Abstract
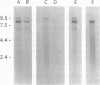
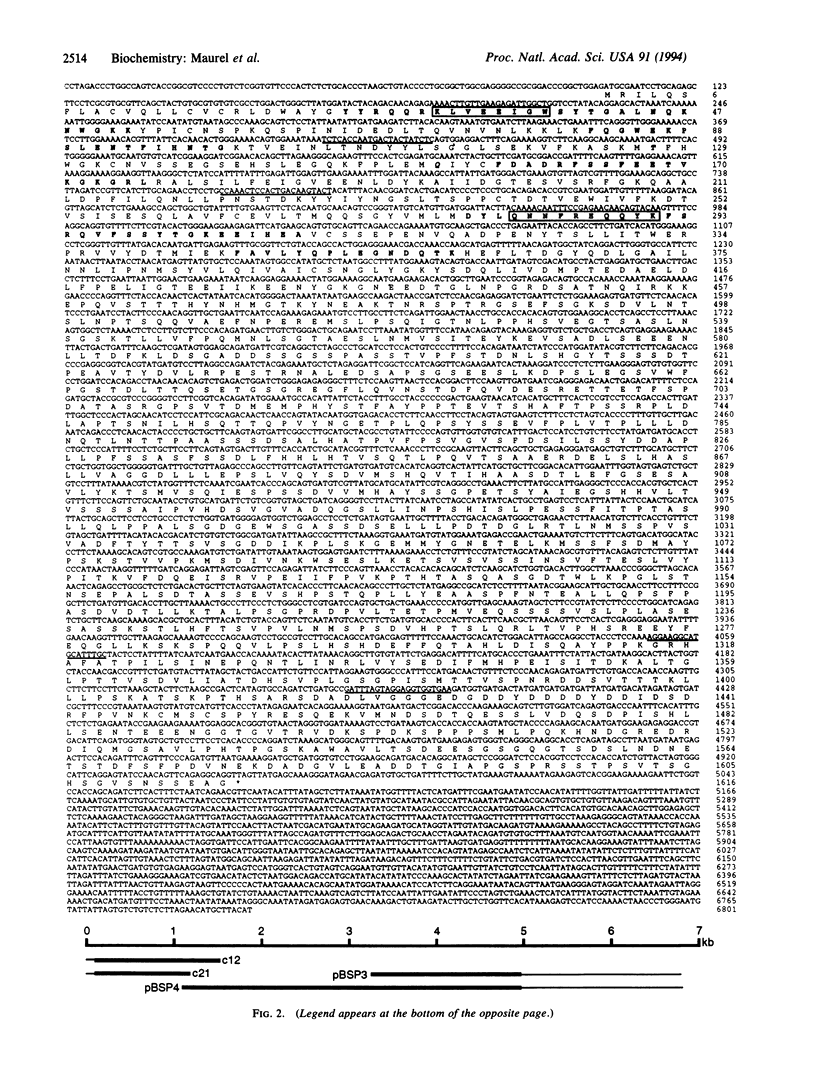
We have identified cDNA clones encoding a chondroitin sulfate proteoglycan of rat brain (previously designated 3F8 and now named phosphacan) that binds to neurons and neural cell-adhesion molecules. A sequence of 1616 amino acids deduced from a 4.8-kb open reading frame contains the N-terminal amino acid sequence of the 3F8 core glycoprotein as well as four internal CNBr, tryptic, and endoproteinase Lys-C peptide sequences from the proteoglycan. The deduced amino acid sequence, beginning with a 24-amino acid signal peptide, reveals an N-terminal domain of 255 amino acids homologous to carbonic anhydrases. The entire amino acid sequence deduced from our cDNA clones corresponds to the extracellular portion of a human receptor-type protein tyrosine phosphatase (RPTP zeta/beta) with which it has 76% identity, and the proteoglycan may represent an mRNA splicing variant of the larger transmembrane protein. RNA analysis demonstrated that a probe to the N-terminal carbonic anhydrase domain of the proteoglycan hybridizes with rat brain mRNA of 9.5, 8.4, and 6.4 kb, whereas probes to the phosphatase domains hybridize with only the 9.5-kb message and with the 6.4-kb message (which corresponds to a previously identified variant of the transmembrane protein in which half of the extracellular domain is deleted). The 30 N-terminal amino acids of the 3H1 chondroitin/keratan sulfate proteoglycan of brain are identical to those of the 3F8 proteoglycan, and six internal tryptic peptide sequences also matched those found in sequenced peptides of the 3F8 proteoglycan and/or amino acid sequences deduced from the cDNA clones. We therefore conclude that the 3H1 chondroitin/keratan sulfate proteoglycan and the 3F8 chondroitin sulfate proteoglycan represent glycosylation and possible extracellular splicing variants of a receptor-type protein tyrosine phosphatase. These proteoglycans may modulate cell interactions and other developmental processes in nervous tissue through heterophilic binding to cell-surface and extracellular matrix molecules, and by competition with ligands of the transmembrane phosphatase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldred P., Fu P., Barrett G., Penschow J. D., Wright R. D., Coghlan J. P., Fernley R. T. Human secreted carbonic anhydrase: cDNA cloning, nucleotide sequence, and hybridization histochemistry. Biochemistry. 1991 Jan 15;30(2):569–575. doi: 10.1021/bi00216a035. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S. M., Flint A. J., Tonks N. K. Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993 Aug;122(4):961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigan D. L. Great expectations: protein tyrosine phosphatases in cell regulation. Biochim Biophys Acta. 1992 Sep 14;1114(1):63–77. doi: 10.1016/0304-419x(92)90007-l. [DOI] [PubMed] [Google Scholar]
