Abstract
Background and Objectives:
Different methods of platelet concentrate preparations leave behind certain number of residual leukocytes, accounting for most of the febrile nonhemolytic transfusion reactions, especially in multitransfused patients. Various inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 are generated during storage and have been implicated for these adverse effects. We have studied the levels of these cytokines and their correlation with leucocyte contents in platelet concentrates prepared by three different methods.
Study Design and Methods:
Five pools of platelet rich plasma platelet concentrates (PRP-PC) and buffy-coat platelet concentrates (BC-PC) each were prepared and divided into two halves. One half of the pool was leucofiltered (LF), whereas the other half was stored as such. Ten apheresis units were also included in the study. All the platelet concentrates were assessed for leucocyte load and cytokine content (IL-1β, IL-6, and TNF-α) on different days of storage (0, 3, and 5) using Nageotte chamber and commercially available immunoassays respectively.
Results:
There was a statistically significant rise in cytokine levels (IL-1β, IL-6, and TNF-α) in nonleucofiltered (NLF) random donor platelet concentrates (RDPs) (PRP-PC and BC-PC) during storage (day 3 and 5) whereas LF RDP concentrates (PRP-PC and BC-PC) and apheresis platelet concentrates (AP-PC) did not show any significant rise in cytokine levels (on day 3 and 5) over the baseline values at day 0.
Conclusion:
This data suggests that although AP-PCs are superior to PRP-PC (NLF) and BC-PC (NLF) in terms of in vitro quality control parameters and cytokine generation during storage, BC-PC mode of platelet preparation followed by leucofiltration is the best method to store platelets and minimise the cytokine accumulation. This strategy is best suited for transfusion in multitransfused hematooncologic patients, who cannot afford single donor apheresis platelets.
Keywords: Cytokines, leucocytes, platelet concentrate
Introduction
Platelet transfusion plays a vital role in thrombocytopenic patients in various clinical settings; however, nonhemolytic febrile transfusion reaction associated with them remains a cause of concern. Various inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 are generated during storage and have been implicated for these adverse effects as they exhibit in vivo pyrogenic activity.[1] These pyrogenic cytokines induce fever by mediating upregulation of the thermostatic set point for body temperature in the thermoregulatory center of the hypothalamus.[2] Therefore, we planned a study to assess the cytokine levels in different types of platelet concentrates during storage.
Material and Methods
A total of 50 platelet concentrate units were included in the study, of which 20 were platelet rich plasma platelet concentrates (PRP-PC) units, 20 buffy-coat platelet concentrates (BC-PC) units prepared from whole blood and 10 were AP-PCs derived from healthy apheresis donors through continuous flow double venous access automated blood cell separator (CS-3000 plus). All the blood donors met the requisite criteria for blood donation as has been laid down in Directorate General Of Health Services (DGHS) technical manual, Ministry of Health and Family Welfare, Govt. of India.
Sampling and storage
Five platelet rich pools were prepared by pooling 4 units of PRP-PC for each pool and similarly 4 units of BC-PC each for another 5 pools with the help of a sterile connecting device (Compo Dock, Fresinius HemoCare). Then each pool was separated into two equal halves, of which one half was leucoreduced with 3rd generation leucofilter (Terumo Penpol) and the other was kept as such [Figure 1]. Ten apheresis platelet concentrates (AP-PC) were also included in the study without leucofiltration as they were harvested on the cell separators having inline leucoreduction technology. All the platelet preparations tested for cytokines met the routine quality control criteria recommended by DGHS, Govt. of India for various parameters like volume, swirling, pH, platelet count, and white blood cell (WBC) count and were stored in flat bed platelet agitator incubators (Terumo Penpol Ltd.-Model Compo Safe) at 22 ± 2°C. The quality checks were performed on storage days 0, 3, and 5 by taking 2 ml sample from each PC aseptically. After initial quality checks, the sample was centrifuged at 3000 rpm (rcf = 604g) for 5 min (Bench top centrifuge, REMI) and the platelet poor plasma was stored in cryovials in deep freezer (–80°C) for cytokine assessment at a later date.
