Abstract
Over the last four decades, increases in the incidence of immune-mediated diseases in the Western world have been linked to changes in microbial exposure. It is becoming increasingly clear that the normal microbiota in the gut can profoundly alter susceptibility to a wide range of diseases, such as asthma, in which immune homeostasis is disrupted, yet the mechanisms governing this microbial influence remains poorly defined. In this study, we show that gastrointestinal exposure to PSA, a capsular polysaccharide derived from the commensal bacterium Bacteroides fragilis, significantly limits susceptibility to the induction of experimental asthma. We report that direct treatment of mice with PSA generates protection from asthma, and this effect can be given to a naïve recipient by adoptive transfer of CD4+ T cells from PSA-exposed mice. Remarkably, we found that these PSA-induced T cells are not canonical FoxP3+ regulatory T cells, but that they potently inhibit both Th1 and Th2 models of asthma in an IL-10-dependent fashion. These findings reveal that bacterial polysaccharides link the microbiota with the peripheral immune system by activating CD4+Foxp3− T cells upon exposure in the gut, and they facilitate resistance to unnecessary inflammatory responses via the production of IL-10.
Keywords: asthma, Bacteroides fragilis, hygiene hypothesis, PSA
Introduction
When compared with developing and agrarian societies, Western societies have seen a dramatic increase in the prevalence of allergy and asthma in the past several decades. Among other factors influencing this trend, commensal bacteria, parasitic helminthes and the molecules they produce have been shown to play a significant role in immune system development (Mazmanian et al. 2005) and promoting homeostasis (Mazmanian and Kasper 2006; Maizels 2009; McSorely and Maizels 2012). The impact these components have on the immune system follows general predictions first made in 1989, now known as the hygiene hypothesis (Strachan 1989, 2000), which postulated that limiting exposure to microbes may lead to greater susceptibility to immune-mediated disease.
Connections between exposure to microbes and susceptibility to airway inflammation and asthma indicate that antigens from gut-resident commensal flora may be key in maintaining homeostasis within the lung environment (Ly et al. 2011). Beginning in the 1990s, studies on abscess responses (Tzianabos et al. 1993, 1994), postsurgical adhesion formation (Chung et al. 2002; Ruiz-Perez et al. 2005) and more recently inflammatory bowel disease (IBD) (Mazmanian et al. 2008) suggest that the polysaccharide PSA from the commensal anaerobic bacterium Bacteroides fragilis is a potent immunomodulatory molecule which induces an anti-inflammatory immune response. Although none of these prior disease models fit into the foundation of the hygiene hypothesis, it is clear that B. fragilis can influence the localized immunologic environment and that it is conceivable that such protective responses may also impact peripheral tissues and their susceptibility to inflammatory disease, as predicted by the hygiene hypothesis.
The protective function of commensal polysaccharide antigens like PSA is linked to their ability to activate CD4+ T cells (Chung et al. 2002, 2003). Upon uptake by professional antigen presenting cells, these “glycoantigens” are processed to low-molecular-weight fragments and loaded onto MHC class II molecules for recognition by αβ T-cell receptors (Cobb et al. 2004). This interaction with MHCII is high affinity (Cobb and Kasper 2008), promoted by the classical antigen exchange factor human leukocyte antigen DM (Cobb et al. 2004; Cobb and Kasper 2008; Velez et al. 2009), and is remarkably sensitive to the glycosylation pattern of MHCII (Ryan et al. 2011, 2013), yet the exact nature of the responding T cells is not fully understood. They have been characterized as T helper type 1 (Th1) cells based on their interferon gamma (IFNγ) expression (Brubaker et al. 1999; Wang et al. 2006) and proinflammatory impact (Tzianabos et al. 1993), but have also been identified as potent anti-inflammatory regulatory T cells (Tregs) which suppress lymphocyte activation and inflammation via IL-10 (Chung et al. 2002; Ruiz-Perez et al. 2005; Mazmanian et al. 2008; Kreisman and Cobb 2011). Some reports suggest that PSA responding T cells are Tregs that express the transcription factor FoxP3 most closely associated with the regulatory T-cell phenotype; however, the change in these classically defined FoxP3+ Tregs in these murine models are seldom >1–2% overall (Ochoa-Reparaz et al. 2010; Round and Mazmanian 2010). In contrast, we have previously found a distinct lack of FoxP3 expression in PSA responding T cells in human studies (Kreisman and Cobb 2011). To date, it remains unclear whether PSA and other glycoantigens induce traditional FoxP3+ or FoxP3− Tregs in murine models of disease.
