Abstract
Background:
Transfusion of blood remains the gold standard for fluid resuscitation from hemorrhagic shock. Hemoglobin (Hb) within the red blood cell transports oxygen and modulates nitric oxide (NO) through NO scavenging and nitrite reductase.
Aims:
This study was designed to examine the effects of incorporating a novel NO modulator, RRx-001, on systemic and microvascular hemodynamic response after blood transfusion for resuscitation from hemorrhagic shock in a hamster window chamber model. In addition, to RRx-001 the role of low dose of nitrite (1 × 10−9 moles per animal) supplementation after resuscitation was studied.
Materials and Methods:
Severe hemorrhage was induced by arterial controlled bleeding of 50% of the blood volume (BV) and the hypovolemic state was maintained for 1 h. The animals received volume resuscitation by an infusion of 25% of BV using fresh blood alone or with added nitrite, or fresh blood treated with RRx-001 (140 mg/kg) or RRx-001 (140 mg/kg) with added nitrite. Systemic and microvascular hemodynamics were followed at baseline and at different time points during the entire study. Tissue apoptosis and necrosis were measured 8 h after resuscitation to correlate hemodynamic changes with tissue viability.
Results:
Compared to resuscitation with blood alone, blood treated with RRx-001 decreased vascular resistance, increased blood flow and functional capillary density immediately after resuscitation and preserved tissue viability. Furthermore, in RRx-001 treated animals, both mean arterial pressure (MAP) and met Hb were maintained within normal levels after resuscitation (MAP >90 mmHg and metHb <2%). The addition of nitrite to RRx-001 did not significantly improve the effects of RRx-001, as it increased methemoglobinemia and lower MAP.
Conclusion:
RRx-001 alone enhanced perfusion and reduced tissue damage as compared to blood; it may serve as an adjunct therapy to the current gold standard treatment for resuscitation from hemorrhagic shock.
Keywords: Blood flow, emergency medicine, hemorrhage, microcirculation, nitrite, shock, transfusion, trauma
Introduction
Hemorrhagic shock results from loss of large volumes of blood and consequent drop of blood pressure, oxygen and nutrient delivery to the tissues to meet metabolic demands.[1,2,3] Compensatory mechanisms are activated to preserve perfusion to vital organs at the expense of peripheral tissues with progressive development of cell and tissue damage due to poor perfusion.[4] In the microcirculation, hypovolemia induces capillary collapse due to a decrease capillary hydrostatic pressure and is accompanied by a significant decrease in functional capillary density (FCD) and tissue oxygen pressure (pO2).[5,6,7] During the shock period, the vascular endothelial shear stress (ESS) is reduced due to drop in arteriolar and capillary volumetric flow rates. ESS levels determine endothelium dependent nitric oxide (NO) synthase (eNOS) function.[8] In addition, shock limits tissue oxygenation and oxygen is a cofactor for eNOS production, which catalyzes the production of NO from L-arginine.[8] NO plays several major roles in cardiovascular physiology.[9,10] Studies of hemorrhagic shock and resuscitation indicate that NO supplementation during the early phase post resuscitation improves microvascular function, tissue viability, and overall outcome.[11]
The purpose of this study was to examine the therapeutic potential of RRx-001, a modulator of hemoglobin (Hb) nitrite (NO2−) to NO reductase. RRx-001 was developed as an anti-cancer drug and has successfully completed Phase 1 clinical trials in patients with advanced, refractory cancer.[12,13] RRx-001 consists of two functional ends, a highly electrophilic center that rapidly and irreversibly binds to Hb beta subunits cysteine 93 (β-Cys 93) residues.[14] β-Cys 93 residue substitutions with alanine, glycine or leucine increase Hb nitrite to NO reductase.[15] RRx-001 produces similar allosteric changes to the Hb as the residue substitutions [Figure 1]. Previous studies have demonstrated that RRx-001 can rapidly traverse the red blood cell (RBCs) membrane, where it binds to and modifies Hb.[16] Hb nitrite reductase reaction produces NO and met Hb.[17] RRx-001-modified Hb is able to generate high levels of NO from nitrite. The generation of NO from nitrite under hypoxic conditions from RRx-001 modified Hb can enhance blood transfusion outcomes during resuscitation from hemorrhagic shock. The addition of RRx-001 to banked blood prior to be used during resuscitation from traumatic bleeding could eliminate the vasoconstriction and tissue hypoperfusion established during the hypovolemic shock, thereby reducing tissue injury.
