Abstract
Severe sepsis often leads to multiple organ dysfunction syndromes (MODS) with acute kidney injury (AKI). AKI affects approximately, 35% of Intensive Care Unit patients, and most of these are due to sepsis. Mortality rate of sepsis-induced AKI is high. Inappropriate use of antimicrobials may be responsible for higher therapeutic failure, mortality rates, costs and toxicity as well as the emergence of resistance. Antimicrobial treatment is particularly difficult due to altered pharmacokinetic profile, dynamic changes in patient's clinical status and, in many cases, need for renal replacement therapy. This article aims to describe the appropriate antimicrobial dosing and reviews the factors contributing to the difficulties in establishing precise guidelines for antimicrobial dosing in sepsis-induced AKI patients. Search strategy: Text material was collected by systematic search in PubMed, Google (1978–2013) for original articles.
Keywords: Acute kidney injury, antimicrobial agents, critically ill patient, multiple organ dysfunction syndromes, pharmacodynamics, pharmacokinetics, sepsis
Introduction
Sepsis is a common heterogeneous clinical entity that is defined by the physiological changes collectively known as a systemic inflammatory response syndrome, which occurs in response to a presumed infectious etiology.[1] Severe sepsis and septic shock are frequent reasons for patient's admission to Intensive Care Units (ICU). In septic shock, patients fail to maintain their blood pressure despite adequate hydration. Severe sepsis is defined as sepsis plus sepsis-induced organ dysfunction or tissue hypoperfusion.[2] Severe sepsis often leads to multiple organ dysfunction syndromes (MODS) with acute kidney injury (AKI).[3] AKI affects approximately, 35% of ICU patients,[4] and around 50% of these are due to sepsis.[5] AKI has an overall mortality rate of 45%, mortality rate of sepsis-induced AKI is much higher, at over 70%.[4,6] A study from the USA by Angus and Wax[7] has reported that there are approximately, 7.5 lakh new cases of severe sepsis annually, with an economic impact approaching $17 billion, and it is the 3rd leading cause of infectious death and the 10th leading cause of death overall. There is a paucity of data in India regarding the true incidence and prevalence of AKI. In a study from North India, Kohli et al.,[8] reported the incidence of hospital-acquired AKI was 2.1/1000 admissions and the incidence of community-acquired AKI (CAAKI) was 6.6/1000 admissions. Similarly in recent study by Kaul et al. from North India, the prevalence of sepsis-induced CAAKI was 13.9% and overall mortality rate among patients with CAAKI was 26.2% but sepsis-induced CAAKI had the highest mortality. Majority of patients with CAAKI required dialysis mainly hemodialysis.[9] Thus, it has an enormous impact on resource depleted ICUs in developing the country like India. The only solution of such huge problem is an early institution of appropriate resources. Recent published Surviving Sepsis Campaign (SSC) guideline hoped that over time, particularly through education programs and feedback performance improvement initiatives, the guideline will influence bedside health care practitioner behavior that will reduce the burden of sepsis worldwide.[2] Appropriate antimicrobial treatment in terms of spectrum of activity or dose and frequency of administration will result in better outcomes in such patients.[10] However, because of various alterations in the pharmacokinetics (PK) of antimicrobials during sepsis, standard antimicrobial regimens can result in subtherapeutic serum drug concentrations in such patients.[11,12]
Disturbances of Renal Function in Critically Ill Patients
Critically ill-patients are diagnosed with various stages of AKI. Kidney Disease: Improving Global Outcomes have recently published new stages of AKI and their diagnostic criteria [Table 1].[13] Approximately, two-third of patients are diagnosed within the first 24 h after admission to the ICU.[6,14] It is emphasized that disturbances of renal function are not limited to glomerular filtration rate, but also affect the process of tubular secretion and reabsorption.
Table 1.
