Abstract
Stimulation of the vagus nerve has been previously reported to promote neural plasticity and neurogenesis in the brain. Several studies also revealed plastic changes in the spinal cord after injuries to somatosensory nerves originating from both the brachial and lumbo-sacral plexuses. However, the neurogenic responses of the brain to the injury of the viscerosensory innervation are not as yet well understood. In the present study, we investigated whether cells in the dentate gyrus of the hippocampus respond to a chemical and physical damage to the vagus nerve in the adult rat. Intraperitoneal capsaicin administration was used to damage non-myelinated vagal afferents while subdiaphragmatic vagotomy was used to damage both the myelinated and non-myelinated vagal afferents. The 5-bromo-2-deoxyuridine (BrdU) incorporation together with cell-specific markers was used to study neural proliferation in subgranular zone, granule cell layer, molecular layer and hilus of the dentate gyrus. Microglia activation was determined by quantifying changes in the intensity of fluorescent staining with a primary antibody against ionizing calcium adapter-binding molecule 1. Results revealed that vagotomy decreased BrdU incorporation in the hilus 15 days after injury compared to the capsaicin group. Capsaicin administration decreased BrdU incorporation in the granular cell layer 60 days after the treatment. Capsaicin decreased the number of doublecortin-expressing cells in the dentate gyrus, whereas vagotomy did not alter the expression of doublecortin in the hippocampus. Both the capsaicin- and the vagotomy-induced damage to the vagus nerve decreased microglia activation in the hippocampus at 15 days after the injury. At 30 days post injury, capsaicin-treated and vagotomized rats revealed significantly more activated microglia. Our findings show that damage to the subdiaphragmatic vagus in adult rats is followed by microglia activation and long-lasting changes in the dentate gyrus, leading to alteration of neurogenesis.
Keywords: vagus injury, hippocampus, vagotomy, capsaicin, vagal afferents, microglia, rat
Abbreviations
BrdU, 5-bromo-2-deoxyuridine; CNS, central nervous system; VNS, vagus nerve stimulation; DCX, doublecortin; Iba1, ionizing calcium adapter binding molecule-1
INTRODUCTION
The communication between the brain and the periphery is a two-way road. The hypothesis that visceral afferents exert a significant influence on the central nervous system (CNS) plasticity has been tested by many investigators. Recent studies have already showed that vagus nerve stimulation (VNS) can induce neurogenesis and plasticity in the hippocampus[1]. In particular, it was demonstrated that acute VNS induces cell proliferation in the dentate gyrus of the adult rat hippocampus and an increase of both the total amount of doublecortin (DCX) immunoreactivity and the number of DCX-positive neurons in the dentate gyrus[1]. In contrast, chronic VNS did not alter the numbers of 5-bromo-2- deoxyuridine (BrdU) + DCX + cells in the dentate gyrus of the rat hippocampal formation but induced an increase of the brain-derived neurotrophic factor expression, which may serve to promote and maintain new neuronal connections formed in response to chronic VNS[1]. Moreover, it was shown that acute VNS increased the expression of genes for brain-derived neurotrophic factor and basic fibroblast growth factor in the rat hippocampus, both of which are important modulators of hippocampal plasticity and neurogenesis[2].
Several studies have revealed that injury to somatosensory nerves is followed by plastic changes in the CNS. That is, it was previously demonstrated that after severe brachial plexus injury, the remarkable restoration of sensory function occurred in avulsed spinal root dermatomes in human patients[3] indicates the immense plasticity of the CNS. Another study showed that in obstetric brachial plexus palsy, changes in spinal cord architecture reflected the subsequent regeneration process[4]. However, little is known about plasticity of the CNS following injuries to the viscerosensory innervation. Ryu et al[5] recently reported that capsaicin-induced damage to gastric viscerosensory innervation in rats was followed by restoration of VR1-immunopositive projections by 37 days, whereas both total afferent innervation and VR1-immunopositive innervation failed to reach control levels, even by 67 days after subdiaphragmatic vagotomy. It has also been shown that central viscerosensory projections in the nucleus of the solitary tract could be restored by 300 days after capsaicin-induced nerve damage[6].