Figure 1.
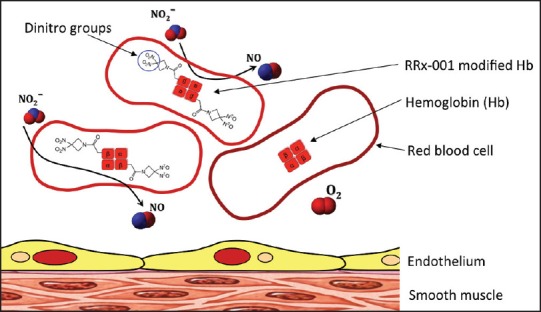
RRx-001 increases nitric oxide (NO) generation in two ways: (i) As an NO donor: Through metabolism of the dinitro groups released from the compound, and (ii) as an NO promoter: RRx-001 modifies beta subunits cysteine 93 increasing nitrite reduction to NO by deoxyhemoglobin
Materials and Methods
Animal preparation
Experiments were performed in 55-65 g male Golden Syrian Hamsters (Charles River Laboratories, Boston, MA, USA) fitted with a dorsal skinfold window chamber. Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals and the experiments were approved by the Local Animal Care and Use Committee. The hamster window chamber model is widely used for microvascular studies in the unanesthetized state. In addition to the window chamber, arterial (carotid) and venous (jugular) catheters were implanted. The complete surgical technique is described in detail elsewhere.[18] Three to four days after the initial surgery, the chamber microvasculature was examined at ×40, and systemic parameters monitored. Only animals meeting established inclusion criteria, as previously described,[19] were entered into the study.
Acute hemorrhage and resuscitation protocol
Acute hemorrhage was induced by withdrawing 50% of estimated total blood volume (BV) through the carotid artery catheter within 5 min. Total BV was estimated as 70 mL/kg of body weight. One hour after hemorrhage induction, animals received 25% of BV of resuscitation fluids (infusion rate: 200 μl/min) through a jugular vein catheter.
Experimental protocol
Conscious hamsters were placed in a restraining tube with the window chamber protruding and fixed to the stage of a transillumination intravital microscope (BX51WI, Olympus, New Hyde Park, NY, USA). Animals were given 20 min to adjust to the tube environment before any measurements were made. The tissue image was projected onto a charge-coupled device camera (4815, COHU, San Diego, CA, USA) and viewed on a monitor. Measurements were carried out using a ×40 (LUMPFL-WIR, 0.8 NA, Olympus) water immersion objective. Systemic (mean arterial pressure [MAP], heart rate [HR], hematocrit [Hct], Hb, PaO2, PaCO2, pH, lactate, plasma nitrite, and metHb) and microvascular (arteriolar and venular diameters, blood flow, and FCD) parameters were analyzed as previously described,[20,21] before hemorrhage (baseline), after hemorrhage (shock), and up to 90 min after volume replacement (resuscitation). Tissue viability (number of necrotic and apoptotic cells) was measured at 8 h following hemorrhage, as described previously.[22,23]
Experimental groups
Animals were randomly divided into four experimental groups before hemorrhage using a random ordering scheme for the resuscitation fluid used, namely: Blood (fresh blood only); nitrite (fresh blood followed by nitrite infusion 10−9 moles/animal); RRx-001 (fresh blood treated with RRx-001 2 mg/mL) and RRx-001 + N (fresh blood treated with RRx-001 2 mg/mL followed by nitrite 10−9 moles/animal) as summarized in Table 1. Fresh blood was collected from a donor, adult Golden Syrian Hamster (60-80 g), by centrifugation (2700 rpm, 7 min), buffy coat was discarded, and packed RBCs stored at 4°C until needed. To limits the effects of RRx-001 to the RBC and to establish the role of increased Hb nitrite to NO reductase during resuscitation, RBCs were incubated (1 mL of packed cells with 2 mg of RRx-001 for 30 min at 4°C), then cells were rinsed ×2 by centrifugation at 3000 rpm using phosphate buffered saline with 0.5% albumin (prefiltered 0.22 μm, pH 7.4) to remove any un-reacted RRx-001. After the final wash, RRx-001 treated RBCs were adjusted to a 30% Hct with fresh plasma. Nitrite was infused as 1 × 10−9 moles of sodium nitrite (NaNO2 10 μM in 100 μL of saline) through the carotid artery catheter 10 min after resuscitation. An equal volume of saline was given to the other groups. To address effects of instrumentation and observation, an additional Sham group was included.
