Abstract
Introduction:
Thalassemia is one of the most common genetic disorder of hemoglobin synthesis in Jammu region. Although RBC transfusion is life saving for these patients, it may be associated with some complications like RBC alloimmunization. Thus, the aim of this study was to determine the frequency of alloimmunization and the most common alloantibodies involved.
Material and Methods:
This was a descriptive study involving a total of 70 thalassemic patients in the age range of 2-17 years receiving regular blood transfusions, registered at SMGS Blood Bank, Jammu. Relevant clinical and laboratory data was collected with reference to age at the start of transfusions, total number of transfusions received and splenectomy status. Antibodies screening, antibody identification, and cross matching was done on allpatient samples included in the study, during the period between November 2009 and October 2010.
Results:
In this study, a total of six alloantibodies six patients (8.5%) and one autoantibody (1.42%) was detected. All identified alloantibodies belonged to Rh system (i.e. anti-E, in 3 patients (50%), anti D, in one patient (16.66%)) and Kell system (anti-K, in two patients (33.34%)). Higher frequency of alloimmunization was found, with increase in number of transfusions and in those who received transfusions after 1 year of age. Alloimmunization was not significantly associated with gender and splenectomy status (P-value > 0.05).
Conclusion:
Red cell alloantibodies developed in 8.5% of thalassemic patients and 1.42% had autoantibodies. The most common alloantibodies identified were anti Rh system antibodies (anti-E and anti-D) present in 50% and 16.66% of patients respectively. Alloimmunization is not an uncommon problem faced by blood banks and finding compatible units for regularly transfused thalassemic patients may become very difficult. In order to reduce alloimmunization, a policy for performing extended red cell phenotyping of these patients is essential and at least antigen E and Kell negative blood should be provided for transfusion to these patients.
Keywords: Alloimmunization, multitransfused, thalassemia
Introduction
Thalassemia is a heterogeneous group of genetic disorders of hemoglobin synthesis of globin chains. In India, it is estimated that nearly 8000-10000 new thalassemics (homozygous) are born every year and beta thalassemia gene is found more commonly in Punjabis, Sindhis, Bengalis, and Gujratis.[1] The conventional treatment of beta thalassemia major is based on regular blood transfusions from early childhood. Although blood transfusion is life saving for thalassemia patients, it may be associated with some complications such as iron overload, platelet, and RBC alloimmunization.[2] Therefore, screening for unexpected antibodies should be a part of all pretransfusion testing, with antibody identification in the event of a positive result.[3] Thalassemia patients enrolled for regular transfusions in our hospital belong mainly to Jammu region that lies in the thalassemic zone. This study was therefore designed to find out the frequency of alloimmunization, most common alloantibodies involved to red cell antigens and the factors that might contribute to their development, so that present transfusion policies can be reviewed.
Material and Methods
This study was conducted over a period of one year from November 2009 to October 2010 in Department of Immunohematology and Blood Transfusion Medicine, GMC, Jammu. The study was approved by Hospital ethics committee. Written consent was taken from each patient.
Patients
A total of 70 thalassemia patients in the age ranging from of 2-17 years receiving multiple blood transfusions at interval of 2-4 weeks, or those who had received at least 10 transfusions, were included in this study.
Clinical transfusion records were analyzed for the presence of alloimmunization/autoimmunization with antibody specificity, age at start of transfusion, number of blood units received, and status of splenectomy.
As per transfusion strategy of our institute, all thalassemia patientswere given ABO and RhD-matched packed red cells after compatibility testing by gel card technique in the AHG phase (type and crossmatch policy).
Investigations
Using standard blood bank methods, plasma was separated and analyzed to detect antibodies to RBC antigens. Prior to every transfusion, plasma was tested for the presence of alloantibodies using commercial three cell panel, Diamed-Diacell I, II, III with homozygous expression of the antigens. Any pretransfusion sample with positive antibody screen was subjected to antibody identification. The antibody identification test was performed by acommercial Diamed-Diapanel 1 to 11, with known antigens against the patient's sample. The tests were done using the gel card method by Diamed ID (Switzerland), as per manufacturer's guidelines. A polyspecific direct antiglobulin test was performed using 0.8% cell suspension of the patient's RBC with antihuman globulin. Elution and adsorption methods were employed in patients with suspected autoantibodies.