Stages of acute kidney injury according to KDIGO

Need for Individualized Antimicrobial Dosing in Sepsis-Induced Acute Kidney Injury
In addition to the in vitro susceptibility of the isolated strains and timely antimicrobial administration,[15] antimicrobial efficacy is dependent on the serum and tissue concentrations of the agent used.[16] Sepsis significantly alters the PK of antimicrobial agents, including increasing the volume of distribution (Vd), protein binding and drug clearance. The effect of hypoproteinemia, organ dysfunction and the presence of augmented renal clearance may lead to unexpectedly high or low plasma antimicrobial concentrations.[17] The problem of optimal antimicrobial doses becomes even more complex when there is concomitant renal failure because drug clearance is reduced, and accumulation of antimicrobials in the blood and tissues may potentially contribute to increased adverse side effects.[16]
Pharmacokinetics of antimicrobials in a heterogeneous group is altered to varying extent compared with a healthy population and their clinical state and drug PK can fluctuate significantly on the day to day basis. Therefore, indicators routinely employed in designing the antimicrobial regimen in individuals without organ dysfunction are entirely inadequate. In such patient, these disparities can result in inappropriate antimicrobial treatment.
Antimicrobial agents are a group of drugs with “silent” pharmacodynamics (PD) (i.e. the pharmacologic effect is not perceivable immediately after administration), it is almost impossible to assess whether therapeutic concentrations are being achieved during the early phase of therapy. Therefore, situations likely to alter antimicrobial PK [Figure 1] and necessitate dosage adjustment are necessary to enable the individualization of antimicrobial therapy.[18]
Figure 1.

Clinical scenarios likely to alter antimicrobial pharmacokinetics in Multiple Organ Dysfunction Syndrome[18]
Factors Affecting Antimicrobial Dosing in Patients with Acute Kidney Injury
Factors contribute to the difficulties in establishing precise guidelines for antimicrobial dosing in critically ill-patients with AKI [Table 2] are mainly: (1) Patient related (2) hemofiltration-related and (3) drug-related variables.
Table 2.
Factors contributing to the difficulties in establishing precise guidelines for antimicrobial dosing in patients with acute kidney injury

Patient-related variables
Most of the antimicrobials are acidic and protein binding is often significantly altered in critical illness due to the fall in serum albumin, decreased systemic pH and the presence of uremic toxins, bilirubin and free fatty acids, all of which may be present in renal failure and sepsis.[19,20,21] Most antimicrobial agents are eliminated via the kidney, and therefore a significant reduction in creatinine clearance may result in an extensive prolongation of the half-life of some antimicrobials. Hepatic metabolism and biliary or gut excretion may substantially increase in the presence of renal failure. Sepsis causes the damage of vascular endothelium with an increase of capillary permeability and redistribution of fluid into the extracellular compartment. As a result, Vd of water soluble antimicrobials increases with a subsequent drop in their concentration to the subtherapeutic level.
Hemodialysis and hemofiltration-related variables
Continuous venovenous hemofiltration (CVVH) removes plasma water by producing an ultrafiltrate and clears molecules of varying sizes by convection - by dragging molecules with the fluid. This process of the molecular clearance is influenced by:
-
The sieving coefficient of the molecules removed:
- Sieving coefficient is defined as the concentration of drug in the ultrafiltrate divided by mean of concentrations in pre-and post-filter blood and it reflects the capacity of a drug to pass through a hemofilter membrane. It varies from 0 (drugs that do not pass) to 1 (drugs that pass freely).
-
The ultrafiltration rate:
- In addition, drug clearance is directly proportional to the ultrafiltration rate; a higher proportion of the drug is removed at higher filtration rates.
-
The proportion of replacement fluid given predilution:
- Transfer of drug across the filter membrane depends on the concentration of drug. Infusion of a proportion of the total replacement fluid before the filter (predilution) may decrease local concentration and results in a decrease in drug clearance.
-
Membrane characteristics:
- Use of large surface area membranes and frequent changes of the filter membrane will increase the amount of drug being removed. Solute-membrane interaction, leading to the formation of plasma protein layers on the membrane and reduce its permeability.[22]
Drug-related variables
Several drug factors [Figure 2] play an important role in determining the final amount of drug removed by hemofiltration, notably:
Figure 2.

