Abstract
Allo-anti-M often has an immunoglobulin G (IgG) component but is rarely clinically significant. We report a case of hemolytic disease of the fetus and newborn along with prolonged anemia in newborn twins that persisted for up to 70 days postbirth. The aim was to diagnose and successfully manage hemolytic disease of newborn (HDN) due to maternal alloimmunization. Direct antiglobulin test (DAT), antigen typing, irregular antibody screening and identification were done by polyspecific antihuman globulin cards and standard tube method. At presentation, the newborn twins (T1, T2) had HDN with resultant low reticulocyte count and prolonged anemia, which continued for up to 70 days of life. Blood group of the twins and the mother was O RhD positive. DAT of the both newborns at birth was negative. Anti-M was detected in mothers as well as newborns. Type of antibody in mother was IgG and IgM type whereas in twins it was IgG type only. M antigen negative blood was transfused thrice to twin-1 and twice to twin-2. Recurring reduction of the hematocrit along with low reticulocyte count and normal other cell line indicated a pure red cell aplastic state. Anti-M is capable of causing HDN as well as prolonged anemia (red cell aplasia) due to its ability to destroy the erythroid precursor cells. Newborns with anemia should be evaluated for all the possible causes to establish a diagnosis and its efficient management. Mother should be closely monitored for future pregnancies as well.
Keywords: Anti-M alloimmunization, hemolytic disease of newborn, pure red cell aplasia
Introduction
Anemia in a newborn can be severe to present as an acute life-threatening event or as a mild incidental finding. The management approach to these two conditions is different; primarily priority of stabilizing the infant is more in the former, whereas in the other, the clinician has time to develop a diagnostic plan before the need for a therapeutic intervention. A thorough maternal family history and history of pregnancy is of paramount importance in work-up of a newborn with anemia.
Most cases of neonatal anemia with fetal erythroblastosis are caused by alloimmunization to antigens in Rh blood group, most commonly by anti-D alloantibody, which was first reported by Levine et al. in 1941.[1] With advancement in the treatment strategies and maternal prophylaxis with anti-D (Rh immunoglobulin, RhIg), the incidence of hemolytic disease of newborn (HDN) has successfully decreased. However, there are still many other blood group incompatibilities, for example, antibodies against the Kell, Duffy, Kidd, and the MNS blood group system, that may be the cause of hemolysis in newborn but there is no consensus on management of such pregnancies. Antibodies with anti-M specificity, usually IgM, have been reported to be detected in 10% of pregnant women with a positive antibody screen. However, 0.01-0.7% of pregnant women would trigger anti-M IgG that can cross the placenta, resulting in variable degrees of hemolysis in fetuses.[2]
We present a case of newborn twins presenting with initial features of hemolysis followed by prolonged anemia due to maternal alloimmunization to M antigen. Twins required blood transfusion and were followed-up for antibody titers along with the mother.
Case Report
A pregnant women (gravida 2) with a 3-year-old daughter delivered twins (diamniotic dichorionic) at 38 weeks of gestation as normal vaginal delivery. The newborns were normal at the time of birth, but presented with hyperbilirubinemia at 16 h of life. Mother was never transfused previously. Clinically both the newborns were stable hemodynamically with no hepatosplenomegaly, hydrops, hematoma, polycythemia, or twin discordance on examination and were adequately taking breast milk. Blood bank received a requisition for reconstituted whole blood for an exchange transfusion for the twins (T1, T2) as their billirubin [Figure 1] was in exchange zone.
Figure 1.
