 (25) | |
| Vulva | 2 (4) | 1 (2) | 3 (3) | |
| FIGO stage, n (%) | ||||
| I | 42 (78) | 36 (61) | 0·15 | 78 (69) |
| II | 4 (7) | 8 (14) | 12 (11) | |
| III | 6 (11) | 14 (24) | 20 (18) | |
| IV | 2 (4) | 1 (2) | 3 (3) | |
| Educational level, n (%) | ||||
| Elementary school | 8 (15) | 6 (9) | 0·71 | 14 (12) |
| Secondary school | 23 (42) | 29 (45) | 52 (44) | |
| College/university | 24 (44) | 29 (45) | 53 (45) | |
| Treatment modalities, n (%) | ||||
| Surgery only | 30 (55) | 26 (42) | 0·63 | 56 (48) |
| Surgery and chemotherapy | 18 (33) | 22 (35) | 40 (34) | |
| Surgery, chemotherapy and radiation | 4 (7) | 5 (8) | 9 (8) | |
| Chemotherapy and radiation | 2 (4) | 4 (6) | 6 (5) | |
| Surgery and radiation | 1 (2) | 3 (5) | 4 (3) | |
| Chemotherapy only | 0 (0) | 2 (3) | 2 (2) | |
Diagnosis and treatment characteristics
The participants were on average 16 months post-treatment, and there was no relationship between fatigue and time post-treatment, measured either using a t-test of time postdiagnosis against caseness of fatigue (mean difference 1·9 months, p = 0·25) or by Kendall's tau (τ = −0·10, p = 0·13) on time and fatigue score. The majority (93%) were treated with surgery; 49% had received chemotherapy, and 16% had received radiation. Two-thirds (69%) had been diagnosed with early stage (FIGO stage I) disease.
As shown in Table 1, 46% of the women (n = 54, mean age 61) were diagnosed with uterine cancer. These women were mainly treated with surgery, and 28% had additionally received chemotherapy, whereas only 8% had received radiation. Most (80%) were diagnosed in FIGO stage I.
Twenty-seven per cent (n = 32, mean age 57) of the women were treated for ovarian cancer. All were treated with surgery, and most of them (81%) were treated with adjuvant chemotherapy, while only one person received radiation. About half (47%) were diagnosed in FIGO stage I.
One-quarter (25%) (n = 29, mean age 47) were diagnosed with cervical cancer. Seventy-five per cent were diagnosed in stage I of the disease, and 76% of the women had been treated with surgery. About 52% of the women had been treated with chemotherapy, and 45% had received radiotherapy. There were significantly more women treated for cervical cancer who received radiation compared to uterine and ovarian cancer (Fisher's exact test: p < 0·001).
Three women (3%) were diagnosed with vulval cancer (mean age 55). One was diagnosed in stage I. All were treated with surgery, one was treated with radiation and one with chemotherapy.
Variables associated with fatigue
Socio-demography
Women with fatigue were on average eight years younger than nonfatigued women (95% CI: 3·2–12·1, p < 0·001). They also reported statistically significantly higher income as compared to the nonfatigued women (p = 0·02).
There were no statistically significant differences in caseness of fatigue according to socio-demographic variables, such as marital status, educational level or employment status.
Diagnosis and treatment
The distribution of participants reporting fatigue or nonfatigue according to diagnosis and treatment-related variables is shown in Table 1. A total of 53% of the women reported cancer-related fatigue. There were statistically significant differences in reports of fatigue according to the diagnosis (Fisher's exact test, p = 0·04), with the highest proportion of fatigue among women with cervical (69%) and ovarian cancer (62%).
There were no differences in caseness of fatigue according to treatment (surgery, radiation, chemotherapy) (all p-values > 0·27) or in relation to time since diagnosis (p = 0·31) or FIGO stage (p = 0·15).
Quality of life
As shown in Table 2, there were differences in all of the eight domains in SF-36, with fatigued women reporting on average lower levels of quality of life. Figure 1 displays the detailed score distributions on the eight domains. Due to an administrative error, some response forms were missing some of the SF-36 items. This explains the low number of responses on one of the sum scales, but it has no effect on the interpretation of the results (as all the differences are statistically significant, and most of them are highly significant).