Previous reports indicate that circulating cytokines and other inflammatory molecules that are produced by insults in the peripheral nervous system (PNS) can affect the brain[7]. Several reports indicated that spinal nerve damage resulted in activation of microglia in the dorsal horn of the spinal cord containing the central terminals of the damaged nerve[8,9,10]. Gallaher et al[11] found that subdiaphragmatic vagotomy significantly activated microglia in the hindbrain and nodose ganglion, supporting the hypothesis of CNS plasticity following damage to the PNS. Hippocampal microglia are distributed in the hilus and molecular layer of the dentate gyrus and concentrated on the hilar and molecular layer borders of the granule cell layer[12]. They are rapidly activated in response to acute neuropathological events following CNS insults of various natures, such as traumatic brain injury, ischemia or seizures[13,14,15]. Following such insults, microglia rapidly proliferate[16,17] and undergo morphological alterations[18,19], becoming hypertrophic and increasing or expressing de novo a plethora of surface markers[20,21].
In the present study, we investigated whether damage to the subdiaphragmatic vagus induces neural plasticity in the dentate gyrus of the hippocampus. The neurotoxin capsaicin was used to destroy unmyelinated axons of small primary afferent neurons[22]. Capsaicin treatment of adult rats was shown to produce extensive degeneration of vagal afferent axons and terminals[23]. We also performed a subdiaphragmatic vagotomy to destroy myelinated and non-myelinated axons of the vagus nerve. To study cell proliferation and differentiation, we evaluated the BrdU incorporation and DCX- immunoreactivity. Microglia activation was determined by quantifying changes in the ionizing calcium adapter binding molecule-1 (Iba1) immunoreactivity.
RESULTS
Capsaicin treatment and vagotomy verification
All the vehicle treated rats immediately wiped the eye where drop of 1% ammonium hydroxide was applied. Systemic administration of capsaicin abolished eye wipe reflex in all the i.p. injected rats. All the vagotomized rats met the criteria of complete vagotomy described previously[24].
The number of BrdU-labeled cells changes in the hilus and granule cell layer of the dentate gyrus after vagus nerve injury
To study if an injury applied peripherally to the vagus nerve could cause changes in the number of newly generated cells in the hippocampus, BrdU-labeled cells were quantified at several time points (3, 15, 30 and 60 days) after injury (Figure 1). No significant differences in BrdU immunoreactivity (BrdU-ir) were detected in the subgranular zone (SGZ) and molecular layer (Mol) layers between capsaicin treated, vagotomy and control groups at each considered time point (data not shown). Interestingly, intraperitoneal capsaicin administration significantly decreased BrdU incorporation in the granule cell layer (GCL) of the dentate gyrus after 60 days from treatment (P < 0.05, Figure 1D). Moreover, a significant difference (P < 0.05) in BrdU-ir between vagotomized animals and capsaicin-treated animals was found in the hilus at 15 days, where the vagotomized group showed significantly fewer BrdU-positive cells compared to the capsaicin-treated group. Capsaicin treatment slightly increased while vagotomy slightly decreased the number of BrdU-labeled cells; however these differences were statistically insignificant (Figure 1F).
Figure 1.
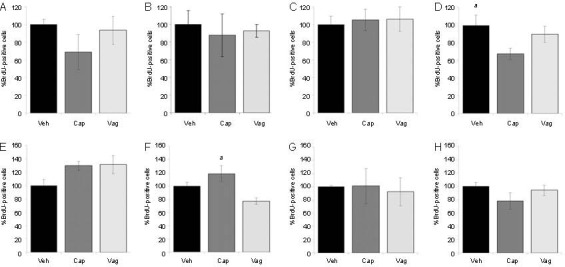
Bromodeoxyuridine (BrdU) quantification in the dentate gyrus. Quantification of BrdU-positive cells in the granular cell layer 3 days (A), 15 days (B), 30 days (C) and 60 days (D) after injury. Capsaicin treatment significantly decreased BrdU incorporation in the granular cell layer 60 days after injury. Quantification of BrdU-positive cells in the hilus 3 days (E), 15 days (F), 30 days (G) and 60 days (H) after injury.