Here, we have utilized a two variations of the ovalbumin (OVA)-induced model of asthma to investigate the influence of gastrointestinal exposure to a commensal glycoantigen on a peripheral tissue. We found that oral exposure to glycoantigen (GlyAg) robustly protects against asthma induction in an IL-10 and T-cell-dependent mechanism, and that the responding regulatory T cells do not express FoxP3. These findings demonstrate that a commensal-derived glycoantigen directly influences immune homeostasis in peripheral tissues and limits susceptibility to asthma, thereby demonstrating the power of the microbiota to shape disease outcome.
Results
Oral exposure to commensal-derived glycoantigen protects against airway inflammation
A Th1-driven OVA-induced model of asthma in C57Bl/6 mice is generally characterized by proinflammatory leukocyte infiltration into the alveolar space, pathologic changes in and around the airways, increased cytokine production, and activation of T cells within the lung-draining mediastinal lymph nodes. In order to begin understanding the relationship between microbiota-derived antigens and susceptibility to the development of asthma, we examined the impact of oral exposure to the GlyAg PSA from B. fragilis on preventing onset of inflammation. Compared with positive asthmatic controls, we found significant reductions in the infiltration of leukocytes (white blood cells; WBC; Figure 1A), neutrophils (Neuts; Figure 1B), lymphocytes (Lymph; Figure 1C) and monocytes/macrophages (Macs; Figure 1D) found within the bronchoalveolar lavage (BAL) samples of mice orally exposed to GlyAg. Due to this model being Th1 skewed, eosinophil numbers did not increase upon the induction of asthma, and they remain unchanged between all groups (data not shown).
Fig. 1.

Oral exposure to GlyAg suppresses asthmatic lung inflammation. (A–D) Differential analysis of cellular infiltrates from BAL samples of mice treated with GlyAg or vehicle control, with (gray bars) and without (white bars) intranasal OVA challenge to induce asthma, to quantify total WBCs, Neuts, Lymph and Macs. Pretreatment with GlyAg prevented leukocyte infiltration. (E) Lung homogenate supernatants were analyzed by ELISA for IFNγ, revealing the lack of significant inflammatory cytokine production in GlyAg-treated recipients. (F) CD4+ T cells were purified from the mediastinal lymph nodes from each experimental group and restimulated with anti-CD3/CD28 antibodies or media alone for 72 h to determine the activation status of regional T cells. GlyAg treatment maintained homeostatic T-cell activity levels compared with asthmatic mice showing expanded IFNγ-producing T cells. (G) CD4+ T cells from GlyAg or saline-treated mice were isolated from the spleen and restimulated with anti-CD3/CD28 antibodies. The IL-10 response of GlyAg-exposed T cells was greatly enhanced, suggesting that GlyAgs prevent asthmatic inflammation by T-cell-derived IL-10 secretion. (H) H&E stained sections (4×) and confocal IHC (40×) using EpCAM (green) and MPO (red) as markers to detect tissue pathology and cellular infiltrates. GlyAg prevented tissue pathology and activated leukocyte infiltration. These data demonstrate the prophylactic efficacy of GlyAg pretreatment. #P > 0.05, *P < 0.05.
Cytokine analyses of lung tissue homogenates further revealed that the IFNγ response seen in the positive asthmatic mice was eliminated in mice treated with GlyAg (Figure 1E). Moreover, CD4+ T cells that were purified from mediastinal lymph nodes and stimulated with anti-CD3/CD28 antibodies also showed baseline levels of IFNγ, in contrast to the 3-fold increase in cytokine seen from cells isolated from asthmatic mice (Figure 1F). Consistent with a shift in response towards an increasingly anti-inflammatory environment, anti-CD3-mediated re-activation of CD4+ T cells from the spleen of mice exposed to GlyAg showed marked increase in the production of the suppressive cytokine IL-10 (Figure 1G), supporting the notion that GlyAg stimulation leads to IL-10 secretion by T cells. Finally, myeloperoxidase-positive (MPO+) infiltrating leukocytes around the airways, while apparent in saline-treated mice, are absent in mice treated with GlyAg, in both hematoxylin and eosin (H&E) and confocal immunohistochemistry (IHC) studies (Figure 1H). Together, these data show the ability of microbial products to prevent inflammatory responses in the lung after exposure in the gut.