Table 2.
Mean values and differences for the self-report scale SF-36 in fatigued and nonfatigued gynaecological patients with cancer. Positive differences indicate worse health for women with fatigue.
| Nonfatigued Mean, SD, n (95% CI) | Fatigued Mean, SD, n (95% CI) | p-value | Difference (95% CI) | |
|---|---|---|---|---|
| Physical function | 89, 12, 56 (85–92) | 83, 15, 63 (79–87) | 0·02 | 6 (0·9–10·6) |
| Role physical | 81, 34, 56 (72–90) | 48, 40, 61 (38–58) | <0·001 | 33 (19·2–46·2) |
| Bodily pain | 81, 24, 56 (74–87) | 71, 27, 64 (64–78) | 0·04 | 10 (0·4–18·7) |
| General health | 80, 16, 42 (75–85) | 68, 20, 47 (62–74) | 0·002 | 12 (4·3–19·3) |
| Vitality | 70, 17, 55 (65–74) | 43, 19, 64 (39–48) | <0·001 | 27 (20·3–33·1) |
| Social function | 94, 15, 56 (90–98) | 74, 22, 64 (68–79) | <0·001 | 20 (13·4–26·8) |
| Role emotional | 91, 22, 56 (85–97) | 62, 39, 61 (52–72) | <0·001 | 29 (17·8–40·9) |
| Mental health | 84, 12, 55 (81–87) | 68, 18, 64 (64–72) | <0·001 | 16 (10·5–21·5) |
Figure 1.
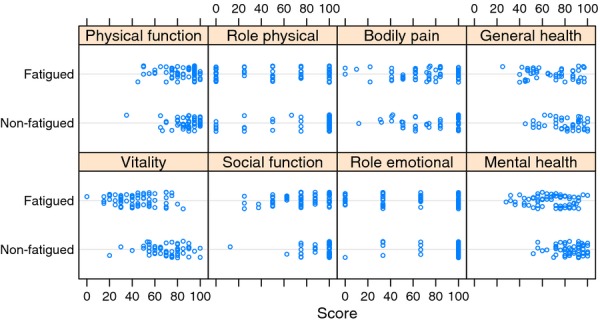
Dot plot (strip chart) showing scores on the eight quality of life domains of the self-report scale SF-36 in fatigued and nonfatigued gynaecological patients with cancer. Higher values indicate better quality of life. The dots have been slightly jittered vertically to reduce the effect of overplotting.
Anxiety and depression
As shown in Table 3, the total mean sum scores of HADS were 5·9 points (95% CI: 3·9–8·0, p < 0·001) higher in women reporting fatigue. The women had a higher score on both subscales of HADS (both p-values < 0·001). Almost all of the women (14/15, 94%) with depression (HADS-D score ≥ 8) reported fatigue, and a majority (21/27, 78%) of the women with anxiety (HADS-A score ≥ 8) also reported fatigue.
Table 3.
Mean values and differences for the self-report scale Hospital Anxiety and Depression Scale (HADS) in fatigued and nonfatigued gynaecological patients with cancer. Positive differences indicate more anxiety/depression for women with fatigue.
| Nonfatigued Mean, SD, n (95% CI) | Fatigued Mean, SD, n (95% CI) | p-value | Difference (95% CI) | |
|---|---|---|---|---|
| HADS anxiety | 3·5, 2·9, 56 (2·8–4·3) | 6·4, 3·6, 63 (7·3–5·5) | <0·001 | 2·9 (1·7–4·0) |
| HADS depression | 1·6, 2, 56 (1·0–2·1) | 4·7, 3·7, 64 (5·6–3·7) | <0·001 | 3·1 (2·1–4·2) |
| HADS total | 5·1, 4·4, 56 (3·9–6·3) | 11, 6·9, 63 (12·7–9·3) | <0·001 | 5·9 (3·9–8) |
Exercise
There were statistically significant differences in the number of weekly exercises, where nonfatigued women reported to exercise more frequently (p = 0·03); see Table 1.