Drug related factors affecting antimicrobial dosing in critical ill patients
The molecular weight of the drug
Protein binding
The degree of renal clearance.
Molecular size influences drug clearance, as the contribution of convective transport relative to diffusion increases with increasing molecular weight of the drug.
Dosage Adaptations
Commonly used drug-dosing technique involves calculating the total creatinine clearance rate by adding any estimated residual renal creatinine clearance to the expected extracorporeal creatinine clearance. The extracorporeal creatinine clearance rate can be assumed to be approximately equivalent to the dialyzate, ultrafiltrate, or effluent rate, and medication dosing guidelines specified for the total creatinine clearance can be used to guide dose selection. This method assumes that drugs only undergo glomerular filtration, not tubular secretion or reabsorption.[23] For drugs that do undergo tubular secretion, this method could lead to increased drug exposures, and in patients with impaired reabsorption, underdosing can potentially occur. Thus, drug dosing technique on the basis of creatinine clearance rate is not effective.
Pharmacokinetics and Pharmacodynamic of Antimicrobials in Sepsis-Induced Acute Kidney Injury Patients
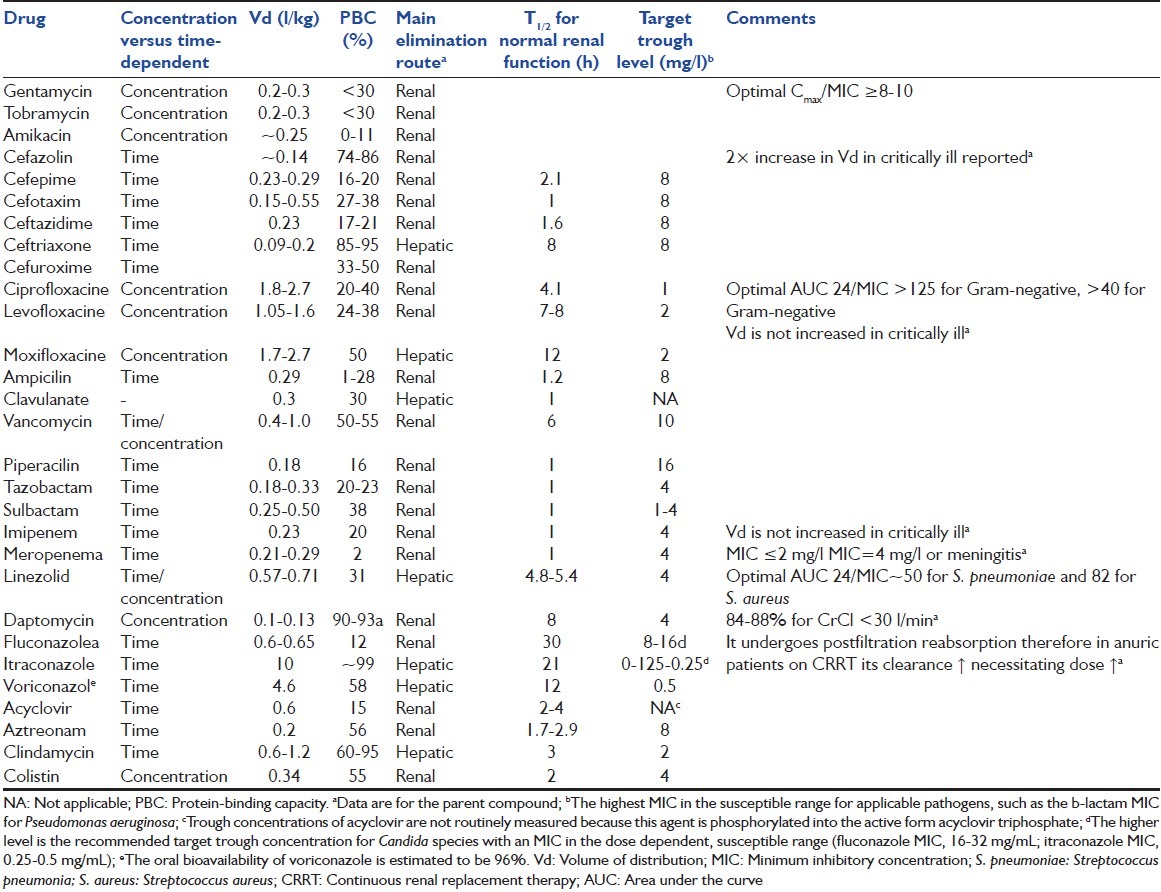
Intrinsic PK and PD properties are the major determinants of in vivo efficacy of antimicrobial agents.[24] The PK and PD properties of each antimicrobial agent and the typical susceptibilities of relevant pathogens are considered in Table 3.[25,26,27,28,29,30]
Table 3.