GlyAg-mediated asthma protection is driven by T cells
Previous data have suggested that the immunomodulatory properties of GlyAgs are T-cell mediated (Chung et al. 2002, 2003). However, this has primarily been shown for gut and peritoneal-localized inflammatory events, and the ability to inhibit peripheral tissue inflammation in a T-cell-dependent fashion is unclear. WT mice were orally treated with GlyAg as before, and splenic CD4+ T cells were adoptively transferred into naive WT mice 24 h prior to asthma induction. Transfer of GlyAg-experienced T cells robustly prevented cellular infiltration into the airways (Figure 2A–D). As before, eosinophil numbers were unchanged from baseline in all mice tested (data not shown). Inflammatory cytokines within the lung tissue (Figure 2E and F) as well as lung histology and IHC (Figure 2G) were also significantly reduced in GlyAg-experienced T-cell recipients. These data demonstrate that the inhibition of asthma induction by commensal-derived GlyAg is T-cell dependent.
Fig. 2.

GlyAg limits airway inflammation in a T-cell-dependent fashion. (A–D) Differential analysis of cellular infiltrates from BAL samples of WT mice receiving purified CD4+ T cells from WT mice treated with GlyAg or saline vehicle control, with (gray bars) and without (white bars) intranasal OVA challenge to induce asthma, showing that GlyAg-mediated protection from cellular infiltration into the airway is driven by T cells. (E and F) Lung homogenate supernatants were analyzed by ELISA for IFNγ and IL-4 as a measure of inflammatory state. GlyAg-experienced T cells suppressed expression of both T-cell cytokines. (G) H&E stained sections (4×) and confocal IHC (40×) using EpCAM (green) and MPO (red) showing the ability of GlyAg-experienced T cells to prevent tissue pathology and activated leukocyte infiltration. #P > 0.05, *P < 0.05.
In order to establish protective efficacy in an asthma model with a varied etiology, such as allergic T helper type 2 (Th2)-skewed asthma, WT mice were treated as before and CD4+ T cells were adoptively transferred directly into the transgenic OTII mice carrying a germ-line encoded T cells receptor specific for OVA. Despite a significantly more robust phenotype and inflammatory state, mice that received T cells from GlyAg-treated mice were still protected from asthmatic induction (Figure 3A–G). Unlike the WT model, the OTII mice show eosinophil infiltration as well as IgE production. Remarkably, we found that T cells from GlyAg-treated mice are able to inhibit eosinophil infiltration as well as serum IgE elevation (Figure 3E and F). Similarly, both H&E and confocal imaging of tissue sections show a significant reduction in inflammatory cells around both airways and blood vessels (Figure 3G). Together, these data establish the profound protective efficacy and T-cell dependency of oral GlyAg treatment in two murine models of asthma with varied etiology.
Fig. 3.

GlyAg is able to suppress both Th1- and Th2-skewed asthma. (A–E) Differential analysis of cellular infiltrates from BAL samples of OT-II mice receiving purified CD4+ T cells from WT mice treated with GlyAg or saline vehicle control, with (gray bars) and without (white bars) intranasal OVA challenge to induce asthma, to quantify leukocyte infiltration, including eosinophils (Eos), showing the suppression of Th2-driven leukocyte infiltration into the airways. (F) As a hallmark of Th2-skewed asthma, serum immunoglobulin titers were analyzed by ELISA for total IgE concentration. GlyAg-experienced T cells prevented IgE production. (G) H&E stained sections (4×) and confocal IHC (40×) using EpCAM (green) and MPO (red) showing that despite the altered etiology (Th2 versus Th1 as in Figures 1–2) and the more severe level of inflammation, GlyAg-experienced T cells robustly prevented the onset of all classical asthma inflammatory symptoms. #P > 0.05, *P < 0.05.
GlyAg-responding T cells are not canonical FoxP3+ regulatory T cells
There are disparities in the literature as to the role of the common CD4+FoxP3+ regulatory T cells (Tregs) in the suppressive effects of the GlyAg PSA. In humans, the responding population were clearly identified as FoxP3− (Kreisman and Cobb 2011), whereas murine reports have varied somewhat (Ochoa-Reparaz et al. 2010; Round and Mazmanian 2010). To determine whether GlyAg-induced FoxP3+ Tregs were responsible for asthma inhibition, FoxP3-GFP reporter mice were orally treated with GlyAg as before. Using sterile flow sorting, CD4+FoxP3− T cells were purified and transferred into naive recipients. Asthma pathogenesis in all measures (Figure 4A–E) was blocked equally well compared with the transfer of bulk CD4+ T cells (Figures 2 and 3), demonstrating that FoxP3+ Tregs are not the responding population to GlyAg stimulation, and are not directly responsible for the inhibition of asthma induction.
