Abstract
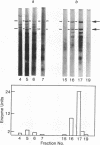
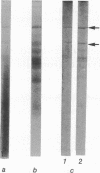
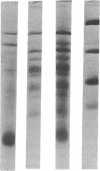
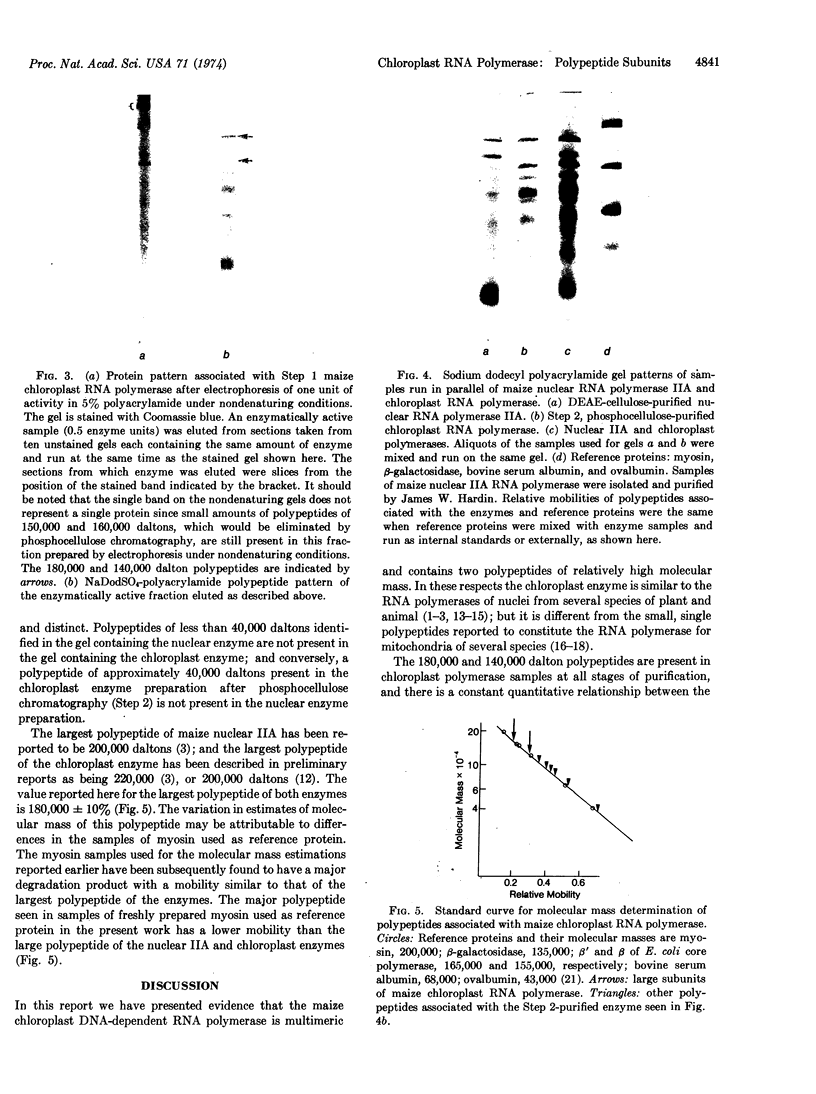
Analysis of the maize chloroplast DNA-dependent RNA polymerase by electrophoresis on polyacrylamide gels that contain sodium dodecyl sulfate shows that the enzyme is multimeric. It contains at least two polypeptides of 180,000 and 140,000 daltons. Polypeptides of lower molecular mass that are associated with the enzyme at several stages of purification may also be subunits of the enzyme.
Electrophoresis of a mixture of purified maize nuclear DNA-dependent RNA polymerase IIA and chloroplast polymerase on sodium dodecyl sulfate-polyacrylamide gels resolves the 160,000 dalton polypeptide of IIA from the 140,000 dalton polypeptide of the chloroplast enzyme, but does not resolve the 180,000 dalton components found in both enzymes. The polypeptides of less than 40,000 daltons in the highly purified maize nuclear IIA preparations are absent from preparations of purified maize chloroplast RNA polymerase.
Keywords: maize nuclear RNA polymerase IIA
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas B. B. Chromatin and ribonucleic acid polymerases in the eukaryotic cell. Subcell Biochem. 1974 Mar;3(1):27–38. [PubMed] [Google Scholar]
- Bottomley W., Smith H. J., Bogorad L. RNA polymerases of maize: partial purification and properties of the chloroplast enzyme. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2412–2416. doi: 10.1073/pnas.68.10.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. Separation and characterization of the subunits of ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6168–6176. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Protein subunits: a table (revised edition). Arch Biochem Biophys. 1972 Mar;149(1):1–14. doi: 10.1016/0003-9861(72)90293-7. [DOI] [PubMed] [Google Scholar]
- Heil A., Zillig W. Reconstitution of bacterial DNA-dependent RNA-polymerase from isolated subunits as a tool for the elucidation of the role of the subunits in transcription. FEBS Lett. 1970 Dec;11(3):165–168. doi: 10.1016/0014-5793(70)80519-1. [DOI] [PubMed] [Google Scholar]
- Jendrisak J. J., Becker W. M. Purification and subunit analysis of wheat-germ ribonucleic acid polymerase II. Biochem J. 1974 Jun;139(3):771–777. doi: 10.1042/bj1390771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H., Schäfer K. P. Mitochondrial RNA polymerase from Neurospora crassa. Nat New Biol. 1971 Jun 30;231(26):265–269. doi: 10.1038/newbio231265a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mullinix K. P., Strain G. C., Bogorad L. RNA polymerases of maize. Purification and molecular structure of DNA-dependent RNA polymerase II. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2386–2390. doi: 10.1073/pnas.70.8.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Reid B. D., Parsons P. Partial purification of mitochondrial RNA polymerase from rat liver. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2830–2834. doi: 10.1073/pnas.68.11.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain G. C., Mullinix K. P., Bogorad L. RNA polymerases of maize: nuclear RNA polymerases. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2647–2651. doi: 10.1073/pnas.68.11.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wu G. J., Dawid I. B. Purification and properties of mitochondrial deoxyribonucleic acid dependent ribonucleic acid polymerase from ovaries of Xenopus laevis. Biochemistry. 1972 Sep 12;11(19):3589–3595. doi: 10.1021/bi00769a015. [DOI] [PubMed] [Google Scholar]