Pharmacokinetics
Pharmacokinetics is the study of the interrelationship between drug dose and variations in concentrations in plasma and tissue over time. The most relevant PK parameters [Figure 3] include the following.[31]
Figure 3.

Basic pharmacokinetic and pharmacodynamic parameters
Cmax: Peak concentration achieved after a single dose
Cmin: The lowest (trough) concentration that a drug reaches before the next dose is administered
Vd: The apparent volume of fluid that contains the total drug dose administered at the same concentration as in plasma
Clearance (CL): Quantification of the irreversible loss of drug from the body by metabolism and excretion
Elimination half-life: Time required for the plasma concentration to fall by one-half
Protein binding: Proportion of drug binding to plasma proteins
Area under the curve (AUC) 0-24: Total area under the concentration curve over 0-24 h.
Pharmacodynamics and pharmacokinetics/pharmacodynamics models
Pharmacodynamics is the study of the relationship between drug concentrations and effect [Figure 4].[31] Several PK/PD models have been constructed using the three most popular parameters: Cmax/minimum inhibitory concentration (MIC), %T > MIC, and AUC24/MIC.
Figure 4.

Interrelationship among pharmacokinetics, pharmacodynamic, and pharmacokinetics/pharmacodynamics[31]
Cmax/MIC: How many times the peak serum concentration of a given antimicrobial is higher than MIC
%T > MIC: Percentage of a dosage interval in which the serum drug concentration remains above the MIC
AUC24/MIC: Area under the concentration curve over 0-24 h-to-minimum inhibitory concentration ratio.
Classification of Antimicrobials Based on Pharmacokinetics-Pharmacodynamics Models Associated with their Optimal Killing Activity[32,33,34,35]
Time-dependent antimicrobial agents
Optimal activity is achieved when unbound plasma concentrations are maintained above the MIC of the bacteria for the longest period, and %T > MIC is the best predictor of their efficacy. Time - dependent antimicrobials, including cephalosporins, carbapenems, and penicillins. After administering the loading dose, timedependent antimicrobials should be readministered in several lower doses per 24 h.[32]
Concentration-dependent antimicrobial agents
Concentration - dependent antimicrobials, including aminoglycosides, fluorochinolones, daptomycin, amphotericin B, should be administered in high doses once per 24 h in order to obtain optimal activity of Cmax/MIC to maximize killing, followed by very low troughs to minimize toxicity.[33]
Time and concentration dependent antimicrobial agents
AUC24/MIC is the most reliable predictor of antimicrobial (vancomycin, linezolid, tetracyclines, azithromycin) efficacy, and it is also related to the type of the pathogen involved.[34,35]
Antimicrobial Dosing in Sepsis-Induced Acute Kidney Injury on Renal Replacement Therapy
In patients with sepsis, sustained oliguria or severe metabolic acidosis, refractory volume overload and severe electrolyte disarray may be the reasons enough to start renal replacement therapy (RRT).[36] In sepsis-induced AKI, therapeutic antimicrobial drugs are often required, but standard dosing regimens are affected by RRT. Continuous renal replacement therapy, particularly CVVH, is becoming more commonly used in the routine management of critically ill patients with AKI.[37] These modalities [Table 4] may change dosing of antimicrobial agents.[38] The SSC recommends that intravenous antimicrobials are begun within the 1st h after diagnosis of severe sepsis and septic shock.[39] Dosage of antimicrobial by type of RRT are showed in Table 5.[18,26,30,40,41,42,43,44,45,46,47,48,49,50]
Table 4.