Fig. 4.
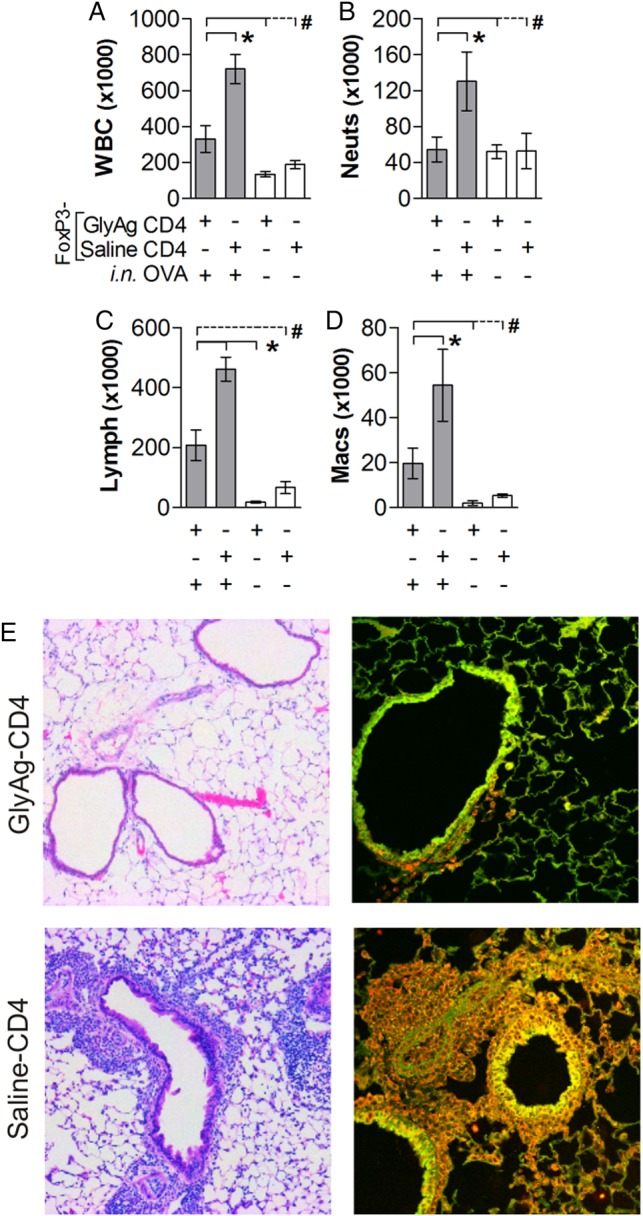
GlyAg-responding CD4+ T cells do not require FoxP3 to inhibit asthma. (A–D) Differential analysis of cellular infiltrates from BAL samples of mice receiving purified FoxP3-depleted CD4+ T cells from GlyAg or saline-treated FoxP3-eGFP reporter mice, with (gray bars) and without (white bars) intranasal OVA challenge to induce asthma. CD4+FoxP3− T cells from GlyAg-treated mice inhibited cellular infiltration despite lacking canonical regulatory T cells. (E) H&E stained sections (4×) and confocal IHC (40×) using EpCAM (green) and MPO (red) further demonstrate the lack of activated leukocytes within the tissues of mice provided GlyAg-experienced CD4+FoxP3− T cells. #P > 0.05, *P < 0.05.
In order to fully establish the lack of direct FoxP3+ Treg response against GlyAg, splenic T cells isolated from mice orally treated with GlyAg were also analyzed by flow cytometry. Consistent with the protection shown in Figure 4 and despite the lack of Foxp3+ T cells, we found no significant change in CD25+FoxP3−, CD25+FoxP3+, or CD25−FoxP3+ populations of Tregs resulting from GlyAg treatment (Figure 5), although we cannot rule out FoxP3 expression at later time points.
Fig. 5.