Table 1.
Definition of the experimental groups and the treatment associated to each one of them

Data analysis
Table and figure results are presented as mean ± standard deviation. Data within each group was analyzed using analysis of variance (ANOVA) for repeated measurements (two-way ANOVA). When appropriate, post-hoc analyses were performed with Bonferroni posttest. The same vessels and capillary fields were followed throughout the experiment so that direct comparisons to their baseline levels could be performed, allowing for more robust statistics. All statistics were calculated using Graph Pad Prism 4.03 (GraphPad Software, Inc., San Diego, CA, USA). Changes were considered statistically significant if P < 0.05. Significance is reported for each group compared with the blood group only.
Results
Systemic response to hemorrhage and resuscitation
Systemic hemodynamic and blood parameters are presented in Table 2. During shock, Hct, and Hb dropped to about 50% of baseline. After resuscitation, MAP was significantly lower in the RRx-001 + N group at 30 and 60 min compared with blood [Figure 2]. HR in the Nitrite, RRx-001, and RRx-001 + N groups was significantly increased for all the time points compared to blood. Furthermore, HR was significantly higher in the RRx-001 + N group when compared to RRx-001 and nitrite.
Table 2.
Systemic parameters during the hemorrhagic shock and resuscitation protocol, which include Hct, Hb, nitrite, MAP, HR, pH, pO2, pCO2, and lactate
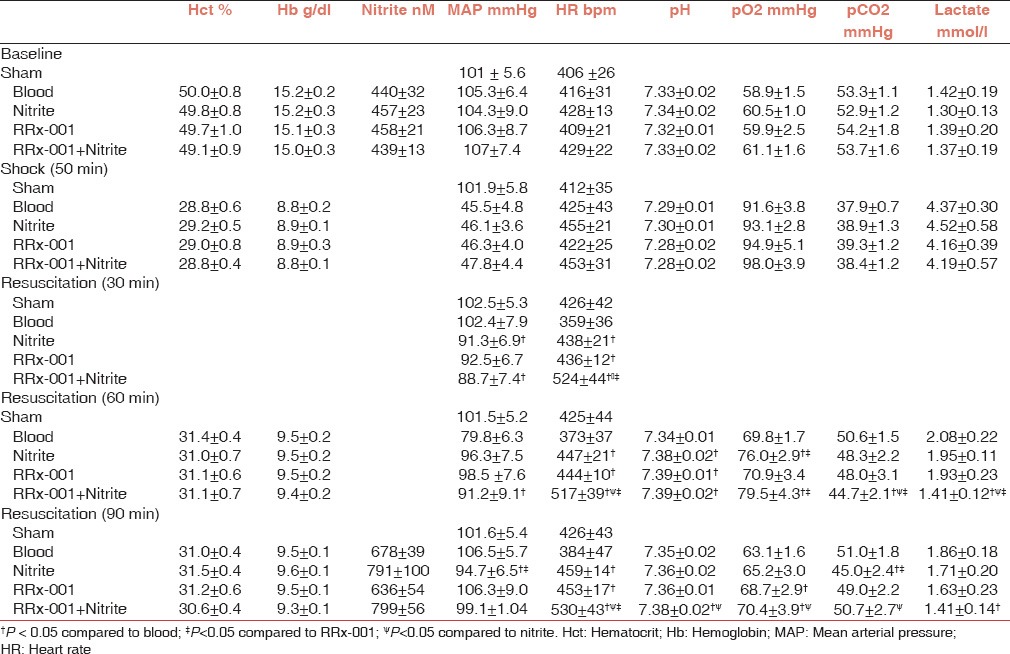
Figure 2.