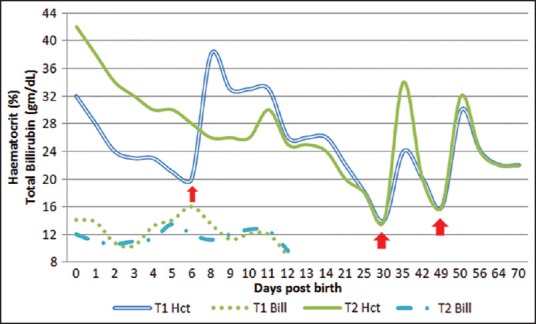
Variation of total billirubin and hematocrit of both the twins (arrow represents the transfusion received by them)
Twins did not require exchange transfusion as phototherapy which was given over 4 days showed a reduction in the bilirubin levels. Both the newborns further showed progressive decrease in hematocrit (HCT) and later developed hepatosplenomegaly. Peripheral smears revealed no evidence for hemolysis, low reticulocyte count (T1-4%, T2-3%). The TORCH titers, serology for parvovirus, sepsis screen were all negative. Quantitative G6PD and thyroid profile were within normal range. Bone marrow examination showed predominantly normoblasts with few cells showing dyserythropoiesis. The other cell lines such as megakaryocytes and cells of lymphoid lineage were normal. Further adenosine deaminase levels were also done to rule out Diamond-Blackfan anemia. The mother and father also had normal beta and alpha thalassemia screen.
Sample and methods
Blood sample of the newborn twins as well as the mother were sent to the blood bank. ABO and D blood typing was performed routinely by both manual tube and column agglutination technology (CAT). Direct antiglobulin test (DAT) was done in CAT cards containing polyspecific antihuman globulin (AHG) (anti-IgG and anti-C3d). Screening of irregular antibody and identification was done using surgiscreen and resolve Panel A, ortho-clinical diagnostics (USA) with polyspecific AHG cards. Rh, MNSs and Kell phenotypes were performed in tubes according to manufacturer's instruction by direct agglutination (Rh, K, M, N, S) and by IAT(s). CAT cards (plain and AHG) and rare antisera used for phenotyping were from ortho-clinical diagnostics (USA). Antibody titration was performed on CAT polyspecific cards and by direct agglutination on tubes. The titer was determined by serial two-fold dilutions in saline, tested against selected M+N− red blood cells (RBCs). The reciprocal of the highest dilution that gave a 1+ reaction is referred to as the titer.
Immunohematological work-up
Twins
Blood group of twins was O RhD positive. DAT was negative for both the twins. Elution of the DAT negative sample was not done.
Mother
Blood group of mother was O RhD positive. On antibody screening, mother showed a positive reaction with screening red blood cells, the reaction strength being cell I− (2+); cell II− (4+); cell III− (4+) at both room temperature and AHG phase, indicating presence of an alloantibody in maternal sample of both IgM and IgG type, which was duly confirmed on 1,4-dithiothreitol treatment.
Further antibody identification panel showed antibody specificity to M antigen, reacting at both room temperature and AHG phase. Similar antibody was identified in the serum of both the twins as well, but reacting at AHG phase only (IgG type). Both antibody screen and identification done on the CAT and manual tube showed similar results.
Phenotype of the twins was a heterozygous state of M and N antigen (M+N+). The mother was homozygous for N antigen (M−N+) and father was homozygous for M antigen (M+N−) [Table 1].
Table 1.
Antigenic phenotypes
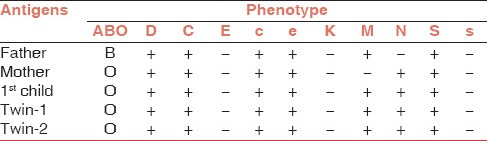
Serology of anti-M alloantibody
The M alloantibody (both IgG and IgM types) showed clear evidence of dosage phenomena with a weaker reaction with the heterozygous cells (M+N+) when compared to a homozygous cell (M+N−). The antibody identification was further enhanced by adding one part (0.1 N) HCl to four parts of the serum before testing (to lower the pH at 6.5). Alloantibody also showed a stronger reaction in albumin than in saline.