Capacity for work
A total of 41% of the women in paid work reported that it was harder to perform the same job after returning from sick leave than before they became ill, whereas the fatigued were more negatively affected compared with the nonfatigued (p < 0·001). Approximately 26% reported that they had reduced their working hours because of the disease, and 18% had received work accommodation because of the illness. However, there were no significant differences in reports of having reduced capacity for work according to fatigue.
Logistic regression
When adjusted for age or for age and HADS in a logistic model, the association between fatigue and diagnosis disappeared; see Table 4. This indicates that the type of cancer matters little in determining whether a woman will experience fatigue, and the apparent association is mainly a result of different types of cancers occurring for women in different age groups. However, this does not imply that (younger) age is a causal mechanism in fatigue. All the women had experienced cancer and cancer treatment, which is undoubtedly the main cause of the fatigue. But for a woman of a given age, the type of cancer she has cannot be used to predict whether she will experience post-treatment fatigue.
Table 4.
Logistic regression analysis of factors related to self-reported fatigue (n = 117). Tjur's D = 0·29 (Tjur 2009)
| Variables | Odds ratio (adjusted) | p-value (adjusted) | Odds ratio (unadjusted) | p-value (unadjusted) |
|---|---|---|---|---|
| Age | 0·96 (0·92–0·99) | 0·04 | 0·95 (0·92–0·98) | 0·002 |
| Diagnosis | 0·32 | 0·06 | ||
| Cervical (ref.) | 1 | 1 | ||
| Uterine | 0·77 (0·22–2·71) | 0·34 (0·12–0·86) | ||
| Ovarian | 1·78 (0·50–6·68) | 0·83 (0·28–2·40) | ||
| Vulva | 0·30 (0·01–4·60) | 0·24 (0·01–2·79) | ||
| HADS | 1·22 (1·12–1·34) | <0·001 | 1·22 (1·12–1·34) | <0·001 |
Discussion
The main findings in this study show that 53% of the women reported cancer-related fatigue following gynaecological cancers. This is a higher percentage than in comparable studies on gynaecological cancers, reporting a prevalence from 17–33% over the different cancer types (Holzner et al. 2003, Liavaag et al. 2007, Vistad et al. 2007, Harrington et al. 2010). This difference in prevalence may partly be explained by differences in time from treatment to follow-up. In the present study, we had a relatively short follow-up time (mean 16 months). However, for our sample of patients, we found no association between follow-up time and fatigue, and we hypothesise that the reduction in prevalence of fatigue levels off slowly, over many years.
There were significant differences related to fatigue between the groups of gynaecological cancers, with women treated for cervical and ovarian cancer having the highest proportion of fatigue. At first glance, one might think that this makes sense due to the fact that women with cervical and ovarian cancer receive radiation and/or chemotherapy, thus leading to a strong impact on QoL and cancer-related fatigue (Carlsson et al. 2000, Chan et al. 2001, Jereczek-Fossa et al. 2002, Payne 2002, Greimel et al. 2009, Gonçalves 2010, Bjelic-Radisic et al. 2012). However, when adjusting for age, the association between fatigue and diagnosis disappears (while adjusting for diagnosis, there is still a significant association between fatigue and age). Furthermore, while age is associated with diagnosis, there is considerable overlap in age for women with different cancers. All this indicates that for a woman of a given age, the type of cancer she experiences has little impact on her risk of experiencing fatigue.