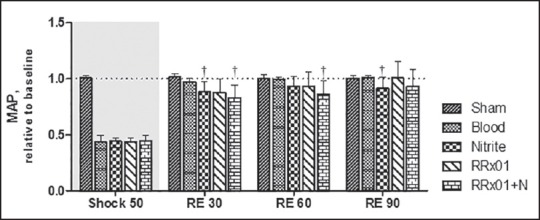
Mean arterial pressure (MAP) relative to baseline for the shock and resuscitation protocol. All the treatments partially restore MAP values to more than 80% of baseline. Baseline MAP was 105 ± 6.5 mmHg. †P < 0.05 compared to blood
Shock decreased arterial pH and pCO2 significantly from baseline in all groups. Resuscitation partially recovered blood gas parameters. Compared to the blood group, pH was significantly increased post resuscitation in the nitrite and RRx-001 groups at 60 min and in the RRx-001 + N group at 60 and 90 min. Arterial pO2 were significantly increased the nitrite group compared to blood at 60 min post resuscitation; in the RRx-001 group at 90 min post resuscitation and in the RRx-001 + N group, 60 and 90 min post resuscitation. In the RRx-001 + N group, lactate levels were significantly decreased at 60 min compared to blood, RRx-001 and nitrite and 90 min pos tresuscitation compared to blood.
Microvascular observations
Changes in arteriolar blood flow, FCD and local vascular resistance (LVR) are presented in Figure 3. Arterial blood flow was significantly increased in all treatment groups compared to blood [Figure 3a]. Arteriolar diameter in the RRx-001 + N group was significantly increased at 60 min compared to blood. Resuscitation partially restored FCD [Figure 3b]. FCD was not different between the blood and the nitrite groups. However, both RRx-001 and RRx-001 + N treatment resulted in significant increases in FCD at 60 and 90 min compared to blood. LVR increased during shock and decreased after resuscitation. The RRx-001 and RRx-001 + N groups showed a significant reduction in the LVR compared to blood during resuscitation; whereas for the nitrite group the LVR was only reduced compared to blood until 60 min.
Figure 3.
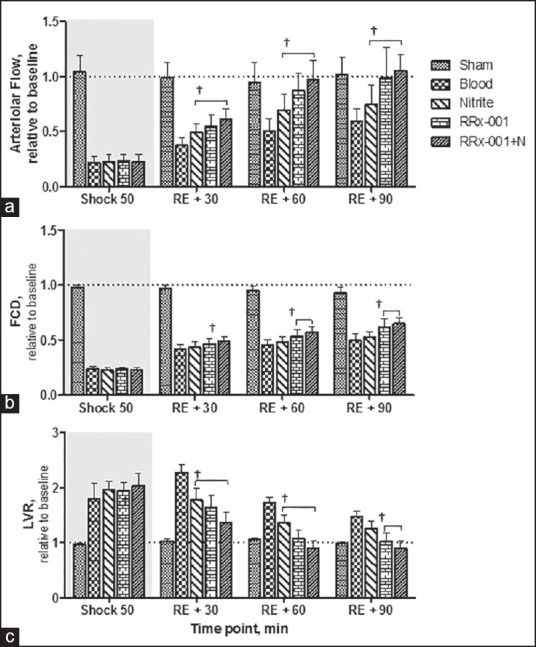
(a) Arteriolar blood flow relative to baseline, baseline arteriolar flow rate is 10.3 ± 3.5 nL/s (b) functional capillary density (FCD) relative to baseline, baseline FCD is 121 ± 15/cm and (c) local vascular resistance during the shock and resuscitation protocol, baseline local resistance is 7.5 1011 mmHg min/L. †P < 0.05, statistically significant compared to blood group
Methemoglobin and tissue viability
Methemoglobin for the nitrite, RRx-001, and RRx-001 + N groups are presented in Figure 4a. Met Hb limits oxygen transport when it exceeds 1.5 g/dL (8-12% of Hb).[24] Therefore, all groups studied had not clinically relevant increase in met Hb.