The antibody titer (using tube and CAT both) during 1st week of life was 16 (IgG) in twin-1 (T1) and 8 (IgG) in twin-2 (T2). The mother had a titer of 16 (IgG) and 8 (IgM) after the delivery. Anti-M was detectable in the twins until 7th weeks of life. The anti-M titer in the mother were constant at 32 (IgG) and 16 (IgM) until 8 weeks of follow-up postbirth [Figure 2].
Figure 2.
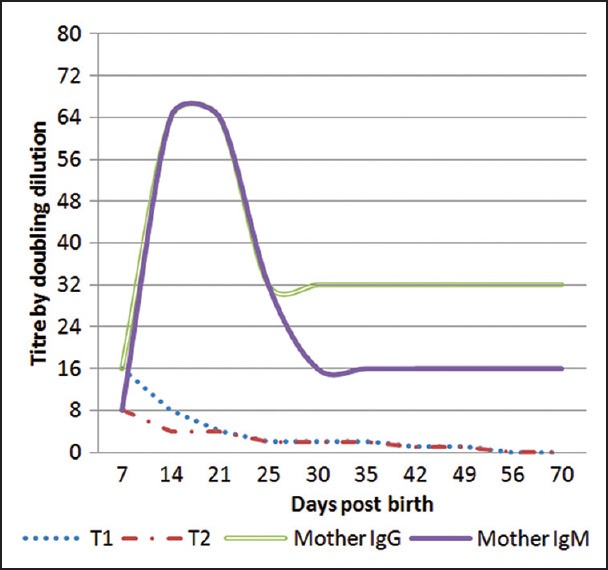
Anti-M alloantibody titre variation in mother and twins
Transfusion management and clinical course
Both the newborns initially showed rise in bilirubin, which subsided following phototherapy (started on day 1) on day 4 and did not require exchange transfusion. Twin-1 showed a decrease in hematocrit to 16% at day 6 and was transfused with 40 mL of (M−N+) packed RBCs (PRBC). On day 32, HCT dropped again to 16% but without any rise in bilirubin and both newborns were transfused with M−N+ PRBC. Repeatedly both showed decrease in the red cells, with all rest other cell lines normal, without any feature of hemolysis. On day 49, postbirth both the twins again had low HCT (15%) and were again transfused with (M−N+) PRBC. Twins continued to have anemia until 70 days of life after birth, their hematocrits stabilized their after [Figure 1].
Discussion
Anemia at birth can be due to various causes, broadly classified on the basis of a decreased production of red cells or an increased destruction. Conditions for decrease in production includes constitutional causes of bone marrow failure as Diamond–Blackfan anemia and Pearson's syndrome were ruled out by specific tests, acquired conditions such as infections and aplastic anemia also were excluded. Nutritional causes were ruled out as iron and folate profiles were in normal range. Bone marrow examination excluded the marrow infiltrative disorders such as leukemia, neuroblastoma and histocytosis.
Anemia due to increased destruction of red cells includes the intrinsic red cell defect, which was ruled out by peripheral blood smear and hemoglobin electrophoresis. Infection/disseminated intravascular coagulation were also excluded as the acquired causes of hemolysis. Immune causes of hemolysis for persistent anemia were considered as the mother showed the presence of an alloantibody against M antigen and M antigen was detected on the twin red cells.
The first case of alloantibody against M antigen causing HDN was reported in 1959 where anti-M with a high titer resulted in a fetal death and a severely affected the child (a twin) at 35 weeks of pregnancy. The woman had a previous miscarriage at 20 weeks and a healthy child. Her husband was heterozygous for the M antigen.[3] Alloantibody against M antigen is repeatedly reported in the literature, but most of them are of IgM type causing grouping discrepancy.[4] Very few cases have been reported of anti-M to be IgG type and there is no clinical consensus on the management of HDN caused by anti-M alloantibody.