Modalities of renal replacement therapy[38]

Table 5.
Dose adjustments of selected intravenous antimicrobials in patients with renal dysfunction and hepatic failure[18,26,30,40,41,42,43,44,45,46,47,48,49,50]

Dosing of Antimicrobials in Sepsis Related Multiple Organ Dysfunction Syndromes
In sepsis-related MODS, homeostasis cannot be maintained without intervention, usually involving two or more organ systems.[51] Hemodynamic alterations lead to sepsis-induced tissue hypoperfusion, which affect PK leading to inadequate dosing of antimicrobials [Figure 1]. Several pathophysiological conditions may increase Vd and an increase in dosage should be considered with the intent of ensuring optimal care [Figure 5]. [52] Dose recommendations and general principles for loading and maintenance dosing of antimicrobial agents [18,53,54,55] in patients with renal failure and hepatic failure are showed in Tables 5 and 6.
Figure 5.

Schematic representation of the pathophysiological or iatrogenic conditions in patients affecting drug distribution and elimination[52]
Table 6.
Antibiotic dosing adjustment for critically ill patients with multiple organ involvement[18,53,54,55]

Conclusion
Sepsis is a common and remains a major cause of multiorgan dysfunction syndrome, indicating a crucial role in efficient antimicrobial treatment. Inappropriate use of antimicrobials may be responsible for higher therapeutic failure, mortality rates, costs and patient toxicity as well as the emergence of resistance. Antimicrobial treatment is particularly difficult due to altered PK profile, dynamic changes in patient's clinical status and, in many cases, need for RRT. Instructions on antimicrobial dosing in package inserts provided by drug manufacturers are frequently insufficient to guide dosing in the critically ill patients appropriately and current guidelines concerning the dosing of antimicrobials are not particularly reliable because they are based on studies involving small and heterogeneous groups of patients, often treated with different RRT modalities.
Shortage of reliable data regarding antimicrobial dosing in critically ill patients possesses an enormous clinical dilemma. Consequently, there is an urgent need for establishing new sets of recommendations corroborated by large-scale prospective clinical studies conducted in homogenous patient populations treated according to the uniform RRT procedures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS).A prospective study. JAMA. 1995;273:117–23. [PubMed] [Google Scholar]
- 4.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–43. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 5.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 6.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angus DC, Wax RS. Epidemiology of sepsis: An update. Crit Care Med. 2001;29:S109–16. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 8.Kohli HS, Bhat A, Jairam A, Aravindan AN, Sud K, Jha V, et al. Predictors of mortality in acute renal failure in a developing country: A prospective study. Ren Fail. 2007;29:463–9. doi: 10.1080/08860220701260651. [DOI] [PubMed] [Google Scholar]
- 9.Kaul A, Sharma RK, Tripathi R, Suresh KJ, Bhatt S, Prasad N. Spectrum of community-acquired acute kidney injury in India: A retrospective study. Saudi J Kidney Dis Transpl. 2012;23:619–28. [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 11.Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, et al. Insufficient ß-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2010;14:R126. doi: 10.1186/cc9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care. 2010;14:R53. doi: 10.1186/cc8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John AK, Norbert L, Peter A, Rashad SB, Burdmann EA, Goldstein SL, et al. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:19–36. [Google Scholar]