Figure 4.
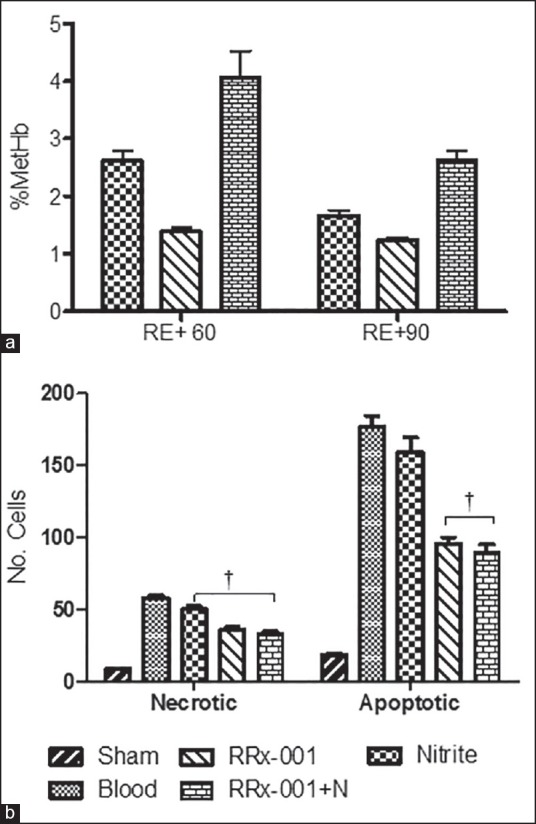
(a) % methemoglobin (MetHb) for the nitrite, RRx-001, and RRx-001 + nitrite (RRx-001 + N) groups at 60 and 90 min postfluid resuscitation. % MetHb in normal, healthy animals is about 2%. (b) The number of apoptotic and necrotic cells at 8 h following resuscitation for all groups. Data are presented as the average of fluorescent cells counted in 40 selected visual fields (210 160 μm). †P < 0.05 compared to blood
Tissue viability (number of apoptotic and necrotic cells in 40 microscopic fields) at 8 h following resuscitation is presented in Figure 4b. The number of apoptotic cells in the RRx-001 and RRx-001 + N groups were significantly lower compared blood. The number of necrotic cells was also significantly lower in the RRx-001 and RRx-001 + N compared to the blood. Nitrite supplementation reduced the number of necrotic cells.
Discussion
The principal finding of the study was that RRx-001 treated blood with or without nitrite supplementation provide superior systemic and microvascular hemodynamic responses compared to transfusion of blood alone during resuscitation from hemorrhagic shock. Modifying Hb of blood used for transfusion-based resuscitation with RRx-001 increasing NO generation. RRx-001 generates NO in two-ways:
As an NO donor: Through metabolism of the dinitro groups released from the compound, and
As an NO promoter: RRx-001 modifies β-Cys 93 increasing nitrite reduction to NO by deoxyhemoglobin [Figure 1].[14]
Mechanistic studies have shown the key role of β-Cys 93 residues in regulating deoxyhemoglobin nitrite reductase activity.[25] Our results demonstrate that improving systemic and microvascular conditions after resuscitation ensures tissue viability. Thus, enhanced nitrite reductase to NO by Hb modification with RRx-001 in blood used to resuscitate from severe hemorrhagic shock can minimizing short- and long-term organ damage.
Hemorrhagic hypotension disrupts vascular homeostasis, and monitoring the microcirculation is crucial in determining the effect fluid therapies. Application of various techniques, including intravital microscopy, has shown the presence of major microcirculatory alterations during hemorrhage,[26] and the persistence of these microcirculatory alterations have been associated with multiorgan failure and death.[27] Our results suggest an improvement of the microcirculatory function mediated by a decrease in LVR. Local microvascular resistance and blood flow are both components of systemic vascular resistance and cardiac output regulation; although, they are the first to compromised during the emergency to preserve vital organs down and last to be recovered after resuscitation.
