Abstract
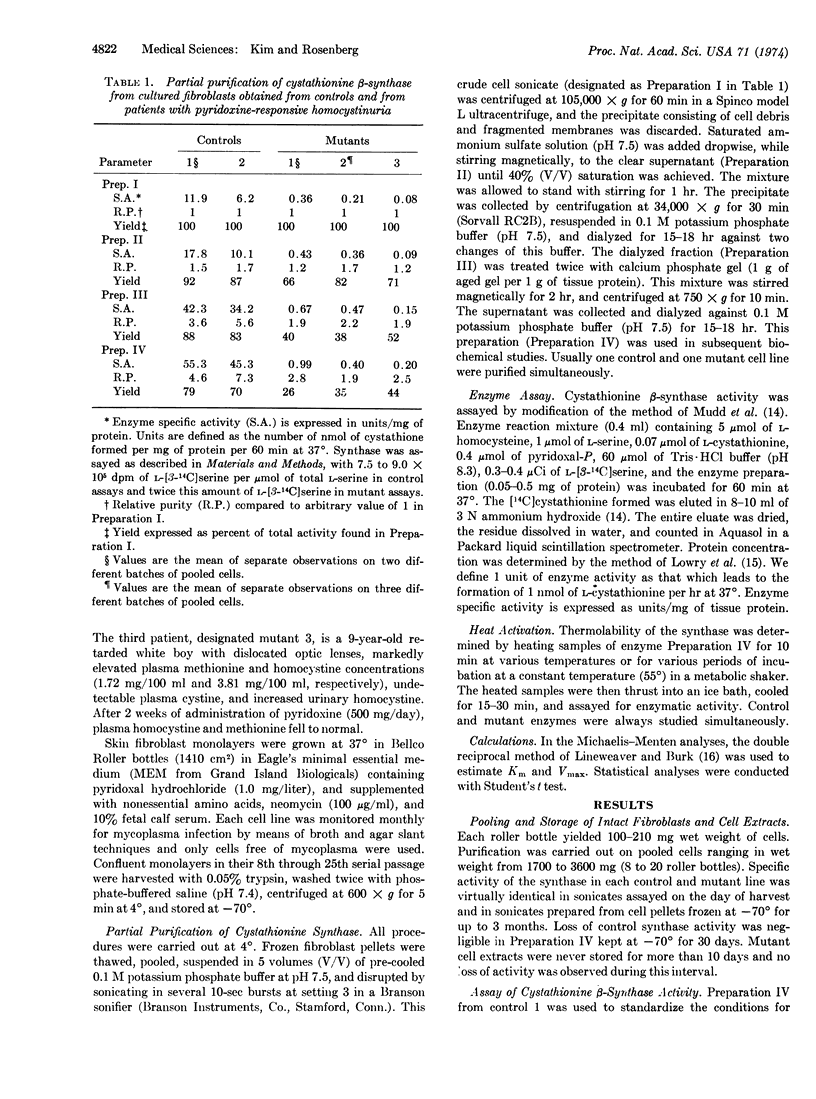
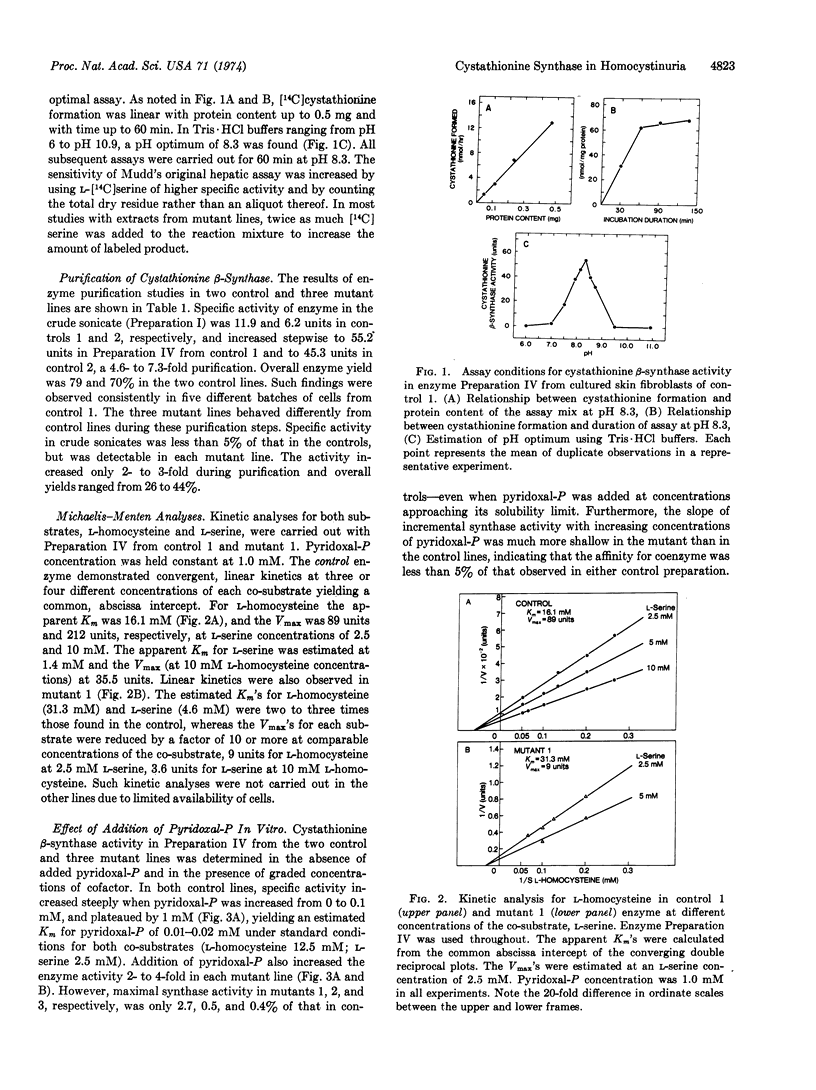
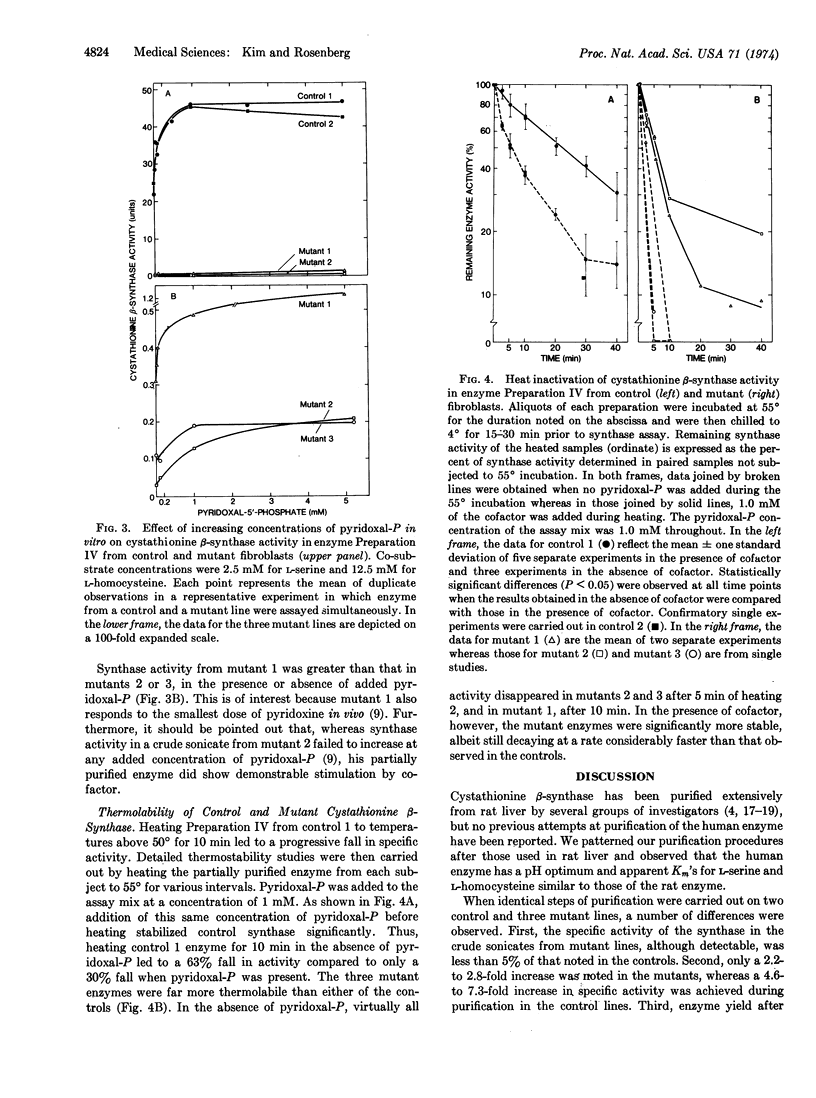
Cystathionine β-synthase [L-serine hydrolyase (adding homocysteine), EC 4.2.1.22] was studied in cultured skin fibroblasts from two control subjects and three patients with pyridoxine-responsive homocystinuria. In crude cell sonicates, cystathionine synthase activity detected in each mutant line was less than 5% of control values. After differential centrifugation, ammonium sulfate fractionation, and calcium phosphate gel treatment, the specific activity of synthase from control lines increased 5- to 7-fold with 70-79% yield. These same steps led to only 2- to 3-fold purification of mutant synthase and a reduced yield (26-44%). Michaelis-Menten analyses with the partially purified enzyme revealed that each mutant synthase had a marked reduction in affinity for its coenzyme, pyridoxal 5′-phosphate, as well as reduced affinity and maximum velocity for both co-substrates, L-homocysteine and L-serine. Even at saturating concentrations of coenzyme, mutant synthase activity was less than 3% of control. Mutant synthase was also far more thermolabile than control enzyme. In the absence of added coenzyme, heating for 10 min at 55° led to complete loss of mutant activity whereas control activity was reduced by 60%. Significantly, addition of saturating concentration of coenzyme prior to heating increased thermostability of both control and mutant synthase, the fractional increase being considerably greater in the mutants. We conclude that these patients suffer from a mutation of the synthase apoenzyme which impairs coenzyme binding, and that this primary abnormality results in reduced total enzyme activity in two ways: by reducing holoenzyme formation; and by accelerating apoenzyme degradation. We propose that pharmacologic amounts of pyridoxine increase holoenzyme formation modestly, thereby enhancing catalytic activity and slowing apoenzyme turnover.