Our study shows that younger women report fatigue and poorer QoL more frequently than older women. Some other recent studies on young adults with various cancers (Smith et al. 2013, Geue et al. 2014), breast cancer (Arndt et al. 2004) and gynaecological cancer (Bifulco et al. 2012) support findings that younger women have lower scores on several QoL dimensions, including fatigue, than older women. In the latter comparison study between young and midlife survivors of gynaecological cancers, Bifulco et al. (2012) concluded that younger women (below age 45) were more affected by fatigue and global health status. Furthermore, in a systematic review on health-related QoL in younger breast cancer survivors (Howard-Anderson et al. 2012) and, more specifically, in a longitudinal study on gynaecological cancers (Chan et al. (2001), the overall QoL scores were lower for younger patients than for older. On the contrary, other previous studies have shown the opposite results, namely that QoL and fatigue outcome worsen with age (Cella et al. 2002, Butt et al. 2010). Thus, understanding the relationship between age and fatigue has some challenges. Although young age is generally associated with health, vitality and long-term planning (Chan et al. 2001), a possible interpretation is that younger patients find it more difficult to accept a cancer diagnosis during early adulthood and family establishment (Chan et al. 2001). Younger women may also view cancer as a greater threat to their lives than older patients (Arndt et al. 2004). Additionally, one may ask whether older women tend to regard symptoms of fatigue as a normal process of ageing and thus may underreport such symptoms. Older persons may also consider physical health in different ways and assess their health compared with their age peers (Arndt et al. 2004). An overview on QoL in older breast cancer patients indicates that older patients are perhaps better equipped mentally to deal with treatment (Ballinger & Fallowfield 2009). Another explanation might be that younger women have fewer coping strategies and resources that are needed to manage a life-threatening disease (Arndt et al. 2004). Given these results, it is essential that the QoL issues and fatigue are given attention during treatment and follow-up for all age groups and with a special focus on younger women.
In the present study, both depression and anxiety were strongly linked to fatigue. Nearly all of the women with depression (94%) and a majority of the women with anxiety (78%) reported fatigue. This correlation between psychological distress and fatigue is also found in several studies concerning cancer in general (Brown & Kroenke 2009, Oh & Seo 2011), as well as in gynaecological cancer studies (Ferrell et al. 2003, Liavaag et al. 2007, Vistad et al. 2007, Harrington et al. 2010, Prue et al. 2010). A systematic review by Oh and Seo (2011) indicates that psychological distress seems to have a higher level of association with cancer-related fatigue than the more physical symptom distress. A systematic review by Brown and Kroenke (2009) (sample size 12,103) confirms the association of fatigue with depression and anxiety. The review supported the conclusion of psychological correlation of cancer-related fatigue, in line with some other previous studies (Donovan & Ward 2005, Jacobsen et al. 2007). However, the possible relationship and the direction of causality (fatigue, anxiety and depression) are still unclear, despite a recent increase in research interest. Studies with longitudinal designs may give more knowledge about these relationships.
For gynaecological cancer, a strong association between cancer-related fatigue and psychological distress is reported in several studies (Liavaag et al. 2007, Vistad et al. 2007, Prue et al. 2010). Vistad et al. (2007) found that there was an association between cancer-related fatigue and depression and anxiety in survivors after radiation for cervical cancer. Liavaag et al. (2007) showed that symptoms of anxiety and fatigue were more prevalent than those of depression in survivors after ovarian cancers.
The women in our study who suffer from fatigue also scored significantly lower on all eight domains of QoL, as measured by the SF-36 questionnaire. Several other QoL studies of gynaecological cancer have shown similar results (Ahlberg et al. 2003, Holzner et al. 2003, Vistad et al. 2007, Liavaag et al. 2008, Arriba et al. 2010). The fatigued women had lowest scores on the domains ‘physical role function’, ‘emotional role function’ and ‘vitality/energy.’ This confirms that cancer-related fatigue has a strong impact on women's lives after treatment for gynaecological cancer. It might decrease the women's ability to carry out everyday activities and may affect their quality of life. In the qualitative study by Ferrell et al. (2003), the women reported frustration and guilt regarding the impact of fatigue on daily functioning. It is also likely that QoL and particularly ‘role function’ and ‘vitality’ affect deeper layers of a woman's life and identity. Thus, fatigue seems to have a greater impact on daily activities than other conditions associated with cancer.
Limitations of the study
Questionnaire-collected data may have both advantages and disadvantages. The difficulty level might influence forced-choice answers in a negative way. The 120 women who answered the questionnaire had agreed to participate in an intervention study (n = 130). The results may not be generalisable to the general population of gynaecological cancer survivors and must be interpreted with caution. As data were collected retrospectively, we do not know whether quality of life and fatigue differences might have existed in the groups before therapy. Furthermore, selection bias will be present if the participants in this study have a higher or lower quality of life than those who have refused to participate.