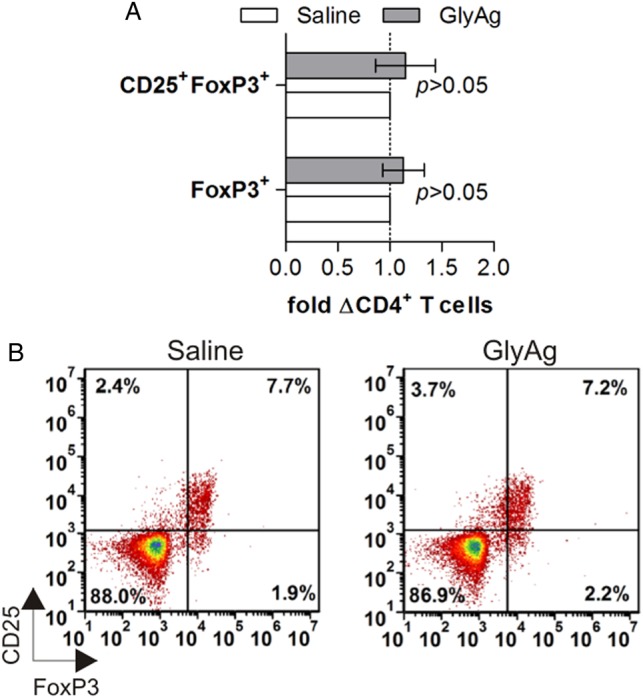
Oral GlyAg treatment does not alter FoxP3+CD4+ T cells. T cells were isolated from GlyAg-treated mice and stained for markers of regulatory T cells. (A) No change in the number of CD4+CD25+FoxP3+ or CD4+CD25−FoxP3+ across multiple experiments and mice were seen upon GlyAg exposure, consistent with the FoxP3-independent protection from asthma (P < 0.05). (B) Representative flow plots of T cells from GlyAg- and saline-treated mice.
Asthma inhibition by GlyAg is IL-10 dependent
Our previous work indicated that GlyAgs suppress proinflammatory T-cell activation through an IL-10-mediated mechanism in humans (Kreisman and Cobb 2011). In order to determine the mechanism by which GlyAg T cells suppress asthma, IL-10-null mice (IL-10n) were sensitized with OVA-Alum, and then OVA-specific enriched CD4+ cells were transferred into an naive IL-10n recipient which had been pretreated with GlyAg as before. We found that the ability to suppress asthma was completely lost in this IL-10-deficient model (Figure 6A–E). Finally, in order to determine whether GlyAg treatment induced OVA-specific T cells to switch to a regulatory/tolerant phenotype, IL-10n mice were directly treated with GlyAg as before, and then received IL-10-sufficient CD4+ OTII T cells prior to asthma induction. Despite the OTII cells being IL-10 sufficient, oral GlyAg treatment was unable to generate a protective response in the absence of IL-10 in the treated mice (Figure 6F–J). These data illustrate that GlyAg induces a separate population of non-canonical Tregs that lack FoxP3 expression but rely upon IL-10 production to reduce susceptibility to asthma.
Fig. 6.

Inflammatory inhibition requires IL-10 production. (A–D) Differential analysis of cellular infiltrates from BAL samples of GlyAg or saline-treated IL-10n mice receiving purified CD4+ T cells from OVA-sensitized IL-10n mice, with (gray bars) and without (white bars) intranasal OVA challenge to induce asthma. (E) H&E stained sections (4×) and confocal IHC (40×) using EpCAM (green) and MPO (red) from these same mice. These data demonstrate that expression of the inhibitory cytokine IL-10 is necessary for the suppressive activity of GlyAgs. In order to test whether the protective IL-10 is derived from GlyAg-induced changes in the OVA-specific T cells that normally cause the inflammation, IL-10n mice were first treated with GlyAg or saline control, then provided IL-10-sufficient OVA-specific OT-II mice. These mice were then challenged with and without intranasal OVA to induce asthma. (F–I) Differential analysis of cellular infiltrates from BAL samples (gray = OVA challenged; white = saline challenge control) demonstrate that GlyAg was unable to protect these mice from leukocyte infiltration. (J) H&E stained sections (4×) and confocal IHC (40×) using EpCAM (green) and MPO (red) confirm that GlyAg treatment requires IL-10 production, and that GlyAg treatment does not directly induce changes in the OVA-specific T cells causing disease.
Discussion
In this study, we show that intestinal exposure to the capsular glycoantigen PSA is able to inhibit asthma induction in a T-cell-dependent fashion. Moreover, we found that this protection is IL-10 dependent, and that the GlyAg-responding population of T cells are lacking the expression of the canonical transcription factor Foxp3, which directly recapitulates our observations with human cells (Kreisman and Cobb 2011). Importantly, asthma blockade was seen for both Th1- and Th2-skewed asthma, illustrating the robust bystander nature of the suppression mediated by PSA.