Clinically significant anti-M causing HDN in mothers have been always reported due to the previous birth as in the case this first pregnancy induced the formation of the alloantibody.[5,6,7,8] In all the reported case, the mother was homozygous for N antigen and fetus heterozygous for M and N antigen. The frequency of M+N− phenotype in our donor population is reported between 38% and 42% and M−N+ phenotype is 14-24% and the heterozygous state (M+, N+) is reported to be between 36% and 43%, respectively.[9,10] M+N−S+s− (Father) and M−N+S+s− (Mother) phenotype is of very low frequency 7% and 1%, probably, the reason why these cases are very rarely reported in our country.
One of the key features for diagnosing immune cause of HDN is a positive DAT, indicating that the fetal red blood cells are coated with the IgG type of maternal antibody. HDN caused by anti-M antibody is generally presented with a negative DAT even though the MNS blood group system is completely developed at birth. It has been previously reported that the DAT might be negative in anti-M-mediated hemolysis.[3,5,11,12] One hypothesis states that it is because of very rapid intravascular hemolysis, while the other states that it could be similar to anti-K and anti-Ge HDN, where erythroid progenitor cells possess Kell and Gerbich antigens.[13,14,15] Both MNSs and Gerbich antigens are present on glycophorins (glycophorins A, B, C, and D, respectively).[16,17] Unlike Rh, the glycophorins also appear on erythroid progenitor cells as well.[16] The hemolytic disease due to anti-M shows two features that resemble ABO hemolytic disease rather than Rh hemolytic disease: First, the DAT is only weakly positive or negative and second, the osmotic fragility of the red cells may be greatly increased.[3,18]
As all other cell lines were in normal range with a low reticulocyte count since birth indicating a possible condition of transient pure red cell aplasia in the twins. Hemolysis presents with a raised reticulocyte count, which was probably masked in this case due to effect of anti-M on the erythroid precursor cells. The M antigen is expressed on immature erythroid precursors[12] and it is possible that precursor cell growth would be inhibited by maternal anti-M, which has been previously also reported.[19] It was observed that the anemia with the twins persisted for up to 70 days of life, which coincided with the possible presence of alloantibody in the newborn until that time, as detectable anti-M was observed until almost 50 days (7 weeks) of life.
The anti-M titer seems to be an unreliable predictor, and more importantly previous reports of titration were performed by different techniques, temperature, and suspension media against MN or MM RBCs resulting in various titers. Reviewing the literature, out of the few cases reported on anti-M associated with fetal anemia, five of six reports describe intrauterine death in Gestational weeks 10-35, with the majority affected around 6 months’ of gestation.[3,5,8] The anti-M IgG titer was only 16 and IgM was 8 was in mother at birth although, it is reported that a titer of 16 IgM to cause intrauterine deaths. Titers in the mother showed a typical anamnestic response as they went up until 64 (both IgG and IgM) in the following week postdelivery due to exposure of M+ cells during birth. The anti-M titers were still measurable with the strength of 16 (IgM) and 32 (IgG) in the mother until 10 weeks postbirth. Wikman et al.[20] tried to prove the activity of anti-M in maternal blood by infusion of 51Cr-labeled M+ and M− RBCs. Which showed the IgM anti-M may have an important role in the hemolysis, whereas in the antenatal situation the IgG component only is of importance. The interpretation of his work was that the anti-M can cause severe fetal anemia, which was also supported by the rapid clearance of M+ RBCs in vivo.
Anti-M antibodies have often been detected in antenatal mothers. In some case reports in the literature, this antibody is described as the second most common non-Rh antibody after anti-Kell[2] but only sporadic cases of anti-M isoimmunization have been reported during the past few decades. Various authors have also proposed that like anti-K, anti-Ge[3], anti-M may cause HDN primarily by destroying erythroid progenitors rather than mature erythrocytes hence might not present with typical features of HDN, but with a prolonged anemia in the neonatal period.