Conclusion
The findings underscore the importance of and need for follow-up in which screening for QoL, fatigue and symptom management should be a standard procedure. Both somatic and psychological aspects of fatigue must be emphasised. Nurses, as well as other health personnel, should make additional efforts to ask and educate patients about fatigue and to provide self-care advice for coping with fatigue.
Relevance to clinical practice
Health personnel need to pay special attention to fatigue and QoL issues during the entire trajectory of women diagnosed with gynaecological cancers, and likewise to the need for screening, information, guidance and symptom management. This concerns women of all age groups and particularly younger women.
Acknowledgments
We wish to thank the women who participated in the intervention study.
Disclosure
The authors have confirmed that all authors meet the ICMJE criteria for authorship credit (http://www.icmje.org/ethical_1author.html) as follows: (1) substantial contributions to conception and design of, or acquisition of data or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content and (3) final approval of the version to be published.
Funding
The main funding is provided by the Norwegian Cancer Society and Grieg Foundation. The study is also supported by the Department of Obstetrics and Gynecology, Haukeland University Hospital.
Conflict of interest
The authors are solely responsible for the content and writing of the paper. The authors report no conflicts of interest.
The Journal of Clinical Nursing (JCN) is an international, peer reviewed journal that aims to promote a high standard of clinically related scholarship which supports the practice and discipline of nursing.
For further information and full author guidelines, please visit JCN on the Wiley Online Library website: http://wileyonlinelibrary.com/journal/jocn
Reasons to submit your paper to JCN:
High-impact forum: one of the world's most cited nursing journals, with an impact factor of 1·316 – ranked 21/101 (Nursing (Social Science)) and 25/103 Nursing (Science) in the 2012 Journal Citation Reports® (Thomson Reuters, 2012).
One of the most read nursing journals in the world: over 1·9 million full text accesses in 2011 and accessible in over 8000 libraries worldwide (including over 3500 in developing countries with free or low cost access).
Early View: fully citable online publication ahead of inclusion in an issue.
Fast and easy online submission: online submission at http://mc.manuscriptcentral.com/jcnur.
Positive publishing experience: rapid double-blind peer review with constructive feedback.
Online Open: the option to make your article freely and openly accessible to non-subscribers upon publication in Wiley Online Library, as well as the option to deposit the article in your preferred archive.
References
- Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. The Lancet. 2003;362:640–650. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- Ahlberg K, Ekman T, Gaston-Johansson F. Fatigue, psychological distress, coping resources, and functional status during radiotherapy for uterine cancer. Oncology Nursing Forum. 2005;32:633–640. doi: 10.1188/05.ONF.633-640. [DOI] [PubMed] [Google Scholar]
- Arndt V, Merx H, Stürmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. European Journal of Cancer. 2004;40:673–680. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Arriba LN, Fader AN, Frasure HE, von Gruenigen VE. A review of issues surrounding quality of life among women with ovarian cancer. Gynecologic Oncology. 2010;119:390–396. doi: 10.1016/j.ygyno.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Ballinger RS, Fallowfield LJ. Quality of life and patient-reported outcomes in the older breast cancer patient. Clinical Oncology. 2009;21:140–155. doi: 10.1016/j.clon.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Beesley V, Eakin E, Steginga S, Aitken J, Dunn J, Battistutta D. Unmet needs of gynaecological cancer survivors: implications for developing community support services. Psycho-Oncology. 2008;17:392–400. doi: 10.1002/pon.1249. [DOI] [PubMed] [Google Scholar]