While several groups have examined the immunomodulatory properties of PSA, none have connected exposure to this commensal bacterial product to a condition directly tied to the hygiene hypothesis, such as asthma. Although it is all but certain that multiple pathways are likely at play with respect to how exposure to microbes and their products drive susceptibility to inflammatory disease, our findings provide one mechanism by which the hygiene hypothesis can be realized. In fact, a model system which replicates the ‘hygiene’ phenomenon (Strachan 1989, 2000) using the most relevant disease (asthma) allows for further investigation of how T cell responses in the mucosa driven by the microbiota actually communicate with the peripheral immune system. Moreover, it provides a means by which to understand how exposure to certain flora and environments provide protection from allergic diseases (Schaub et al. 2006; Illi et al. 2012), while exposure to other environmental stimuli, such as cockroaches, pet dander and house dust mites, promotes susceptibility to asthma (Sporik et al. 1990; Wahn et al. 1997).
Another aspect to consider is timing of antigen or bacterial exposure. The manner of initial colonization upon delivery, vaginal delivery when compared with a cesarean section, has implications for base line cytokine levels (Ly et al. 2006) and susceptibility to the development of asthma (Pistiner et al. 2008; Neu and Rushing 2011; Kristensen et al. 2014). Early exposure to prebiotics, such as non-digestible oligosaccharides, has been shown to reduce wheezing episodes 2 years after treatment (Arslanoglu et al. 2008), but there is little data to indicate if this protection persists into adulthood. Interestingly, recent epidemiological data indicates that although owning dogs provide a benefit for children in terms of reduced susceptibility to asthma, this protection is only true if the individual lives in a home with dogs within the first 2 years of life (de Meer et al. 2005). After this developmental period, the presence or absence of a household dog has no positive or negative impact on asthma susceptibility, indicating that the responses to microbial products are key to the appropriate development of a healthy immune system, and that they must occur very early in life. Consistent with this, there is conflicting evidence about the effectiveness of probiotics on protection from allergic diseases (reviewed in Hörmannsperger et al. 2012), suggesting that maximal benefit is obtained only while the immune system is in rapid development. With PSA as a model microbiota antigen, the critical window of exposure, as well as the critical components required, could be more clearly defined and earlier steps could be taken to harness the beneficial aspects of bacterial exposure.
Finally, the cellular mechanism of PSA action still needs to be further defined. The work centering around B. fragilis and PSA has been used as a model for the effect of gut commensals on overall health (Chung et al. 2002; Cobb et al. 2004; Mazmanian et al. 2005; Ruiz-Perez et al. 2005; Wang et al. 2006; Cobb and Kasper 2008; Velez et al. 2009; Kreisman and Cobb 2011; Ryan et al. 2011); however, even within this body of work, the apparent duality of proinflammatory versus anti-inflammatory effects of the microbiota are readily observed. Early work with B. fragilis focused on activating CD4+ T cells instrumental in generating proinflammatory responses leading to intra-abdominal abscesses (Tzianabos et al. 1993; Gibson et al. 1998; Chung et al. 2003) and later these same T cells were identified as the causative agents for postsurgical adhesions (Chung et al. 2002); yet colonization with B. fragilis or treatment with purified PSA in the absence of an adjuvant can result in exactly the opposite by limiting or preventing the induction of inflammatory conditions such as IBD (Mazmanian et al. 2008), intra-abdominal abscesses (Tzianabos et al. 1995, 1998), and postsurgical adhesions (Chung et al. 2002). If probiotic-styled approaches from either live bacteria or their products are to be translated into the clinic as treatments for inflammatory disease, this apparent duality in response must be addressed. In fact, critical questions remain about the subset of T cells directly responding to PSA and B. fragilis, a gap in our knowledge that is also true for essentially all microbiota studies. Indeed, the global influence of the microbiota on the physiology of the host is now clear, yet our understanding of the regulatory mechanisms at play is critical in furthering the development of PSA, other glycoantigens, and all other probiotics as potential therapeutics for asthma and other immune-mediated diseases.
In summary, we demonstrate that gastrointestinal exposure to a capsular polysaccharide from a commensal bacterium inhibits asthma induction, and that this process is dependent on the activation of CD4+Foxp3− T cells and the subsequent T-cell-driven production of the anti-inflammatory cytokine IL-10. This glycoantigen-driven inhibition was shown to be robust in both Th1- and Th2-skewed disease models, providing the basis for the use of PSA and the downstream immune pathway for the potential treatment of the wide manifestations of human asthma.