With the improvement of immunohemaotlogical standards in the country, the detection of such rare causes of HDN are now being reported. With the interest of the clinician the guideline for the treatment and antenatal monitoring of these cases needs to be worked upon in order to manage such patients efficiently. Fetal surveillance using either middle cerebral artery peak systolic velocity measurements or fetal hemoglobin results obtained by fetal blood sampling is important for management of alloimmunized pregnancies. Intraperitoneal transfusion was the first method used for intrauterine transfusion of compatible red cells, was also used as a method to treat such fetuses. Plasmapheresis of the mother also is a method, which aims to decrease the target antibody titer in the mother by direct plasma replacement. Intravenous Ig (IVIG) blocks the Fc receptors of the fetal reticulo-endothelial system, thereby reducing the degree of phagocytosis of sensitized fetal red cells. Both plasmapheresis and IVIG have been utilized individually for their effect of prolongation of gestation prior to the need for intrauterine therapy. Combined plasmapheresis and IVIG therapy has also been utilized in a variety of antibody-mediated diseases. Only few cases have been reported in the literature using combined treatment in maternal red cell alloimmunization, with favorable outcome.[21] Plasmapheresis may be started as soon as possible right after conformation of pregnancy to lower the anti-M IgG titer. Treatment should be given in cases of high titer, before the onset of hydrops with serial intrauterine transfusion.
Anemia at birth is a diagnostic and management challenge in the newborn. Anti-M is usually reported to be a naturally occurring antibody of IgM and IgG type but has been rarely reported to be clinically significant. Anti-M alloimmunization as the cause of the HDN and persistent anemia (transient pure red cell aplasia) in the twins was evident in our case. Deteriorating hematocrit with a low reticulocyte count and normal other cell lines indicated a possible reduction of erythroid precursors by the anti-M alloantibody causing anemia to persist up to 70 days of life with the twins. This case reflects the importance of exclusion of all the possible causes of anemia for diagnosing and clinical management. From immunohematology point of view, an antibody screen of the mother even when DAT of the newborn is negative is of utmost importance. Follow-up of the mother for serial titers until detectable and serial antenatal and serological monitoring will be necessary in case she plans another pregnancy.
Footnotes
Source of Support: Nil
Conflicting Interest: None declared.
References
- 1.Levine P, Vogel P, Katzin EM, Burnham L. Pathogenesis of erythroblastosis fetalis: Statistical evidence. Science. 1941;94:371–2. doi: 10.1126/science.94.2442.371. [DOI] [PubMed] [Google Scholar]
- 2.Kornstad L. New cases of irregular blood group antibodies other than anti-D in pregnancy. Frequency and clinical significance. Acta Obstet Gynecol Scand. 1983;62:431–6. doi: 10.3109/00016348309154215. [DOI] [PubMed] [Google Scholar]
- 3.Stone B, Marsh WL. Haemolytic disease of the newborn caused by anti-M. Br J Haematol. 1959;5:344–7. doi: 10.1111/j.1365-2141.1959.tb04044.x. [DOI] [PubMed] [Google Scholar]
- 4.Khalid S, Dantes R, Varghese S, Al Hakawati I. Naturally occurring anti M complicating ABO grouping. Indian J Pathol Microbiol. 2011;54:170–2. doi: 10.4103/0377-4929.77394. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto H, Tamaki Y, Sato S, Shibata K. A case of hemolytic disease of the newborn caused by anti-M: Serological study of maternal blood. Acta Obstet Gynaecol Jpn. 1981;33:525–8. [PubMed] [Google Scholar]