At 15 days, a significant difference was found in the hilus between vagotomized animals and capsaicin-treated animals. Graphs represent percentages of BrdU-positive cells, where the average number in VEH animals was taken as 100%. Values in the graphics are expressed as mean ± SEM. VEH: vehicle treatment; CAP: capsaicin treatment; VAG: vagotomy, aP < 0.05.
To visualize the different layers of the dentate gyrus (DG), including SGZ, GCL, Mol and hilus, hippocampal sections were immunostained for a nuclear marker of mature neurons (NeuN). All hippocampal sections revealed multiple layers of NeuN-immunoreactive nuclei, forming the GCL of the DG. Hippocampal sections collected from vehicle- and capsaicin-treated rats as well as from vagotomized rats at 30 minutes after BrdU injection revealed no BrdU and NeuN double-labeled cells.
Doublecortin expression decreases after neurotoxic damage to the vagus nerve
To address the neuronal development, we used DCX, a microtubule-associated protein expressed by neuronal precursor cells and immature neurons. Neuronal precursor cells begin to express DCX while actively dividing, and their neuronal daughter cells continued to express DCX for 2-3 weeks as the cells matured into neurons[25,26,27]. The data indicated that capsaicin administration significantly decreased the number of doublecortin-positive cells in the DG after 3, 30 and 60 days from the injury (P < 0.05). At 15 days, the number of DCX-positive cells was not significantly different from that of control animals. No significant differences in DCX-positive cell numbers were seen between vagotomy group and control group for each considered time point (Figure 2).
Figure 2.
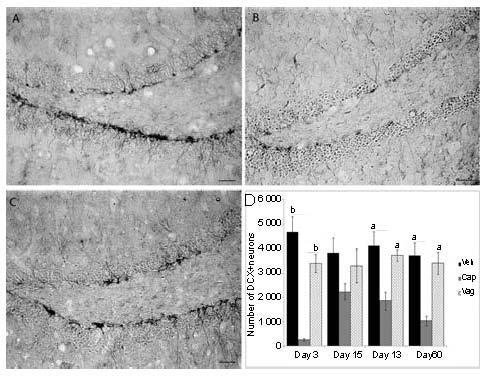
Immunohistochemistry for doublecortin in the dentate gyrus in control (A), capsaicin-treated (B) and vagotomy groups (C) 3 days after injury. (D) Quantification of DCX-positive cell numbers.
In the capsaicin-treated group, BrdU-positive cells are fewer than in the control group for each considered time point.
Values are expressed as mean ± SEM. VEH: Vehicle treatment; CAP: capsaicin treatment; VAG: vagotomy. aP < 0.05, bP < 0.001. Scale bar: 50 μm.
Microglia are activated after a vagus nerve injury
To investigate a possible change in glial cell activity, we focused our attention on the microglia since injury is often associated with an increase in microglia activation. We evaluated the relative absorbance of ionized calcium-binding adapter molecule 1 (iba1) immunoreactivity, a specific marker of microglial activation at 3, 15 and 30 days after injury. Sections collected from vehicle treated rats revealed microglia in maintenance state. Their morphology was indicated by cells with small perikarya and radially branching processes. Sections collected 30 days after capsaicin treatment and vagotomy revealed microglia with larger perikarya and fewer and shorter processes, characteristics of activation. Quantification analysis of Iba1 immunoreactivity revealed that the intensity of staining in the DG was significantly decreased (P < 0.05) 15 days after injury in both experimental groups. At 30 days, Iba1 immunoreactivity was significantly increased (P < 0.05) in both capsaicin-treated and vagotomy groups. The increased overall staining seemed to reflect the increased activation of labeled microglia rather than an increased number of labeled cells (Figure 3).
Figure 3.