Statistical Analysis
Descriptive statistics and fishers exact test was used to calculate P-value, wherever necessary.
Results
During the study period, a total of 70 thalassemia patients were reviewed. Fifty-nine (84.28%) patients were of thalassemia major, eight (11.42%) were of thalassemia intermedia and three (4.28%) were of thalassemia minor. Though thalassemia minor patients rarely require blood transfusions but in this study, out of three patients two had chronic kidney disease due to which these children often presented with symptomatic anemia (Hb 5 g/dl) and were given transfusions. Third child had moderate to severe anemia of unknown etiology due to which this patient more often presented with symptomatic anemia, thus requiring blood transfusions. Her Lab workup findings were as follows: Hb — 6.5 g/dl; bone marrow picture showed hyperplasia; PBF — normocytic normochromic picture, reticulocyte counts on higher side.
Male to female ratio in this study was 1.91. The mean (standard deviation) age of patients was 9.27 (4.58) years with age ranged from 2-17 years. Eighteen patients (25.71%) were of blood group A, twenty five (35.71%) were of blood group B, eight (11.44%) were of blood group AB, and nineteen (27.14%) had O blood group. The mean (standard deviation) age at start of transfusion was 1.96 years (2.25) with interquartile range of 5.35-20.83.
In this study, red cell alloimmunization was found in 6 of 70 patients (8.5%), [Table 1] and only one patient (1.42%) developed autoantibodies.
Table 1.
Demographic data of alloimmunized patients have been shown
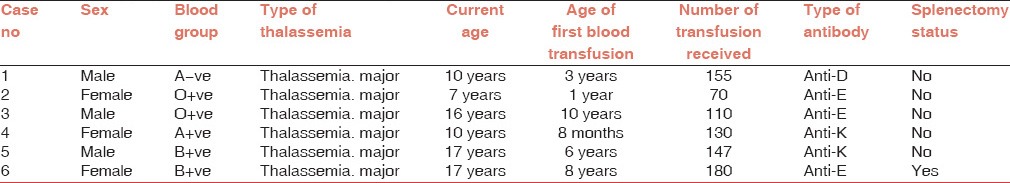
The patients with alloimmunization were in the age range of 7-17 years. Mean age (standard deviation) of patients was 12.83 (4.35) years, 3 patients were <10 years old and 3 (50%) were >10 years old. Male to female ratio was 1:1. Relation between gender and alloimmunization was not statistically significant (P value = 0.33). Mean (S.D) age at first blood transfusion in patients with alloimmunization was 4.7 (3.8) years, with age range from 0.6-10 years. A significant association between alloimmunization and age at start of transfusion was seen. As those patients who started their transfusion at <1 year of age, only 1 out of 33 (3.03%) developed alloantibodies where as those who started their transfusion at >1 year of age, 5 out of 37 (13.57%) developed alloantibodies [Figure 1].
Figure 1.
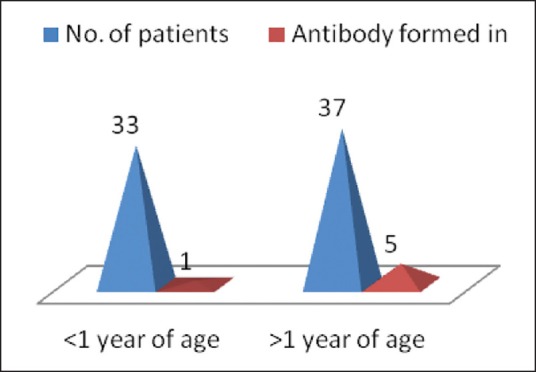
Distribution with respect to age at start of transfusion
As perrecords retrieved from thalassemic patients, the total number of transfusions received by alloimmunized patients was in the range of 71-180, with mean (S.D) of 132 (38.44). The alloimmunization rate was seen higher in those who received >12 transfusions (8.82%) as compared to those who received up to 12 transfusions, (0%). By applying Fischer exact test, P value of 0.01 was calculated which is statistically significant.