- Bifulco G, De Rosa N, Tornesello ML, Piccoli R, Bertrando A, Lavitola G, Morra I, Sardo A, Buonagur FM, Nappi C. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecologic Oncology. 2012;124:444–451. doi: 10.1016/j.ygyno.2011.11.033. [DOI] [PubMed] [Google Scholar]
- Bjelic-Radisic V, Jensen PT, Vlasic KK, Waldenstrom A-C, Singer S, Chie W, Nordin A, Greimel E. Quality of life characteristics inpatients with cervical cancer. European Journal of Cancer. 2012;48:3009–3018. doi: 10.1016/j.ejca.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. Journal of Psychosomatic Research. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Brown LF, Kroenke K. Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics. 2009;50:440–447. doi: 10.1176/appi.psy.50.5.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt Z, Rao AV, Lai J-S, Abernethy AP, Rosenbloom SK, Cella D. Age-associated differences in fatigue among patients with cancer. Journal of Pain and Symptom Management. 2010;40:217–223. doi: 10.1016/j.jpainsymman.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M, Strang P, Bjurström C. Treatment modality affects long-term quality of life in gynaecological cancer. Anticancer Research. 2000;20:563–568. [PubMed] [Google Scholar]
- Cella M, Chalder T. Measuring fatigue in clinical and community settings. Journal of Psychosomatic Research. 2010;69:17–22. doi: 10.1016/j.jpsychores.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Cella D, Lai J-S, Chang C-H, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, Wallace EP. Development of a fatigue scale. Journal of Psychosomatic Research. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- Chan YM, Ngan HYS, Li BYG, Yip AMW, Ng TY, Lee PWH, Yip PSF, Wong LC. A longitudinal study on quality of life after gynecologic cancer treatment. Gynecologic Oncology. 2001;83:10–19. doi: 10.1006/gyno.2001.6345. [DOI] [PubMed] [Google Scholar]
- Donovan HS, Ward S. Representations of fatigue in women receiving chemotherapy for gynecologic cancers. Oncology Nursing Forum. 2005;32:113–116. doi: 10.1188/05.ONF.113-116. [DOI] [PubMed] [Google Scholar]
- Ferrell B, Smith S, Cullinane C, Melancon C. Symptom concerns of women with ovarian cancer. Journal of Pain and Symptom Management. 2003;25:528–538. doi: 10.1016/s0885-3924(03)00148-9. [DOI] [PubMed] [Google Scholar]
- Frumovitz M, Sun CC, Schover LR, Munsell MF, Jhingran A, Wharton JT, Eifel P, Bevers TB, Levenback CF, Gershenson DM, Bodurka DC. Quality of life and sexual functioning in cervical cancer survivors. Journal of Clinical Oncology. 2005;23:7428–7436. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- Geue K, Sender A, Schmidt R, Richter D, Hinz A, Schulte T, Brähler E, Stöbel-Richter Y. Gender-specific quality of life after cancer in young adulthood: a comparison with the general population. Quality of Life Research. 2014;23:1377–1386. doi: 10.1007/s11136-013-0559-6. [DOI] [PubMed] [Google Scholar]
- Gonçalves V. Long-term quality of life in gynecological cancer survivors. Current Opinion in Obstetrics and Gynecology. 2010;22:30–35. doi: 10.1097/GCO.0b013e328332e626. [DOI] [PubMed] [Google Scholar]
- Greimel ER, Winter R, Kapp KS, Haas J. Quality of life and sexual functioning after cervical cancer treatment: a long-term follow-up study. Psycho-Oncology. 2009;18:476–482. doi: 10.1002/pon.1426. [DOI] [PubMed] [Google Scholar]
- Harrington CB, Hansen JA, Moskowitz M, Todd BL. It's not over when it's over: long-term symptoms in cancer survivors – a systematic review. International Journal of Psychiatry in Medicine. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. The Oncologist. 2007;12(Suppl. 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Holzner B, Kemmler G, Meraner V, Maislinger A, Kopp M, Bodner T, Nguyen-Van-Tam D, Zeimet AG, Fleischhacker WW, Sperner-Unterweger B. Fatigue in ovarian carcinoma patients. Cancer. 2003;97:1564–1572. doi: 10.1002/cncr.11253. [DOI] [PubMed] [Google Scholar]