- 6.Furukawa K, Nakajima T, Kogure T, Yazaki K, Yoshida M, Fukaishi T, et al. Example of a woman with multiple intrauterine deaths due to anti-M who delivered a live child after plasmapheresis. Exp Clin Immunogenet. 1993;10:161–7. [PubMed] [Google Scholar]
- 7.Duguid JK, Bromilow IM, Entwistle GD, Wilkinson R. Haemolytic disease of the newborn due to anti-M. Vox Sang. 1995;68:195–6. doi: 10.1111/j.1423-0410.1995.tb03927.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanra T, Yüce K, Ozcebe IU. Hydrops fetalis and intrauterine deaths due to anti-M. Acta Obstet Gynecol Scand. 1996;75:415–7. doi: 10.3109/00016349609033344. [DOI] [PubMed] [Google Scholar]
- 9.Nanu A, Thapliyal RM. Blood group gene frequency in a selected north Indian population. Indian J Med Res. 1997;106:242–6. [PubMed] [Google Scholar]
- 10.Thakral B, Saluja K, Sharma RR, Marwaha N. Phenotype frequencies of blood group systems (Rh, Kell, Kidd, Duffy, MNS, P, Lewis, and Lutheran) in north Indian blood donors. Transfus Apher Sci. 2010;43:17–22. doi: 10.1016/j.transci.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Thompson DJ, Stults DZ, Daniel SJ. Anti-M antibody in pregnancy. Obstet Gynecol Surv. 1989;44:637–41. doi: 10.1097/00006254-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Bony V, Gane P, Bailly P, Cartron JP. Time-course expression of polypeptides carrying blood group antigens during human erythroid differentiation. Br J Haematol. 1999;107:263–74. doi: 10.1046/j.1365-2141.1999.01721.x. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan JI, Manning M, Warwick RM, Letsky EA, Murray NA, Roberts IA. Inhibition of erythroid progenitor cells by anti-Kell antibodies in fetal alloimmune anemia. N Engl J Med. 1998;338:798–803. doi: 10.1056/NEJM199803193381204. [DOI] [PubMed] [Google Scholar]
- 14.Arndt PA, Garratty G, Daniels G, Green CA, Wilkes AM, Hunt P, et al. Late onset neonatal anaemia due to maternal anti-Ge: Possible association with destruction of eythroid progenitors. Transfus Med. 2005;15:125–32. doi: 10.1111/j.0958-7578.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 15.Denomme GA, Shahcheraghi A, Blackall DP, Oza KK, Garratty G. Inhibition of erythroid progenitor cell growth by anti-Ge3. Br J Haematol. 2006;133:443–4. doi: 10.1111/j.1365-2141.2006.06073.x. [DOI] [PubMed] [Google Scholar]
- 16.Poole J. Red cell antigens on band 3 and glycophorin A. Blood Rev. 2000;14:31–43. doi: 10.1054/blre.1999.0124. [DOI] [PubMed] [Google Scholar]
- 17.Daniels G, Green C. Expression of red cell surface antigens during erythropoiesis. Vox Sang. 2000;78(Suppl 2):149–53. [PubMed] [Google Scholar]
- 18.Freiesleben E, Jensen KG. Haemolytic disease of the newborn caused by anti-M. The value of the direct conglutination test. Vox Sang. 1961;6:328–35. doi: 10.1111/j.1423-0410.1961.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 19.Hinchliffe RF, Nolan B, Vora AJ, Stamps R. Neonatal pure red cell aplasia due to anti-M. Arch Dis Child Fetal Neonatal Ed. 2006;91:F467–8. doi: 10.1136/adc.2006.102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikman A, Edner A, Gryfelt G, Jonsson B, Henter JI. Fetal hemolytic anemia and intrauterine death caused by anti-M immunization. Transfusion. 2007;47:911–7. doi: 10.1111/j.1537-2995.2007.01209.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruma MS, Moise KJ, Jr, Kim E, Murtha AP, Prutsman WJ, Hassan SS, et al. Combined plasmapheresis and intravenous immune globulin for the treatment of severe maternal red cell alloimmunization. Am J Obstet Gynecol. 2007;196:138.e1–6. doi: 10.1016/j.ajog.2006.10.890. [DOI] [PubMed] [Google Scholar]