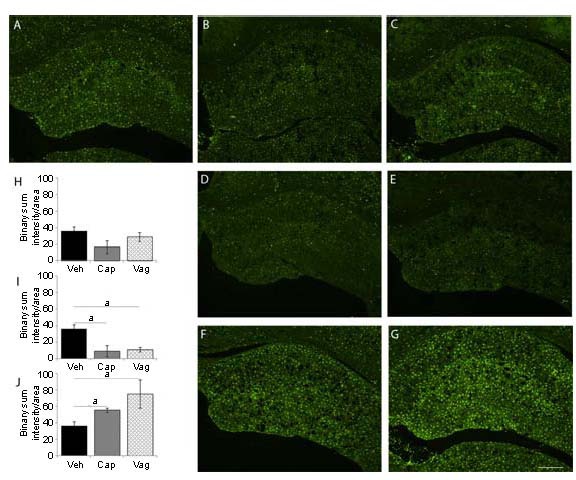
Iba1 quantification in the dentate gyrus.
(A) Vehicle; Iba1 immunoreactivity 3 days after injury in capsaicin (B) and vagotomy group (C); Iba1 immunoreactivity 15 days after injury in capsaicin-treated (D) and vagotomy group (E); Iba1 immunoreactivity 30 days after injury in capsaicin-treated (F) and vagotomy group (G); histograms showing quantification of Iba1 immunoreactivity 3 days (H), 15 days (I) and 30 days (J) after the injury.
No significant difference was seen 3 days after injury (H). At 15 days after injury, Iba1 immunoreactivity was significantly decreased in capsaicin-treated and vagotomy groups (I).
After 30 days from the injury, a significant increase in Iba1 staining was seen in capsaicin-treated and vagotomy groups (J).
Values are expressed as mean ± SEM. VEH: vehicle treatment; CAP: capsaicin treatment; VAG: vagotomy. aP < 0.05. Scale bar: 500 μm.
DISCUSSION
Studies using neuroanatomical, biochemical, electrophysiological and behavioural techniques proved that peripheral injuries trigger alterations of several areas of the CNS, including spinal cord, brainstem, thalamus, cortical and subcortical regions[28,29]. The peripheral and central nervous systems are functionally integrated regarding the consequences of a nerve injury: a peripheral nerve lesion always results in intense and long-lasting central plasticity and reorganization[28,30,31,32]. Moreover, recent experiments on VNS, a well-established adjunct to medical therapy for refractory epilepsy[33,34] and depression[33,35,36,37], demonstrated that acute VNS induces neuronal plasticity in the rat hippocampus. Mechanisms of the plasticity and reorganization occurring after a peripheral nerve injury are still very complex and have not been completely understood.
Since the VNS is stimulating vagal neurons without a damage, in this study, we tested the neurogenic responses of the hippocampus following the damage to the vagus nerve. In the age of obesity, bariatric surgeries, while damaging the vagus, have been proven to be an effective treatment. Our studies revealing the effect of the vagus injury on hippocampal plasticity shed the light on possible complications related to memory, learning and depression following the bariatric surgeries.
In our study, cell proliferation was revealed by using BrdU administered with a single i.p. injection 30 minutes before animal sacrifice. No BrdU/NeuN dual labeled cells were observed. This fact was rather expected because we sacrificed animals 30 minutes after BrdU injection, and since a new generated cell takes more than 2 weeks to differentiate into a mature cell expressing NeuN marker[38]. Moreover, it is highly unlikely that mature neuron will enter the cell cycle and will incorporate BrdU. BrdU incorporates into the nuclear DNA during cell division and labels mitotically active cells; a single injection of BrdU labels all the cells in the S-phase, whereas the proliferating cells in the other phases of the cell cycle remain unlabeled[39]. Thus, since the tissue was collected after a short time, mitotic figures can be seen, and this time point has been proposed as a true measure for the quantification of cells in the S-phase[39]. The results obtained from the distinct examined hippocampus layers showed that 15 days after injury, capsaicin treatment slightly increased while vagotomy slightly decreased the number of BrdU-labeled cells in the hilus. Vagotomized rats showed fewer BrdU-positive cells in the hilus, compared to the capsaicin-treated group. Moreover, intraperitoneal capsaicin administration significantly decreased BrdU incorporation in the GCL of the DG 60 days after treatment. Increased neural proliferation following capsaicin-induced damage to the vagus nerve was previously reported in the nodose ganglion[6,40]. Moreover, VNS has been shown to increase the number of BrdU-positive cells in the DG[1,41], demonstrating a rapid effect of VNS on rat hippocampal progenitor proliferation. Therefore, we conclude that the specific VNS (via the TRPV1 receptor or electric stimuli) rather than just the nerve damage is required to release the neurogenic potential and induce neural proliferation.