Materials and methods
Mice and bacteria
WT C57Bl/6, OTII and IL-10n breeding pairs were obtained from Jackson Labs and housed in specific pathogen free (and B. fragilis-free) conditions according to guidelines established by the Institutional Animal Care and Use Committee of Case Western Reserve University in Cleveland, OH, USA. FoxP3-eGFP reporter mice were a kind gift from Dr. A. Rudensky and were likewise housed. All mice were on the C57Bl/6 background. Experimental mice were 7–12 weeks of age. B. fragilis was grown in anaerobic conditions, and PSA (GlyAg) was purified as previously described (Gibson et al. 1998; Cobb et al. 2004). For all GlyAg treatments, mice were orally gavaged with GlyAg over 12 days (100 μg/dose in saline every 3 days) (Ochoa-Reparaz et al. 2010). Negative controls utilized saline vehicle alone.
Airway inflammation models
CD4+ splenocytes from OT-II mice were purified by magnetic bead-positive selection (Miltenyi), and 2 × 106 cells were transferred intravenously into naive recipient mice. Beginning 24 h later, the mice received intranasal OVA (40 μg/dose in PBS; Sigma) for six consecutive days before being sacrificed on Day 7. For intranasal challenge, mice were anesthetized using a table top anesthesia system (Vet Equip) with 3% isoflurane (Baxter). For co-transfer experiments, CD4+ splenocytes were purified as before from GlyAg or mock saline-treated mice (WT, IL-10n or FoxP3-eGFP), and 2 × 106 T cells were transferred into naive recipient mice at the same time as the OT-II CD4+ splenocytes before beginning the OVA challenges. Similarly, for direct transfer into OTII mice, 2 × 106 CD4+ cells from treated WT mice were purified and transferred as above. Euthanasia was performed with a mixture of 8.6% ketamine (Fort Dodge), 1.7% xylazine (Anased) and 2.9% acepromazine (Boehringer Ingelheim) in sterile saline. Mice were dosed at 0.006 cc/g. Blood was taken via cardiac puncture and centrifuged in Microtainer serum collection tubes (BD Biosciences) and serum was stored at −80°C until use. Mice were then given a tracheotomy, and lungs were flushed with 1 mL PBS containing 0.6 mM EDTA three times. Cells from these washes were collected, resuspended in 50 μL PBS with 0.6 mM EDTA and automated differentials were performed on a Hemavet 950 Hematology Analyzer. Right lungs were inflated with OCT (Tissue-Tek) and fixed in 10% formalin prior to paraffin embedding and sectioning at the Case Western Reserve University (CWRU) Tissue Procurement and Histology Core Facility. Left lungs were homogenized and incubated in 500 μL media for 24 h. The media supernatant from these incubations was analyzed for cytokine levels by standard sandwich ELISA (BioLegend).
Sterile T-cell sorting
Sterile flow sorting of cell populations was performed in the Department of Pathology Flow Cytometry Core on a FACSAria (BD Biosciences).
Histology
The H&E staining of lung sections was performed by the CWRU Tissue Procurement and Histology Core Facility. Unstained slides were stained with antibodies specific for EpCAM (6 µg/mL; eBioscience) and MPO (1 : 100, Abcam; anti-rabbit secondary: 1 : 1000, Invitrogen).
General data analyses
All data are shown as mean ± SEM. Mice included a minimum of four animals per group per replicate experiment. Graphs and statistical measures were generated with GraphPad Prism v5.0 graphing software. Comparisons between multiple groups were done using an ANOVA test, whereas comparisons between two groups (where appropriate) were done using a Student's t-test.
Funding
This work was supported by grants from the American Asthma Foundation (#10-0187) and the National Institutes of Health (GM082916, OD004225 to B.A.C.) and National Institutes of Health (AI089474 to J.L.J.).
Conflict of interest statement
None declared.
Abbreviations
BAL, bronchoalveolar lavage; CWRU, Case Western Reserve University; GlyAg, glycoantigen; IBD, inflammatory bowel disease; IHC, immunohistochemistry; Lymph, lymphocytes; Macs, monocytes/macrophages; MPO+, myeloperoxidase positive; Neuts, neutrophils; OVA, ovalbumin; Th1, T helper type 1; Th2, T helper type 2; WBC, white blood cells.
Acknowledgements
The authors thank Drs Elizabeth Pierce and Tracey Bonfield for their assistance in establishing asthma models, and Dr Sean Ryan, Lili Zhang and Lori Kreisman for technical assistance.