Figure 1.
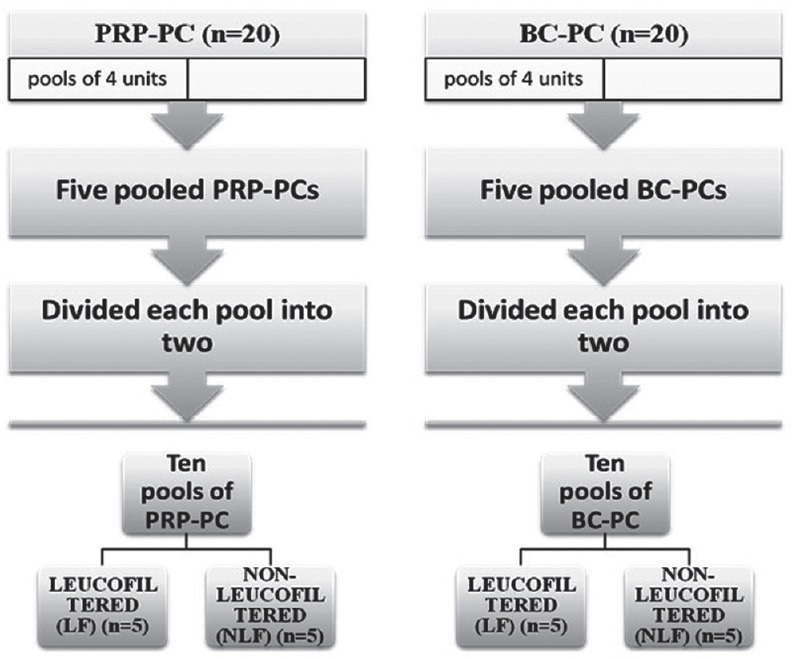
A flow chart for pooling, division and filtration of PRP-PC and BC-PCs
Cytokine assays
Cytokines IL-1β, IL-6, and TNF-α were quantified by specific enzyme-linked immunosorbent assay kit (Immunotech, Beckman-Coulter Company, France). The standard curves were prepared by using recombinant cytokines diluted to an appropriate working range. The minimum detection limits were 1.5 pg/ml for IL-1β, 3 pg/mL for IL-6, and 5 pg/mL for TNF-α.
Statistical analysis
All the observations recorded for platelet quality control parameters and cytokine levels were expressed in terms of mean, standard deviation, standard error, standard error of mean, and range. Since the data was not normally distributed, the intragroup comparison of cytokine levels in the platelet concentrates of different groups was studied by using nonparametric Mann-Whitney U test and within the same group of platelets by using Wilcoxon signed-rank test.
As the data was skewed, the correlation of cytokine level with leucocyte content of the platelet concentrate was studied by applying Spearman's correlation coefficient.
Result
Platelet quality control parameters were assessed on days 0, 3, and 5 of storage.
Platelet count: Platelet count of each pool for PRP-PC, BC-PC [both nonleucofiltered (NLF) and leucofiltered (LF) groups], and individual units for AP-PC was determined and analyzed. All three kinds of platelet preparations met the desired quality control criteria for mean platelet count on days 0, 3, and 5.
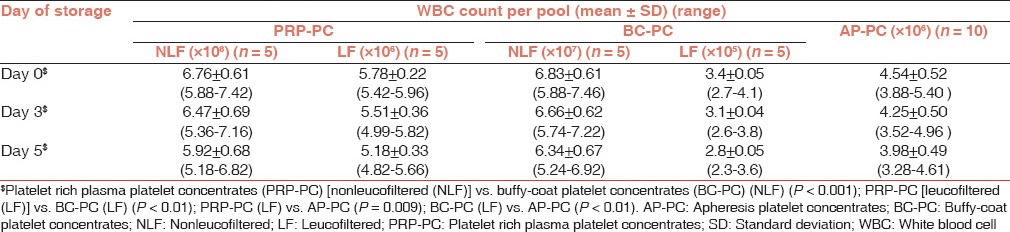
WBC count: The platelet units PRP-PC, BC-PC and AP-PC were comparatively analysed depending on their actual leucocyte content whether LF or NLF. The PRP-PC had the highest leukocyte content (108) and LF BC-PC(105) had the least [Table 1].