- Canoll P. D., Barnea G., Levy J. B., Sap J., Ehrlich M., Silvennoinen O., Schlessinger J., Musacchio J. M. The expression of a novel receptor-type tyrosine phosphatase suggests a role in morphogenesis and plasticity of the nervous system. Brain Res Dev Brain Res. 1993 Oct 15;75(2):293–298. doi: 10.1016/0165-3806(93)90035-9. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–493. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- Gebbink M. F., Zondag G. C., Wubbolts R. W., Beijersbergen R. L., van Etten I., Moolenaar W. H. Cell-cell adhesion mediated by a receptor-like protein tyrosine phosphatase. J Biol Chem. 1993 Aug 5;268(22):16101–16104. [PubMed] [Google Scholar]
- Grumet M., Flaccus A., Margolis R. U. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J Cell Biol. 1993 Feb;120(3):815–824. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Saito H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Finne J., Margolis R. K., Margolis R. U. Identification of an O-glycosidic mannose-linked sialylated tetrasaccharide and keratan sulfate oligosaccharides in the chondroitin sulfate proteoglycan of brain. J Biol Chem. 1986 Jun 25;261(18):8237–8242. [PubMed] [Google Scholar]
- Krusius T., Reinhold V. N., Margolis R. K., Margolis R. U. Structural studies on sialylated and sulphated O-glycosidic mannose-linked oligosaccharides in the chondroitin sulphate proteoglycan of brain. Biochem J. 1987 Jul 1;245(1):229–234. doi: 10.1042/bj2450229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. B., Canoll P. D., Silvennoinen O., Barnea G., Morse B., Honegger A. M., Huang J. T., Cannizzaro L. A., Park S. H., Druck T. The cloning of a receptor-type protein tyrosine phosphatase expressed in the central nervous system. J Biol Chem. 1993 May 15;268(14):10573–10581. [PubMed] [Google Scholar]
- Rauch U., Gao P., Janetzko A., Flaccus A., Hilgenberg L., Tekotte H., Margolis R. K., Margolis R. U. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified with monoclonal antibodies. J Biol Chem. 1991 Aug 5;266(22):14785–14801. [PubMed] [Google Scholar]
- Rauch U., Karthikeyan L., Maurel P., Margolis R. U., Margolis R. K. Cloning and primary structure of neurocan, a developmentally regulated, aggregating chondroitin sulfate proteoglycan of brain. J Biol Chem. 1992 Sep 25;267(27):19536–19547. [PubMed] [Google Scholar]
- Sap J., Jiang Y. P., Friedlander D., Grumet M., Schlessinger J. Receptor tyrosine phosphatase R-PTP-kappa mediates homophilic binding. Mol Cell Biol. 1994 Jan;14(1):1–9. doi: 10.1128/mcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S. S., Tsoulfas P., Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991 Nov 15;67(4):675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- Walton K. M., Dixon J. E. Protein tyrosine phosphatases. Annu Rev Biochem. 1993;62:101–120. doi: 10.1146/annurev.bi.62.070193.000533. [DOI] [PubMed] [Google Scholar]
- Yang X. H., Seow K. T., Bahri S. M., Oon S. H., Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991 Nov 15;67(4):661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]