- 14.Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: A critical care nephrology Italian collaborative effort (NEFROINT) Minerva Anestesiol. 2011;77:1072–83. [PubMed] [Google Scholar]
- 15.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840–51. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JA, Joynt GM, Choi GY, Gomersall CD, Lipman J. How to optimise antimicrobial prescriptions in the intensive care unit: Principles of individualised dosing using pharmacokinetics and pharmacdynamics. Int J Antimicrob Agents. 2012;39:187–92. doi: 10.1016/j.ijantimicag.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Ulldemolins M, Roberts JA, Lipman J, Rello J. Antibiotic dosing in multiple organ dysfunction syndrome. Chest. 2011;139:1210–20. doi: 10.1378/chest.10-2371. [DOI] [PubMed] [Google Scholar]
- 19.Gulyassy PF, Depner TA. Impaired binding of drugs and endogenous ligands in renal diseases. Am J Kidney Dis. 1983;2:578–601. doi: 10.1016/s0272-6386(83)80038-9. [DOI] [PubMed] [Google Scholar]
- 20.Suh B, Craig WA, England AC, Elliott RL. Effect of free fatty acids on protein binding of antimicrobial agents. J Infect Dis. 1981;143:609–16. doi: 10.1093/infdis/143.4.609. [DOI] [PubMed] [Google Scholar]
- 21.Craig WA, Suh B. Changes in protein binding during disease. Scand J Infect Dis Suppl. 1978:239–44. [PubMed] [Google Scholar]
- 22.Golper TA, Bennett WM. Drug removal by continuous arteriovenous haemofiltration. A review of the evidence in poisoned patients. Med Toxicol Adverse Drug Exp. 1988;3:341–9. doi: 10.1007/BF03259889. [DOI] [PubMed] [Google Scholar]
- 23.Schetz M. Drug dosing in continuous renal replacement therapy: General rules. Curr Opin Crit Care. 2007;13:645–51. doi: 10.1097/MCC.0b013e3282f0a3d3. [DOI] [PubMed] [Google Scholar]
- 24.Scaglione F, Paraboni L. Influence of pharmacokinetics/pharmacodynamics of antibacterials in their dosing regimen selection. Expert Rev Anti Infect Ther. 2006;4:479–90. doi: 10.1586/14787210.4.3.479. [DOI] [PubMed] [Google Scholar]
- 25.Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med. 2009;37:2268–82. doi: 10.1097/CCM.0b013e3181aab3d0. [DOI] [PubMed] [Google Scholar]
- 26.Patel K, Roberts JA, Lipman J, Tett SE, Deldot ME, Kirkpatrick CM. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: Using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob Agents Chemother. 2011;55:5868–73. doi: 10.1128/AAC.00424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50:99–110. doi: 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Scaglione F. Can PK/PD be used in everyday clinical practice. Int J Antimicrob Agents. 2002;19:349–53. doi: 10.1016/s0924-8579(02)00020-1. [DOI] [PubMed] [Google Scholar]
- 29.Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis. 2005;41:1159–66. doi: 10.1086/444500. [DOI] [PubMed] [Google Scholar]
- 30.Fish DN, Teitelbaum I, Abraham E. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother. 2005;49:2421–8. doi: 10.1128/AAC.49.6.2421-2428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowland M, Tozer TN. Clinical Pharmacokinetics: Concepts and Applications. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1995. [Google Scholar]
- 32.Craig WA. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 33.Craig WA. Does the dose matter? Clin Infect Dis. 2001;33(Suppl 3):S233–7. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 34.Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003;17:479–501. doi: 10.1016/s0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 35.Pea F, Viale P. The antimicrobial therapy puzzle: Could pharmacokinetic-pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin Infect Dis. 2006;42:1764–71. doi: 10.1086/504383. [DOI] [PubMed] [Google Scholar]
- 36.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, et al. Continuous renal replacement therapy: A worldwide practice survey. The beginning and ending supportive therapy for the kidney (B.E.S.T. kidney) investigators. Intensive Care Med. 2007;33:1563–70. doi: 10.1007/s00134-007-0754-4. [DOI] [PubMed] [Google Scholar]