Table 1.
White blood cell count per pool of random donor platelet concentrates and individual AP-PC
Comparative analysis of various cytokine levels in different platelet preparations during storage (pre and post leucofiltration)
IL-1β: NLF PRP-PC units had significantly higher levels of IL-1β than (NLF) BC-PC units [day 0 (P = 0.004), day 3 (P = 0.035) and day 5 (P = 0.049)] and AP-PC units on all days of storage (P < 0.01). The LF PRP-PC and BC-PC showed a slight rise in IL-1β but was not statistically significant and were comparable to AP-PC units on all days of storage (P > 0.05) [Tables 2 and 3].
Table 2.
Cytokine levels in platelet concentrates (nonleucofiltered) on storage
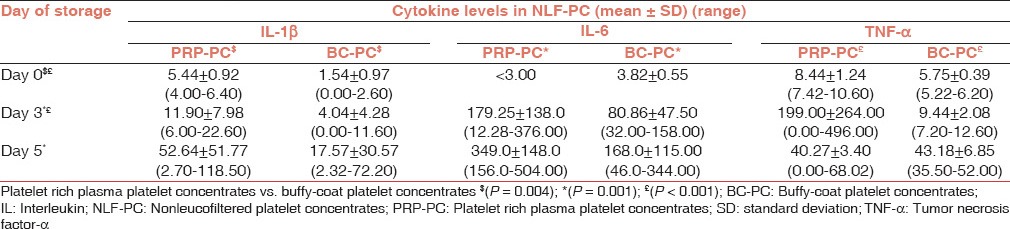
Table 3.
Cytokine levels in platelet concentrates (leucofiltered) and AP-PCs on storage
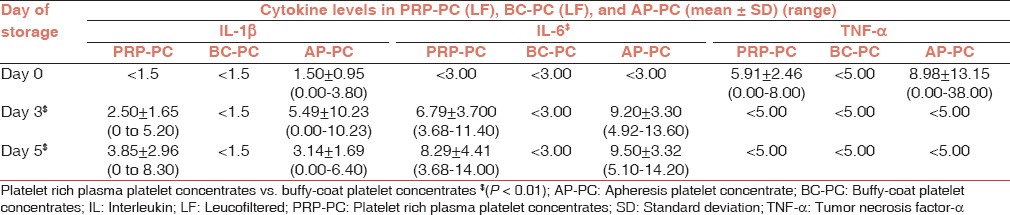
IL-6: The PRP-PC (NLF) and BC-PC (NLF) units showed a statistically significant rise in IL-6 levels on day 3 and 5 of storage (P = 0.001) over the base line on day 0, but the difference between PRP-PC (NLF) and BC-PC (NLF) IL-6 levels was not statistically significant (P > 0.05). All LF PRP-PC and AP-PC units showed a steady rise in IL-6 levels during storage, whereas they were below the detection limit in LF BC-PC units.
TNF-α: The TNF-α levels showed a significant (P < 0.01) rise in NLF PRP-PC and BC-PC units and were significantly higher in PRP-PC than BC-PC units (P < 0.01). TNF-α levels did not show any rise from baseline in AP-PC and LF PRP-PC and BC-PC units.