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. Journal of the National Cancer Institute. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Donovan KA, Vadaparampil ST, Small BJ. Systematic review and meta-analysis of psychological and activity-based interventions for cancer-related fatigue. Health Psychology. 2007;26:660–667. doi: 10.1037/0278-6133.26.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Critical Reviews in Oncology/Hematology. 2002;41:317–325. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- Korfage IJ, Essink-Bot M-L, Mols F, van de Poll-Franse L, Kruitwagen R, van Ballegooijen M. Health-related quality of life in cervical cancer survivors: a population-based survey. International Journal of Radiation*Oncology*Biology*Physics. 2009;73:1501–1509. doi: 10.1016/j.ijrobp.2008.06.1905. [DOI] [PubMed] [Google Scholar]
- Liavaag AH, Dorum A, Fossa SD, Trope C, Dahl AA. Controlled study of fatigue, quality of life, and somatic and mental morbidity in epithelial ovarian cancer survivors: how lucky are the lucky ones? Journal of Clinical Oncology. 2007;25:2049–2056. doi: 10.1200/JCO.2006.09.1769. [DOI] [PubMed] [Google Scholar]
- Liavaag AH, Dørum A, Bjøro T, Oksefjell H, Fosså SD, Tropé C, Dahl AA. A controlled study of sexual activity and functioning in epithelial ovarian cancer survivors. A therapeutic approach. Gynecologic Oncology. 2008;108:348–354. doi: 10.1016/j.ygyno.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Liavaag A, Dørum A, Fosså S, Tropé C, Dahl A. Morbidity associated with “self-rated health” in epithelial ovarian cancer survivors. BMC Cancer. 2009;9:1–11. doi: 10.1186/1471-2407-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loge JH, Ekeberg Ø, Kaasa S. Fatigue in the general norwegian population: normative data and associations. Journal of Psychosomatic Research. 1998a;45:53–65. doi: 10.1016/s0022-3999(97)00291-2. [DOI] [PubMed] [Google Scholar]
- Loge JH, Kaasa S, Hjermstad MJ, Kvien TK. Translation and performance of the Norwegian SF-36 health survey in patients with rheumatoid arthritis. I. Data quality, scaling assumptions, reliability, and construct validity. Journal of Clinical Epidemiology. 1998b;51:1069–1076. doi: 10.1016/s0895-4356(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Oh HS, Seo WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews on Evidence-Based Nursing. 2011;8:191–201. doi: 10.1111/j.1741-6787.2011.00214.x. [DOI] [PubMed] [Google Scholar]
- Payne JK. The trajectory of fatigue in adult patients with breast and ovarian cancer receiving chemotherapy. Oncology Nursing Forum. 2002;29:1334–1340. doi: 10.1188/02.ONF.1334-1340. [DOI] [PubMed] [Google Scholar]
- Prue G, Allen J, Gracey J, Rankin J, Cramp F. Fatigue in gynecological cancer patients during and after anticancer treatment. Journal of Pain and Symptom Management. 2010;39:197–210. doi: 10.1016/j.jpainsymman.2009.06.011. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. URL http://www.R-project.org/ [Google Scholar]
- Smith AW, Bellizzi KM, Keegan THM, Zebrack B, Chen VW, Neale AV, Lynch CF. Health-related quality of life of adolescent and young adult patients with cancer in the united states: the adolescent and young adult health outcomes and patient experience study. Journal of Clinical Oncology. 2013;31:2136–2145. doi: 10.1200/JCO.2012.47.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R, Fitch MI. Supportive care needs of women with gynecologic cancer. Cancer Nursing. 2008;31:284–291. doi: 10.1097/01.NCC.0000305743.64452.30. [DOI] [PubMed] [Google Scholar]
- Stone PC, Minton O. Cancer-related fatigue. European Journal of Cancer. 2008;44:1097–1104. doi: 10.1016/j.ejca.2008.02.037. [DOI] [PubMed] [Google Scholar]
- Tjur T. Coefficients of determination in logistic regression models – a new proposal: the coefficient of discrimination. American Statistician. 2009;63:366–372. [Google Scholar]
- Vistad I, Fosså SD, Kristensen GB, Dahl AA. Chronic fatigue and its correlates in long-term survivors of cervical cancer treated with radiotherapy. BJOG: An International Journal of Obstetrics & Gynaecology. 2007;114:1150–1158. doi: 10.1111/j.1471-0528.2007.01445.x. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