As a next step, we performed DCX-immunohistochemistry to follow the development of proliferating cells in the hippocampus. DCX is a 40 kDa microtubule-associated protein that is associated with migration of neuronal precursors of the developing CNS[26,42,43,44]. In the two neurogenic areas of adult mammalian brain (the SGZ in the DG of the hippocampus and the subventricular zone of the lateral ventricles), DCX is normally expressed by developing neurons[45]. During adult neurogenesis, DCX starts to be expressed when neuroblasts are generated, peaking during the second week, and then decreases, leaving the path to other mature neuronal markers, such as NeuN[25,26,27]. After acute VNS, an increase of both the total amount of DCX immunoreactivity and the number of DCX-positive neurons in the DG has been shown[1,44,46]. In the present study, we found that capsaicin administration significantly decreased the number of DCX-expressing cells in the DG after 3, 30 and 60 days from the injury. No significant differences in DCX-positive cell numbers were seen between the vagotomy group and control group for each considered time point. This fact may suggest that capsaicin-induced damage to vagal afferents may alter the local environment in the hippocampus and accelerate the maturation of newborn neurons, leading to a faster switch from DCX-expressing cells to NeuN-expressing cells. The possible role of capsaicin on neural proliferation has been recently reviewed[47]; however; the molecular mechanisms of its action need to be elucidated to support this hypothesis.
Activation of microglia commonly occurs in an early response of the CNS to a wide variety of pathological stimuli, including axotomy, trauma, inflammation, degeneration, and ischemia[48,49]. They also play a central role in chronic degenerative conditions, such as Alzheimer's disease, Parkinson's diseases, multiple sclerosis, amyotrophic lateral sclerosis, and many other diseases[50]. Since microglial activation usually increases after injury[51,52,53,54,55,56], in this study, we evaluated the intensity of iba1, a specific marker of microglia activation. Our results showed that both the capsaicin- and the vagotomy-induced damage to the vagus nerve first decreased microglia activation in the hippocampus 15 days after the injury. At 30 days post injury, capsaicin-treated and vagotomized rats revealed significantly more activated microglia compared to control animals. These findings suggest that damage to the subdiaphragmatic vagus in adult rats activates the microglia after a period of delay, probably due to the fact that in the first range of time after the injury, the microglia may migrate to other regions of the brain. This hypothesis is also supported by our recently published data[11] showing that subdiaphragmatic vagotomy significantly activated microglia in all the vagal nuclei in the hindbrain 2 weeks post-vagotomy. However, the prolonged microglia activation was only observed in the dorsal motor nucleus of the vagus. Results of the present study together with previously published data suggest that damage to the vagus nerve induces microglia activation progressing retrogradely from the periphery to the CNS. This prolonged activation of microglia in the CNS may have a significant effect on vagal structures through cytokine release and alterations of the microenvironment, leading to changes in adult neurogenesis and neural plasticity.
The results of our study suggest that peripheral nerve injury induces a cascade of events that alter adult neurogenesis in the hippocampus. Observed changes in cell proliferation, differentiation and microglia activation may have significant functional consequences. Therefore, consequences to memory and special navigation that result from PNS damage should be investigated in the future.