Correlation
This was done by categorizing the platelet preparations on the basis of leucocyte contamination (×108, ×107, ×106, and ×105) on day 0 irrespective of the method of their preparation in order to determine a critical level of leucocytes for clinically significant amount of cytokine accumulation [Table 4 and Figure 2]. Platelet preparations with higher leucocyte content (≥107) had significantly higher levels of cytokines as compared to the units with lower (≤106) leucocyte count. The cytokine levels were below the detection threshold in units with ≤105 leucocytes during the entire shelf life.
Table 4.
White blood cell content and cytokine levels on different days of storage
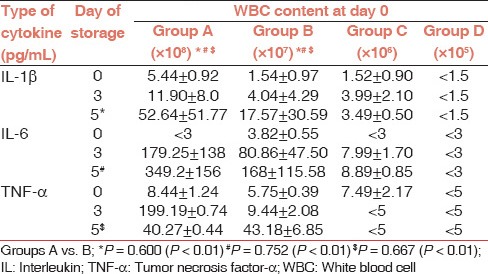
Figure 2.
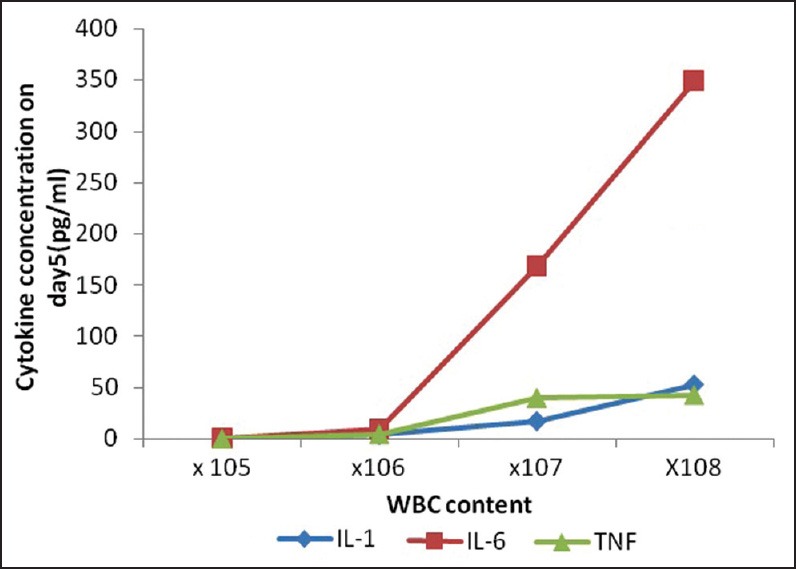
Correlation of white blood cell content (×105, ×106, ×107, and ×108) with cytokine levels in different groups of platelet concentrates
Discussion
The aim of safe transfusion practice is to provide the recipient the desired therapeutic benefit without much untoward effects. Leucoreduction of blood components has proved to be of value in multiply transfused and immune-compromised patients. The contaminating leucocytes in the blood components release various cytokines during storage and upon their disintegration, we could demonstrate this proof of principle in our study as we found that the nonfiltered group of PCs (PRP-PC and BC-PC) had a significant rise in IL-1β and IL-6 levels during storage. These levels were statistically higher (P < 0.05) for all assessment days when compared to the respective LF group of PCs which showed no or minimal rise in their IL-1β level above the baseline (at day 0). The AP-PC units showed a rise till day 3 only from 1.54 ± 0.95 to 5.49 ± 10.23 pg/ml.
A comparative study by Aye et al.,[3] observed significantly higher levels of IL-1β and IL-6 in nonfiltered PRP-PCs on days 3-5 as compared to day 0. Likewise, a rise in TNF-α was observed from day 0 to day 5 in nonfiltered PCs. After prestorage leucofiltration, there was no statistically significant rise in the levels of any of cytokines from day 0 to day 5.