In this study, out of six alloimmunized patients, splenectomy was observed in one female patient (16.6%), but she was alloimmunized before splenectomy. No significant association was observed (P value of 0.16).
Discussion
The frequency of alloimmunization reported by different studies ranged from 5-30% in transfusion dependent thalassemia patients.[4,5,6] However, in this study, the frequency of alloimmunization was 8.5% with C.I of 0.44 to 11.56 and autoimmunization was 1.42%. This study was consistent with study by Ho et al. where frequency of alloimmunization was 7.4%.[7] Haslina et al. in 2006 reported frequency of 8.6% among Malaysian multitransfused thalassemics.[8] Similar study reported from Northern India by Pahuja et al. reported very low alloimmunization rate of 3.79%.[9] However, a study by Ahmed et al. in 2010 reported the alloimmunization rate of 11.3% in Egyptian thalassemic patients.[10]
The most common alloantibody detected was anti-Rh system antibodies (anti-E seen in three patients (50%) and anti-D seen in one patient (16.66%)) followed by anti-K seen in two patients (33.34%). All of our patients received compatible blood for ABO and Rh (D) antigens after major cross matching. Several studies, have shown anti-E as most common alloantibody as reported by Sirchia et al.[3] Ho et al. detected anti-E with a rate of 21%.[10] Ameen et al. detected anti-E with a rate of 46.5%.[7] Haslina et al. detected anti-E with a rate of 44.4%.[8]
One of most important reason for anti-D alloimmunization in ourstudy was transfusion of some red blood cells withRh D incompatible blood, due to false negative results in weak D typing of blood donors. Transfusions of weak D red cells to D negative patients stimulate the immune system for production of anti-D. In weak D individuals, the Dantigen usually requires enhancement with anti human globulin (AHG). It is thus required that more attention should be given to quality control programs in blood banking laboratory especially for determination of weak D positive RBCs and reporting of D negative blood group.
In this study, autoantibodies were found in a 15-year-old girl. She developed autoantibodies without underlying alloantibodies, as determined by positive autocontrol test and persistent positive direct Coombs test with no specific pattern on the red cell elution test and pan agglutination on the adsorption test. The monospecific drect Coombs test was positive for Ig G and C3d in this patient. No secondary causes were identified in this patient.
In study by Ameen et al. in 2003, frequency of autoimmunization was 11% and similar study by Haslina et al. in 2006, frequency of autoimmunization was 1.7%.[8,11]
This study shows no significant association between alloimmunization and gender (P-value = 0.33), as male to female ratio in alloimmunized patients was 1:1. Similar studies by Bilwani et al. 2005 reported M:F ratio 2:1.[12] Bashawari et al. 2005 reported M:F ratio 1:2.[13] Haslina et al. reported no significant association between gender and alloimmunization (P = 0.16).[8]
In this study, mean age at start of transfusion of alloimmunized patients was 4.7 years. The rate ofalloimmunization who started transfusion at <1 year of age was 3.03% compared to those who started at >1 year of age was 13.53% [Figure 1]. Merianou et al. 1987 found that the rate of alloimmunization was significantly lower when age of starting transfusion was <1 year (9% vs 38.7%).[5] Similar study by Sirchia et al. reported that those receiving transfusion before age of 6 years were less prone to antibody formation (1.1% vs 5.7%; P < 0.01).[3] These results support the view that there is some form of immune tolerance induced by animmature immune response to repeated blood transfusions. Spanos et al. reported alloantibody formation was significantly less in patients where blood transfusion was started prior to age three (20.9%) as compared to when transfusion was started later (47.5%). This resistance to alloimmunization is perhaps the consequence of the immaturity of the immunological system and particularly that of antibody production.[6]
In this study, we found the earliest development of antibodies was after transfusion of 12 units of packed red cells. A significant association between alloimmunization and number of transfusions was observed. (P value < 0.05%). Spanos et al. reported earliest sensitization after approximately 10 transfusions in thalassemics patients.[6]
In this study, 2.85% patients underwent splenectomy, only one of them had alloantibody before splenectomy (P value = 0.16). In a study in Hong Kong by Ho et al. splenectomy did not affect the incidence of alloimmunization.[7]
Conclusion
Due to high incidence of anti Rh system (66.66%) and anti-K (33.3%) antibodies in our study population, it is advisable to phenotype patients and donors and matched red cell units for at least Rh blood group system and Kell blood group system antigens in addition to ABO and D antigen should be providedt. Antigen-matched transfusions should effectively prevent alloimmunization for thalassemia patients who have a lifelong, transfusion-dependent disease.