Blood transfusion is currently the gold standard for treatment of severe hemorrhagic shock. When blood is used during resuscitation, intravascular BV and oxygen carrying capacity are restored, cardiovascular function improves, energy requirements are met, and survival is more likely. Transfusion post hemorrhage recovers the microcirculation, but not necessarily to normal levels. The injury resulting from the shock phase prior to fluid therapy limits perfusion during the resuscitation and thus prevents full recovery of the microcirculation. Moreover, when blood is used, baseline MAP levels can be restored; however, restoring MAP is not necessarily accompanied by a better organ perfusion and oxygenation, due to microvascular flow dysfunctions (the so-called “no reflow” phenomenon).[4,28] The results of our study suggest that during the time when NOS is still malfunctioning, modifying the Hb in the blood used for transfusions for resuscitation with RRx-001 will increase perfusion by opening arterioles and reducing microvascular resistance, leading to improved outcome compared to blood transfusion alone.
The use of NO donors under hemorrhagic shock conditions have been shown to result in enhanced myocardial contractile activity and recovery of MAP, despite reduced peripheral vascular resistance. Remizova et al.[29] studied the effects of an NO donor, DNIC-GS (dinitrosyl iron complexes with glutathione) in a hemorrhagic shock model. They found that injection of DNIC-GS in a rat model prior to hemorrhage resulted in increased stroke volume, left ventricular work, and cardiac output. Our results suggest that RRx-001 may also improve cardiac function in the face of decreased vascular resistance, increasing HR and MAP.
Nitrite is a biologic metabolite of NO and a precursor for NO under acidic conditions. Plasma nitrite levels correlate with eNOS activity and are tightly controlled.[30,31] We have previously studied the effects of nitrite supplementation (10 ηmol of nitrite and 50 ηmol of nitrite) on systemic and microvascular parameters after resuscitation from hemorrhagic shock. Similar effects over systemic and microvascular parameters were observed with the administration of 10 ηmol of nitrate per animal compared with the nitrite group in this study. In previous studies, we also administrated 50 ηmol of nitrate that had a more profound effect on arteriolar diameter and blood flow; however, it decreased blood pressure and increased met Hb levels.[19] For 50 ηmol of nitrate the percentage of metHb at 60 and 90 min was 5.8 ± 1.8% and 3.1 ± 1.3%, respectively. In the current study, RRx-001 treatment maintained blood pressure following resuscitation and resulted in met Hb levels of only 1.4 ± 0.1% at 60 min and 1.2 ± 0.1% at 90 min, which corresponds to met Hb levels for healthy individuals. Blood with RRx-001 modified Hb reduces these limitation, while improves microvascular arteriolar diameter and blood flow.
Blood transfusions are a ubiquitous part of healthcare delivery. In the United States, someone needs blood about every 2 s and according to the 2009 National Blood Collection and Utilization Survey Report, a total of 15 million units of blood were transfused that year.[32] The findings of this study may have a direct application in not only treating cases of hemorrhagic shock but for other indications that require blood transfusion such as anemia.[33,34,35,36] In anemia, transfusion of blood + RRx-001 may be a superior therapeutic approach to transfusion of blood alone because it appears to improve the effectiveness of RBCs providing optimal oxygen delivery to tissues. Since NO bioactivity of stored blood decreases rapidly after blood is removed from the organism, RRx-001 can ameliorate negative effect observed after transfusion of stored blood, through preventing vasoconstriction, capillary poor perfusion and tissue hypoxia.[37,38,39] Restoration of NO bioavailability prior or concurrently with the transfusion strategy may reduce the morbidity and mortality associated with blood transfusion. Furthermore, enhancing the ability of blood to generate NO by incubation with RRx-001 may decrease the number of units of blood needed for treatment and reduce healthcare costs. Detailed preclinical toxicity studies of RRx-001 in mice, rats, and dogs were reported before.[14] Briefly, the study found that the maximum tolerated dose was 20 mg/kg for a single-dose intravenous and cumulative toxicity was not observed. The anti-cancer mechanism of action of RRx-001 is not been fully elucidated; although, it includes NO and ROS generation, and modulation of intracellular redox.