Muylle et al.,[4] performed a correlation between leucocyte content and IL-1β levels on different days of storage of their platelet preparations and showed significantly higher levels on day 5 (6375 ng/L) (145-26000 ng/L) as compared to day 0 (60 ng/L) (20-175 ng/L) in units with WBC count >6 × 106/bag, whereas units with lower WBC content did not show much rise in IL-1β levels. This implies that increase in leucocyte content of platelet concentrates above a certain level (>106/bag) leads to significantly higher amounts of IL-1β accumulation during storage. We also observed significant rise in mean IL-6 levels in NLF PRP-PC and BC-PC from day 0 (<3 pg/mL) to day 3 (179.25 ± 138.00 and 349.00 ± 148.00) and day 5 (80.86 ± 47.50 and 168 ± 11.50) (mean ± SD) (pg/mL) of storage, whereas LF PRP-PC, BC-PC, and AP-PC units did not show a significant rise in cytokine levels during storage.
A similar observation was made by Wadhwa et al.,[5] in their study where authors had found an 18 fold rise in the mean IL-6 levels of 2395 pg/mL on day 5/6 over the baseline of 140 pg/ml (day 1) in NLF random donor platelet concentrates. Although cytokine levels in our PRP-PC (NLF) and BC-PC (NLF) were not as high as theirs on last day of storage but we observed a 50-100 fold rise in IL-6 levels on day 5 over the baseline in our preparations. But our LF BC-PCs did not show any detectable IL-6 implying as leucocyte content decreases there is decline in cytokine accumulation in different preparations [Figure 2].
Addas-Carvalho et al.,[6] showed a significant rise for TNF-α in their nonfiltered PRP-PC during 7 days of storage with mean level of 5.68 pg/ml on day 3 to 8.62 pg/ml on day 5 to 48.84 pg/ml on day 7 (P < 0.05). But the TNF-α was undetectable in LF PCs and in AP-PCs, which were having low WBC content (<×106 in all AP-PC). However, we found higher levels of TNF-α levels in PRP-PCs[6,7] on day 3 of storage only.
TNF-α levels in platelet units with WBC content (≥×107) showed a significant rise from day 0 (8.44 ± 1.24) to day 3 (199 ± 264) (P < 0.05), although the levels declined after day 3, they were higher than day 0 and statistically insignificant (P > 0.05). However, the units with WBC content (×106) had a mean TNF-α level of 7.44 ± 2.17 on day 0 and were undetectable on days 3 and 5 of storage. This suggests that platelet preparations with higher leucocyte content accumulated larger amount of TNF-α till mid of their shelf-life and showed some decline in the later half of storage either due to fall in leucocyte metabolism or disintegration.
It is known that the WBCs are metabolically active during storage, especially in platelets which are routinely stored at 20-24°C.[8] The present study demonstrates that WBC cytokine synthesis and release into plasma can be triggered during storage of platelets.
Muylle et al.,[4] suggested a higher threshold of WBC content of 6 × 107 per unit, to prevent accumulation of higher IL-6 levels, whereas we observed that minimal or no cytokine synthesis was observed when WBC content was below ≤106 per platelet pool unit. The difference in our observation may be due to the fact that we also included apheresis PCs and prestorage LF PRP-PC and BC-PCs as well. This shows that the units which were leucoreduced early in the course of storage by use of third-generation filters failed to produce detectable IL-1β, IL-6, and TNF-α during their shelf life suggesting that more than 106 WBCs per unit of PC are necessary for the production of detectable cytokines, since these filters have a capacity to reduce WBCs by about 3 log10. On the contrary, WBC reduction by filtration at the bedside after storage does not offer the same advantage, since cytokines would have already been released due to disintegration of leucocytes during storage of PCs. Thus prestorage leucofiltration in PCs prevent the cytokine accumulation as was evident that they were significantly higher in the nonfiltered PRP-PC and BC-PC than the filtered units during the course of storage.