Acknowledgement
Dr. Dinesh Kumar MD, Department of Community Medicine, Government Medical College, Jammu for statistical analysis.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.Shah D, Chowdhary P, Dubey AP. Current trends in management of beta thalassemia. Indian J Pediatr. 1999;36:1229–42. [PubMed] [Google Scholar]
- 2.Sadeghian MH, Keramati MR, Badiei Z, Ravarian M, Ayatollahi H, Rafatpanah H, et al. Alloimmunization among transfusion-dependent thalassemia patients. Asian J Transfus Sci. 2009;3:95–8. doi: 10.4103/0973-6247.53884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sirchia G, Zanella A, Parravicini A, Morelati F, Rebulla P, Masera G. Red cell alloantibodies in thalassemia major. Transfusion. 1985;25:110–2. doi: 10.1046/j.1537-2995.1985.25285169198.x. [DOI] [PubMed] [Google Scholar]
- 4.Shin JH, Lee JY, Kim JH, Kim HR, Lee JN. Screening and identification of unexpected red cell antibodies by simultaneous LISS/Coombs and NaCl/Enzyme Gel Methods. J Korean Med Sci. 2009;24:632–5. doi: 10.3346/jkms.2009.24.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michail-Merianou V, Pamphili-Panousopoulou L, Piperi-Lowes L, Pelegrinis E, Karaklis A. Alloimmunization to red cell antigens in thalassemia: Comparative study of usual versus better match transfusion programmes. Vox Sang. 1987;52:95–8. doi: 10.1111/j.1423-0410.1987.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 6.Spanos T, Karageora M, Ladis V, Peristeri J, Hatziliani A, Kattamis C. Red cell alloantibodies in patients with thalassemia. Vox San. 1990;58:50–5. doi: 10.1111/j.1423-0410.1990.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 7.Ho HK, Ha SY, Lam CK, Chan GC, Lee TL, Chiang AK, et al. Alloimmunisation in Hong Kong southern Chinese transfusion dependent thalassemia patients. Blood. 2001;97:3999–4000. doi: 10.1182/blood.v97.12.3999. [DOI] [PubMed] [Google Scholar]
- 8.Haslina MN, Ariffin N, Hayati II, Roseline H. Red cell alloimmunization in multiply transfused malay thalassemic patients. South East Asian J Trop Med Public Health. 2006;37:1015–20. [PubMed] [Google Scholar]
- 9.Pahuja S, Pujani M, Gupta KS, Chandra J, Jain M. Alloimmunization and auto immunization in multitransfused thalassemics of Indian origin. Hematology. 2010;15:174–7. doi: 10.1179/102453309X12583347114013. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MA, Hasan SN, Ragab HS, Habib AS, Emara AN, Aly AA. Red cell alloimmunization and autoantibodies in Egyptian transfusion dependent thalassemia patients. Arch Med Sci. 2010;6:592–8. doi: 10.5114/aoms.2010.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameen R, Al-Eyaadi O, Al-Shemmari S, Chowdhary R, Al-Bashir A. Frequency of red blood cell alloantibody in Kuwaiti population. J Medical Prin Pract. 2005;14:230–4. doi: 10.1159/000085740. [DOI] [PubMed] [Google Scholar]
- 12.Bilwan F, Kakepoto GN, Adil SN, Usman M, Hassan F, Khurshid M. Frequency of irregular red cell alloantibodies in patients with thalassemia major: A bicentre study. J Pak Med Assoc. 2005;55:563–5. [PubMed] [Google Scholar]
- 13.Bashawari LA, Ahmed MS, Fawaz NE, Qatarya A, Ak AL, Ahmed MA. Red cell alloimmunization in thalassemia patients. Bahrain Med Bull. 2005;27:1–5. [Google Scholar]