Keywords: apoenzyme-coenzyme interaction, thermostability
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber G. W., Spaeth G. L. The successful treatment of homocystinuria with pyridoxine. J Pediatr. 1969 Sep;75(3):463–478. doi: 10.1016/s0022-3476(69)80274-x. [DOI] [PubMed] [Google Scholar]
- CARSON N. A., NEILL D. W. Metabolic abnormalities detected in a survey of mentally backward individuals in Northern Ireland. Arch Dis Child. 1962 Oct;37:505–513. doi: 10.1136/adc.37.195.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERRITSEN T., VAUGHN J. G., WAISMAN H. A. The identification of homocystine in the urine. Biochem Biophys Res Commun. 1962 Dec 19;9:493–496. doi: 10.1016/0006-291x(62)90114-6. [DOI] [PubMed] [Google Scholar]
- GREENGARD O. THE ROLE OF COENZYMES, CORTISONE AND RNA IN THE CONTROL OF LIVER ENZYME LEVELS. Adv Enzyme Regul. 1963;1:61–76. doi: 10.1016/0065-2571(63)90007-4. [DOI] [PubMed] [Google Scholar]
- Hayashi S. I., Granner D. K., Tomkins G. M. Tyrosine aminotransferase. Purificaton and characterization. J Biol Chem. 1967 Sep 25;242(18):3998–4006. [PubMed] [Google Scholar]
- Hollowell J. G., Jr, Coryell M. E., Hall W. K., Findley J. K., Thevaos T. G. Homocystinuria as affected by pyridoxine, folic acid, and vitamin B12. Proc Soc Exp Biol Med. 1968 Nov;129(2):327–333. doi: 10.3181/00379727-129-33314. [DOI] [PubMed] [Google Scholar]
- Kashiwamata S., Kotake Y., Greenberg D. M. Studies of cystathionine synthase of rat liver: dissociation into two components by sodium dodecyl sulfate disc electrophoresis. Biochim Biophys Acta. 1970 Sep 16;212(3):501–503. doi: 10.1016/0005-2744(70)90256-1. [DOI] [PubMed] [Google Scholar]
- Kimura H., Nakagawa H. Studies on cystathionine synthetase characteristics of purified rat liver enzyme. J Biochem. 1971 Apr;69(4):711–723. doi: 10.1093/oxfordjournals.jbchem.a129520. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litwack G., Rosenfield S. Coenzyme dissociation, a possible determinant of short half-life of inducible enzymes in mammalian liver. Biochem Biophys Res Commun. 1973 May 1;52(1):181–188. doi: 10.1016/0006-291x(73)90971-6. [DOI] [PubMed] [Google Scholar]
- MUDD S. H., FINKELSTEIN J. D., IRREVERRE F., LASTER L. HOMOCYSTINURIA: AN ENZYMATIC DEFECT. Science. 1964 Mar 27;143(3613):1443–1445. doi: 10.1126/science.143.3613.1443. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Edwards W. A., Loeb P. M., Brown M. S., Laster L. Homocystinuria due to cystathionine synthase deficiency: the effect of pyridoxine. J Clin Invest. 1970 Sep;49(9):1762–1773. doi: 10.1172/JCI106394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- Nakagawa H., Kimura H. Purification and properties of cystathionine synthetase synthetase from rat liver: separation of cystathionine synthetase from serine dehydratase. Biochem Biophys Res Commun. 1968 Jul 26;32(2):209–214. doi: 10.1016/0006-291x(68)90370-7. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Rosenberg L. E. Amino acid metabolism and its disorders. Major Probl Clin Pediatr. 1973;10:1–478. [PubMed] [Google Scholar]
- Seashore M. R., Durant J. L., Rosenberg L. E. Studies of the mechanism of pyridoxine-responsive homocystinuria. Pediatr Res. 1972 Mar;6(3):187–196. doi: 10.1203/00006450-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Uhlendorf B. W., Conerly E. B., Mudd S. H. Homocystinuria: studies in tissue culture. Pediatr Res. 1973 Jul;7(7):645–658. doi: 10.1203/00006450-197307000-00008. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Tada K., Yokoyama Y., Arakawa T. Homocystinuria of vitamin B6 dependent type. Tohoku J Exp Med. 1968 Nov;96(3):235–242. doi: 10.1620/tjem.96.235. [DOI] [PubMed] [Google Scholar]