Cytokines such as TNF-α, IL-1β, and IL-6 belong to a group of cytokines with overlapping biologic properties. They share the ability to stimulate T and B-lymphocytes to augment cell proliferation and to promote or suppress gene expression for several proteins.[9] These cytokines act as endogenous pyrogens by inducing fever through increased synthesis of prostaglandin E2 in the hypothalamus followed by a rise in thermostatic set point and have been implicated in febrile nonhemolytic transfusion reactions.[4,10] However, patient-related factors such as the presence of soluble cytokine receptors and of receptor antagonists and the rate of metabolism of cytokines may also interfere in the outcome of the infusion of these cytokines.
On translating the cytokine levels to actual content per 100 ml of PC, it was found that they were (5.1, 1.9, 0.3) ng of IL-1β, (30.3, 16.8, 1.0) ng of IL-6, and (2.8, 3.45, 1.0) ng of TNF-α for nonfiltered PRP-PC and BC-PCs and AP-PC, respectively. For an adequate therapeutic dose, a 70 kg man would generally require 10 such random donor PCs and 2-4 apheresis PCs. Based on above calculations, the estimated cytokines infused would be as 30.6,180, and 16.8 ng for PRP-PC;13.3, 117.6, and 24.15 ng for BC-PC;1.8, 6, and 0 ng for AP-PC for IL-1β, IL-6, and TNF-α, respectively. These levels when compared to studies in literature are sufficient to cause pyrexia, insomnia, arthralgia, anorexia and headache in humans.[9] With respect to the dose equilibration, systemic administration of 10-1000 ng of intravenous IL-1β per kg has produced fever, insomnia, anorexia, arthralgias, and headache in humans. Moreover, it has been shown that TNF-α at concentrations of 0.4-20 pg/ml stimulates the production of plasminogen activator inhibitor and urokinase plasminogen activator in cultured endothelial cells.[11] On the basis of the present study, it can be stated that an individual receiving 10 units of 5-day-old random donor nonfiltered PRP-PCs and BC-PC would receive pyrogenic doses of IL-6 and TNF-α, whereas they were at lower levels in apheresis units and LF platelet units. Thus, it becomes imperative to transfuse either prestorage leucoreduced random donor platelets or apheresis platelets in seriously ill patients requiring prophylactic as well as therapeutic platelet transfusion.
PCs prepared either from whole-blood donations by the BC-PC, or by plateletpheresis are indicated to prevent or treat acute hemorrhage secondary to thrombocytopenia, and there is an ongoing debate about which platelet product should be used. Lozano et al.,[12] found that usage of each of these two products is highly heterogeneous among countries and individual institutions, ranging from 10% to 90%, with a 50:50 ratio in Europe. In comparison of pooled platelets prepared by the BC method and apheresis PCs, data suggest similar efficacy of the products. Regarding recipients adverse reactions, there is no advantage for apheresis concentrates. From the donor's point of view, evidence favors using the abundance of platelets available from whole-blood donation. As residual viral transmission risk continues to fall, the advantage of apheresis products related to the decrease to donor exposure lessens. While the cost-effectiveness of apheresis products is comparable to that of other accepted blood safety interventions, in case of emerging pathogens, probably pathogen inactivation of pooled BC-PCs would be a more desirable strategy.
Another study by Schrezenmeir and Sefried[13] preferred PCs derived from whole-blood donation and prepared by the BC method unless a specific clinical condition such as neonatal immune thrombocytyopenia or platelet refractoriness due to alloantibodies requiring transfusion of matched PCs.
Van der meer[14] observed that the need for pooling of whole-blood-derived platelet concentrates increases donor exposure and thereby potentially increases the risks associated with transfusion of whole-blood-derived platelet concentrates. But alloimmunization rates, acute reaction rates, and transfusion related acute lung injury rates are not different. Apheresis donation procedures have fewer adverse events. The various benefits and disadvantages of the methods have to be balanced when choosing a preferred way of platelet collection.