Conclusion
Resuscitation by transfusion of blood treated with RRx-001, an anti-cancer agent, which has successfully completed Phase 1 clinical trials, with or without nitrite supplementation was superior to transfusion of blood alone or blood + nitrite, in terms of arterial diameter, blood flow, FCD, vascular resistance, and tissue viability. Treatment with RRx-001 modified blood alone was preferred over the combination with nitrite due to the absence of adverse systemic side-effects such as decreased blood pressure and methemoglobinemia. Because RRx-001 enhances perfusion and reduces tissue damage, it may serve as an alternative or an adjunct to the conventional treatment of hypotensive resuscitation and in other medical conditions where anemia requires treatment.
Acknowledgments
This work was supported by program project P01-HL071064, and grants R01-HL52684, and R01-HL62354. The author would like to thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals.
Footnotes
Source of Support: This work was supported by Program project P01-HL071064, and grants R01-HL52684, and R01-HL62354.
Conflicting Interest: B. Oronsky and J. Scicinski have ownership interest in RadioRx, Inc. (including patents and stock option). B. Oronsky is an employee of RadioRx, lnc. as CMO; and J. Scicinski is an employee of RadioRx, lnc. as VP Research and Development. Author declares no competing financial interests by the results presented in this manuscript. No financial support was received from RadioRx Inc for the completion of the study. RadioRx inc did not participate in the design of the experimental study.
References
- 1.Cabrales P, Intaglietta M, Tsai AG. Transfusion restores blood viscosity and reinstates microvascular conditions from hemorrhagic shock independent of oxygen carrying capacity. Resuscitation. 2007;75:124–34. doi: 10.1016/j.resuscitation.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krausz MM. Initial resuscitation of hemorrhagic shock. World J Emerg Surg. 2006;1:14. doi: 10.1186/1749-7922-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrales P, Tsai AG, Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80:707–12. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakaria el R, Garrison RN, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: Effect of time delay in therapy initiation. J Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai AG, Intaglietta M. The unusual properties of effective blood substitutes. Keio J Med. 2002;51:17–20. doi: 10.2302/kjm.51.17. [DOI] [PubMed] [Google Scholar]
- 6.Salazar Vázquez BY, Wettstein R, Cabrales P, Tsai AG, Intaglietta M. Microvascular experimental evidence on the relative significance of restoring oxygen carrying capacity vs. blood viscosity in shock resuscitation. Biochim Biophys Acta. 2008;1784:1421–7. doi: 10.1016/j.bbapap.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundvall J, Gustafsson D. Impairment during marked hypotension of the plasma volume control in hemorrhage. Acta Physiol Scand. 1982;114:371–8. doi: 10.1111/j.1748-1716.1982.tb06997.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaul PW. Regulation of endothelial nitric oxide synthase: Location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 9.Szabó C, Thiemermann C. Invited opinion: Role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock. 1994;2:145–55. [PubMed] [Google Scholar]
- 10.Anggård E. Nitric oxide: Mediator, murderer, and medicine. Lancet. 1994;343:1199–206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 11.Nachuraju P, Friedman AJ, Friedman JM, Cabrales P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation. 2011;82:607–13. doi: 10.1016/j.resuscitation.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ning S, Bednarski M, Oronsky B, Scicinski J, Saul G, Knox SJ. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials. Cancer Res. 2012;72:2600–8. doi: 10.1158/0008-5472.CAN-11-2303. [DOI] [PubMed] [Google Scholar]
- 13.RadioRx; 2014. [Last accessed: June 10, 2014]. RadioRx. Lead Compound RRx-001 Overview. Available from: http://www.radiorx.com/rrx-001overview.html . [Google Scholar]