- 37.Wright SE, Bodenham A, Short AI, Turney JH. The provision and practice of renal replacement therapy on adult intensive care units in the United Kingdom. Anaesthesia. 2003;58:1063–9. doi: 10.1046/j.1365-2044.2003.03449.x. [DOI] [PubMed] [Google Scholar]
- 38.Mushatt DM, Mihm LB, Dreisbach AW, Simon EE. Antibiotic dosing in slow extended daily dialysis. Clin Infect Dis. 2009;49:433–7. doi: 10.1086/600390. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother. 2011;66(Suppl 2):ii25–31. doi: 10.1093/jac/dkq516. [DOI] [PubMed] [Google Scholar]
- 40.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: Implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49:1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549–55. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andes D, van Ogtrop ML, Peng J, Craig WA. In vivo pharmacodynamics of a new oxazolidinone (linezolid) Antimicrob Agents Chemother. 2002;46:3484–9. doi: 10.1128/AAC.46.11.3484-3489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergner R, Hoffmann M, Riedel KD, Mikus G, Henrich DM, Haefeli WE, et al. Fluconazole dosing in continuous veno-venous haemofiltration (CVVHF): Need for a high daily dose of 800 mg. Nephrol Dial Transplant. 2006;21:1019–23. doi: 10.1093/ndt/gfi284. [DOI] [PubMed] [Google Scholar]
- 44.Heintz BH, Matzke GR, Dager WE. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy. 2009;29:562–77. doi: 10.1592/phco.29.5.562. [DOI] [PubMed] [Google Scholar]
- 45.Ahern JW, Possidente CJ, Hood V, Alston WK. Cefazolin dosing protocol for patients receiving long-term hemodialysis. Am J Health Syst Pharm. 2003;60:178–81. doi: 10.1093/ajhp/60.2.178. [DOI] [PubMed] [Google Scholar]
- 46.Aronoff GR, Bennett WM, Berns JS. American College of Physicians. 5th ed. Philadelphia: PA; 2007. Drug prescribing in renal failure: Dosing guidelines for adults and children; p. 97. [DOI] [PubMed] [Google Scholar]
- 47.Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 48.Vilay AM, Grio M, Depestel DD, Sowinski KM, Gao L, Heung M, et al. Daptomycin pharmacokinetics in critically ill patients receiving continuous venovenous hemodialysis. Crit Care Med. 2011;39:19–25. doi: 10.1097/CCM.0b013e3181fa36fb. [DOI] [PubMed] [Google Scholar]
- 49.Salama NN, Segal JH, Churchwell MD, Patel JH, Gao L, Heung M, et al. Intradialytic administration of daptomycin in end stage renal disease patients on hemodialysis. Clin J Am Soc Nephrol. 2009;4:1190–4. doi: 10.2215/CJN.01650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adembri C, Fallani S, Cassetta MI, Arrigucci S, Ottaviano A, Pecile P, et al. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: Intermittent versus continuous infusion. Int J Antimicrob Agents. 2008;31:122–9. doi: 10.1016/j.ijantimicag.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 51.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 52.Scaglione F, Paraboni L. Pharmacokinetics/pharmacodynamics of antibacterials in the intensive care unit: Setting appropriate dosing regimens. Int J Antimicrob Agents. 2008;32:294–301. doi: 10.1016/j.ijantimicag.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Hooper D. Quinolones. In: Mandell GL, Bennett JE, Dolin RM, editors. Douglas, Bennett's. Principles and Practice of Infectious Diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. pp. 404–23. [Google Scholar]
- 54.Greer ND. Tigecycline (Tygacil): The first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent) 2006;19:155–61. doi: 10.1080/08998280.2006.11928154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert SP. The Merck Manual. 19th ed. NJ, USA: Merck and Co. Publication; 2013. Health care professionals: Bacteria and antibacterial drugs. [Google Scholar]