References
- Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- Brubaker JO, Li Q, Tzianabos AO, Kasper DL, Finberg RW. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: Differential stimulatory effect on mouse and rat lymphocytes in vitro. J Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- Chung DR, Chitnis T, Panzo RJ, Kasper DL, Sayegh MH, Tzianabos AO. CD4+ T cells regulate surgical and postinfectious adhesion formation. J Exp Med. 2002;195:1471–1478. doi: 10.1084/jem.20020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DR, Kasper DL, Panzo RJ, Chtinis T, Grusby MJ, Sayegh MH, Tzianabos AO. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Kasper DL. Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology. 2008;18:707–718. doi: 10.1093/glycob/cwn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meer MG, Janssen NA, Brunekreef B. Early childhood environment related to microbial exposure and the occurrence of atopic disease at school age. Allergy. 2005;60:619–625. doi: 10.1111/j.1398-9995.2005.00746.x. [DOI] [PubMed] [Google Scholar]
- Gibson FC, III, Onderdonk AB, Kasper DL, Tzianabos AO. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J Immunol. 1998;160:5000–5006. [PubMed] [Google Scholar]
- Hörmannsperger G, Clavel T, Haller D. Gut matters: Microbe-host interactions in allergic disease. J Allergy Clin Immunol. 2012;129:1452–1459. doi: 10.1016/j.jaci.2011.12.993. [DOI] [PubMed] [Google Scholar]
- Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, Büchele G, Boznanski A, Danielewicz H, Cullinan P, et al. Protection from childhood asthma and allergy in Alpine farm environments—The GABRIEL Advanced Studies. J Allergy Clin Immunol. 2012;129:1470–1477. doi: 10.1016/j.jaci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Kreisman LS, Cobb BA. Glycoantigens induce human peripheral Tr1 cell differentiation with gut-homing specialization. J Biol Chem. 2011;286:8810–8818. doi: 10.1074/jbc.M110.206011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen K, Fisker N, Haerskjold A, Ravn H, Simões FEA, Stensballe L. Caesarean section and hospitalization for respiratory syncytial virus infection: A population based study. Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000552. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: Interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127:1087–1094. doi: 10.1016/j.jaci.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly NP, Ruiz-Pérez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, Laskey D, Delaney ML, DuBois AM, Levy H, et al. Mod of delivery and cord blood cytokines: A birth cohort study. Clin Mol Allergy. 2006;4:13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM. Exploring the immunology of parasitism – from surface antigens to the hygiene hypothesis. Parasitology. 2009;136:1549–1564. doi: 10.1017/S0031182009006106. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6:849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McSorley HJ, Maizels RM. Helminth infections and host immune regulation. Clin Microbiol Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J, Rushing J. Cesarean versus vaginal delivery: Long-term outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38:321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón CJ. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–279. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez B, Chung DR, Sharpe AH, Yagita H, Kalka-Moll WM, Sayegh MH, Kasper DL, Tzianabos AO. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc Natl Acad Sci USA. 2005;102:16753–16758. doi: 10.1073/pnas.0505688102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SO, Bonomo JA, Zhao F, Cobb BA. MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. J Exp Med. 2011;208:1041–1053. doi: 10.1084/jem.20100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SO, Leal SM, Jr, Abbott DW, Pearlman E, Cobb BA. Mgat2 ablation in the myeloid lineage leads to defective glycoantigen T cell responses. Glycobiology. 2013;24:262–271. doi: 10.1093/glycob/cwt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–977. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Sporik R, Holgate ST, Platts-Mills ETA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. N Engl J Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP. Family size, infection and atopy: The first decade of the “hygiene hypothesis”. Thorax. 2000;55(Suppl. 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Gibson FC, Cisneros RL, Kasper DL. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J Infect Dis. 1998;178:200–206. doi: 10.1086/515594. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Kasper DL, Cisneros RL, Smith RS, Onderdonk AB. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Invest. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Onderdonk AB, Zaleznik DF, Smith RS, Kasper DL. Structural characteristics of polysaccharides that induce protection against intra-abdominal abscess formation. Infect Immun. 1994;62:4881–4886. doi: 10.1128/iai.62.11.4881-4886.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez CD, Lewis CJ, Kasper DL, Cobb BA. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology. 2009;127:73–82. doi: 10.1111/j.1365-2567.2008.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahn U, Lau S, Bergmann R, Kulig M, Forster J, Bergmann K, Bauer CP, Guggenmoos-Holzmann I. Indoor allergen exposure is a risk factor for sensitization during the first three years of life. J Allergy Clin Immunol. 1997;99:763–769. doi: 10.1016/s0091-6749(97)80009-7. [DOI] [PubMed] [Google Scholar]
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]