MATERIALS AND METHODS
Materials
All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee and conformed to National Institutes of Health guidelines for the use of vertebrate animals (publication No. 86-23, revised 1985). All efforts were made to minimize both the number of animals used and animal suffering. Experiments were performed on adult male Sprague-Dawley rats (6 weeks old; Simonsen Laboratories, Gilroy, CA, USA). Animals were housed in individual hanging cages in a temperature-controlled vivarium with ad libitum access to food (Harlan Teklad F6 Rodent Diet W; Harlan Laboratories, Madison, WI, USA) and water. Rats were maintained under standard laboratory conditions with a 12-hour light/dark cycle. Rats were divided into three groups. The first two groups received intraperitoneal injections of capsaicin or vehicle; the third group underwent subdiaphragmatic vagotomy.
Methods
Capsaicin and vehicle treatment
Forty rats were anesthetized with isoflurane anaesthetic (3% induction 1–2% maintenance). Twenty rats (five rats for each time point: 3, 15, 30, and 60 days) were injected i.p. with capsaicin (catalogue No. M2028; Sigma-Aldrich, St.Louis, MO, USA) while another twenty rats (five rats for each time point: 3, 15, 30, and 60 days) were injected i.p. with vehicles (10% ethanol in 10% Tween-80; catalog No. P1754; Sigma-Aldrich) in 0.9% saline solution). Three different doses of capsaicin were used in this study (25, 50, and 50 mg/kg; total dose 125 mg/kg) at 0, 6, and 24 hours, respectively, at an injection volume of 1 mL/kg. Twenty minutes before the first injection, animals were injected i.p. with atropine sulfate (3 mg). Artificial ventilation was provided, as required, during the 3- to 5-minute period of respiratory arrest that typically occurred after the first capsaicin injection. Vehicle injections followed the same protocol and schedule as capsaicin treatment.
Finally, to assess the effectiveness of the capsaicin treatment, rats were tested for the corneal chemosensory response (eye wipe) to mild corneal irritation, mediated by the capsaicin-sensitive trigeminal innervation of the cornea[57,58]. A drop of 1% ammonium hydroxide was applied on the corneal surface of one eye, and the number of eye wipes was measured during the 15 seconds immediately after the application. None of the capsaicin-treated rats had any eye-wipe response.
Subdiaphragmatic vagotomy
An additional twenty animals were subjected to a total surgical resection of both the dorsal and ventral subdiaphragmatic vagal trunks in a manner consistent with previously described procedures[59,60]. Briefly, after the animals were anesthetized with a drug cocktail containing ketamine, acepromazine, and xylazine (0.1 mL/100 g body weight), a ventral midline incision was made and the vagus was exposed by gentle retraction of the stomach and liver. A 5-mm section of each vagus nerve was transected and removed from the dorsal trunk above the celiac and gastric branches and from the ventral trunk above the hepatic and accessory celiac branches, as close to the oesophageal hiatus of the diaphragm as possible. Verification of completeness of vagotomy was performed with criteria described previously[24]. Rats were then maintained on the powdered rodent chow for a few days.
BrdU administration
Labelling of proliferating cells was performed by a single i.p. injection of the thymidine analogue BrdU (Sigma, Steinheim, Germany) at 100 mg/kg body weight 30 minutes before sacrifice. This post-BrdU survival time is sufficient to label cells in S-phase but not to allow the labelled cells to divide, thus providing a measure of cell proliferation[61,62].
Tissue fixation and sectioning
At the appropriate time points, animals were deeply anesthetized with ketamine/acepromazine/xylazine cocktail and transcardially perfused with 0.1 M phosphate buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde. After the perfusion, brains were immediately removed, post-fixed with 4% paraformaldehyde for 2 hours and immersed overnight in a cryoprotectant solution of 30% sucrose in PBS with 0.05% sodium azide at 4°C. Free-floating coronal sections (30 μm thick) were obtained using a freezing microtome through the entire hippocampus (-1.80 to -7.64 mm from bregma; Paxinos and Watson, 1997). Sections were stored in cryoprotectant at -20°C until immunohistochemical labelling.