- 14.Scicinski J, Oronsky B, Taylor M, Luo G, Musick T, Marini J, et al. Preclinical evaluation of the metabolism and disposition of RRx-001, a novel investigative anticancer agent. Drug Metab Dispos. 2012;40:1810–6. doi: 10.1124/dmd.112.046755. [DOI] [PubMed] [Google Scholar]
- 15.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–74. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oronsky BT, Scicinski JJ, Reid T, Knox S. Beyond antiangiogenesis: Vascular modulation as an anticancer therapy-a review. Transl Oncol. 2012;5:133–40. doi: 10.1593/tlo.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–8. [PubMed] [Google Scholar]
- 18.Colantuoni A, Bertuglia S, Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984;246:H508–17. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- 19.Cabrales P. Low dose nitrite enhances perfusion after fluid resuscitation from hemorrhagic shock. Resuscitation. 2009;80:1431–6. doi: 10.1016/j.resuscitation.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrales P, Tsai AG, Intaglietta M. Is resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock. 2007;27:380–9. doi: 10.1097/01.shk.0000239782.71516.ba. [DOI] [PubMed] [Google Scholar]
- 21.Cabrales P, Tsai AG, Intaglietta M. Microvascular pressure and functional capillary density in extreme hemodilution with low- and high-viscosity dextran and a low-viscosity Hb-based O2 carrier. Am J Physiol Heart Circ Physiol. 2004;287:H363–73. doi: 10.1152/ajpheart.01039.2003. [DOI] [PubMed] [Google Scholar]
- 22.Yang P, Smith JR, Damodar KS, Planck SR, Rosenbaum JT. Visualization of cell death in vivo during murine endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 2003;44:1993–7. doi: 10.1167/iovs.02-0582. [DOI] [PubMed] [Google Scholar]
- 23.Cabrales P, Tsai AG, Intaglietta M. Deferoxamine lowers tissue damage after 80% exchange transfusion with polymerized hemoglobin. Antioxid Redox Signal. 2007;9:375–84. doi: 10.1089/ars.2006.1379. [DOI] [PubMed] [Google Scholar]
- 24.Hamirani YS, Franklin W, Grifka RG, Stainback RF. Methemoglobinemia in a young man. Tex Heart Inst J. 2008;35:76–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Lui FE, Dong P, Kluger R. Polyethylene glycol conjugation enhances the nitrite reductase activity of native and cross-linked hemoglobin. Biochemistry. 2008;47:10773–80. doi: 10.1021/bi801116k. [DOI] [PubMed] [Google Scholar]
- 26.Sinaasappel M, van Iterson M, Ince C. Microvascular oxygen pressure in the pig intestine during haemorrhagic shock and resuscitation. J Physiol. 1999;514(Pt 1):245–53. doi: 10.1111/j.1469-7793.1999.245af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellis CG, Jagger J, Sharpe M. The microcirculation as a functional system. Crit Care. 2005;9(Suppl 4):S3–8. doi: 10.1186/cc3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezkalla SH, Kloner RA. No-reflow phenomenon. Circulation. 2002;105:656–62. doi: 10.1161/hc0502.102867. [DOI] [PubMed] [Google Scholar]
- 29.Remizova MI, Kochetygov NI, Gerbout KA, Lakomkin VL, Timoshin AA, Burgova EN, et al. Effect of dinitrosyl iron complexes with glutathione on hemorrhagic shock followed by saline treatment. Eur J Pharmacol. 2011;662:40–6. doi: 10.1016/j.ejphar.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 31.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–9. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitaker B. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health; 2011. Report of the US Department of Health and Human Services. The 2009 National Blood Collection and Utilization Survey Report. [Google Scholar]
- 33.Lelubre C, Vincent JL. Red blood cell transfusion in the critically ill patient. Ann Intensive Care. 2011;1:43. doi: 10.1186/2110-5820-1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 35.Spivak JL, Gascón P, Ludwig H. Anemia management in oncology and hematology. Oncologist. 2009;14(Suppl 1):43–56. doi: 10.1634/theoncologist.2009-S1-43. [DOI] [PubMed] [Google Scholar]
- 36.Moliterno AR, Spivak JL. Anemia of cancer. Hematol Oncol Clin North Am. 1996;10:345–63. doi: 10.1016/s0889-8588(05)70342-7. [DOI] [PubMed] [Google Scholar]
- 37.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: A hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51:859–66. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: Role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