Therefore, the present study concludes that the apheresis platelets are better than the random donor platelets [PRP-PC (NLF) and BC-PC (NLF)] in terms of in vitro quality control parameters and cytokine accumulation during storage. However, BC-PC followed by leucofiltration is the best method to store platelets and minimize the cytokine accumulation providing an equivalent dose and efficient alternative to apheresis platelets after pooling, in multitransfused hematooncologic patients in tertiary care hospitals in resource constrained nation like ours.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.Stack G, Synder EL. Cytokine generation in stored platelet concentrates. Transfusion. 1994;34:20–5. doi: 10.1046/j.1537-2995.1994.34194098597.x. [DOI] [PubMed] [Google Scholar]
- 2.Saran RK. Transfusion Medicine–Technical Manual (Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India) 2003;32:353–4. [Google Scholar]
- 3.Aye MT, Palmer DS, Giulivi A, Hasemi S. Effect of filtration of platelet concentrates on the accumulation of cytokines and platelet release factors during storage. Transfusion. 1995;2:117–24. doi: 10.1046/j.1537-2995.1995.35295125733.x. [DOI] [PubMed] [Google Scholar]
- 4.Muylle L, Joos M, Wouters E, De Bock R, Peetermans ME. Increased tumor necrosis factor α (TNF- α), interleukin 1, and interleukin 6(IL-6) levels in the plasma of stored platelet concentrates: Relationship between TNF-α and IL-6 levels and febrile transfusion reactions. Transfusion. 1993;33:195. doi: 10.1046/j.1537-2995.1993.33393174443.x. [DOI] [PubMed] [Google Scholar]
- 5.Wadhwa M, Senghatchhian MJ, Lubenko A, Contreras M, Dilger P, Bird C, et al. Cytokine levels in platelet concentrates: Quantitation by bioassays and immunoassays. Br J Haematol. 1996;93:225–34. doi: 10.1046/j.1365-2141.1996.4611002.x. [DOI] [PubMed] [Google Scholar]
- 6.Addas-Carvalho M, Origa AF, Saad ST. Interleukin 1 beta and tumor necrosis factor levels in stored platelet concentrates and the association with gene polymorphisms. Transfusion. 2004;44:996–1003. doi: 10.1111/j.1537-2995.2004.03257.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary R, Aggarwal A, Khetan D, Dayal R. Cytokine generation in stored platelet concentrates: Comparison of two methods of preparation. Indian J Med Res. 2006;124:427–30. [PubMed] [Google Scholar]
- 8.Gottschall JL, Johnson VL, Rzad L, Anderson AJ, Aster RH. Importance of white blood cells in platelet storage. Vox Sang. 1984;47:101–7. doi: 10.1111/j.1423-0410.1984.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 9.Dinatello CA. Interleukin-1 and Interleukin-1 antagonism. Blood. 1991;77:1627–52. [PubMed] [Google Scholar]
- 10.Muylle L, Wouters E, Peetermans ME. Febrile reaction to platelet transfusion: The effect of increased interleukin 6 levels in concentrates prepared by the platelet-rich plasma method. Transfusion. 1996;36:886–90. doi: 10.1046/j.1537-2995.1996.361097017174.x. [DOI] [PubMed] [Google Scholar]
- 11.van Hinsbergh VW, van den Berg EA, Fiers W, Dooijewaard G. Tumor necrosis factor induces the production of urokinase-type plasminogen activator by human endothelial cells. Blood. 1990;75:1991–8. [PubMed] [Google Scholar]
- 12.Lozano ML, Rivera J, Vicente V. Platelet concentrates from whole blood donations (buffy-coat) or apheresis: Which one to use? Med Clin (Barc) 2012;138:528–33. doi: 10.1016/j.medcli.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Schrezenmeir H, Seifried E. Buffy-coat derived pooled platelet concentrates and apheresis platelet concentrates: Which product should be preferred? Vox Sang. 2010;99:1–15. doi: 10.1111/j.1423-0410.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 14.van der Meer PF. Apheresis versus whole blood derived platelet concentrates: Pros and cons. ISBT Sci Ser. 2012;7:112–6. [Google Scholar]