Immunohistochemistry
For BrdU staining, every tenth free-floating section of the hippocampus (13 sections per animal) was used. For Iba1, the marker of activated microglia, five hippocampal sections from each animal were used. The sections were immersed for 15 minutes in a 1% Triton X-100 in Tris buffered saline. Antigen retrieval was performed by incubating for 90 minutes at 65°C in a solution 50% 0.3 M NaCl/30 mM citrate buffer/50% formamide, and then rinse with 0.3 M NaCl/30 mM citrate buffer. The sections were then incubated in 2 N HCl at 37° for 30 minutes and rinsed in 0.1 M borate buffer for 10 minutes. The sections were incubated for 30 minutes in a blocking solution consisting of 3% normal horse serum in 0.1% Triton X-100 solution. The blocking solution was used to wash the tissues, and each section was incubated overnight at room temperature with primary antibody. Subsequently, the sections were incubated for 2 hours at room temperature with appropriate secondary antibodies. Sections were mounted in ProLong (Invitrogen, Carlsbad, CA, USA) to reduce photo bleaching. Sections stained for BrdU were counterstained with NeuN, a neuronal nuclear marker of mature neurons.
For DCX staining, five sections from each rat were used. Blocking of endogenous peroxidase activity was performed with 0.3% H2O2 for 30 minutes. After washing with PBS, the slides were incubated in 1.5% horse serum in PBS for 30 minutes. Goat polyclonal antibody against doublecortin (1:1 000; Santa Cruz Biotechnologies, Santa Cruz, CA, USA) in 0.04% Triton-X100 in PBS was applied overnight at room temperature. The slides were subsequently exposed to biotinylated anti-goat IgG and streptavidin peroxidase complex (1:800 dilution). They were then visualized by staining with 3,3-diaminobenzidine (Sigma-Aldrich, Deisenhofen, Germany). The slides were finally mounted with Permount after dehydration.
Quantification analysis
Images of the DG were viewed and captured under ×200 and ×400 magnification with a Nikon 80 i imaging photomicroscope (Nikon, Tokyo, Japan) equipped with a digital camera (Nikon Digital Sight DS-Qi1Mc). The number of positive-stained profiles for BrdU was counted with the NIS-Elements AR 3.0 Imaging System (Nikon, Tokyo, Japan).
The number of BrdU-positive cells was determined for each rat by the optical fractionator method. Briefly, the counts were collected from every tenth hippocampal section throughout the anterior–posterior extent of the hippocampus (-1.80 to -7.64 mm from bregma; Paxinos and Watson, 1997). Resulting cell counts were multiplied by the fraction of the hippocampus examined (e.g., 10)[63,64]. Four discrete regions of the DG were examined[65]: the SGZ, GCL, Mol, and the hilus.
The number of DCX-positive cells was determined on five sections evenly spaced throughout the entire hippocampus. For quantification of Iba1 expression in the DG of each rat, the total fluorescence intensity was measured by the NIS-Element AR 3.0 Imaging system in five evenly spaced sections throughout the entire hippocampus; the intensity values were then averaged for each group of animals and expressed as the mean densitometric gray scale values.
Statistical analysis
Data were analyzed by one-way analysis of variance and preplanned comparisons with the controls performed by the post hoc Fisher and Bonferroni protected least significant difference test via StatView software (SAS Institute, Cary, NC, USA). Significance was set at P < 0.05. All values are presented as mean±SEM.
Acknowledgments
Authors would like to thank Jeanne Jensen for being always ready to “Polish my English”
Footnotes
Funding: Washington State University Start-up Funds, George W. Bagby Research Fund and Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR).
Conflict of interests: None declared
Ethical approval: All animal procedures conformed to National Institutes of Health guidelines for the use of vertebrate animals of USA.
(Edited by Magnaghi V/Oliveira T/Zhao LJ/Song LP)
REFERENCES
- 1.Biggio F, Gorini G, Utzeri C, et al. Chronic vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Int J Neuropsychopharmacol. 2009;12(9):1209–1221. doi: 10.1017/S1461145709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 3.Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125(Pt 1):113–122. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- 4.Korak KJ, Tam SL, Gordon T, et al. Changes in spinal cord architecture after brachial plexus injury in the newborn. Brain. 2004;127(Pt 7):1488–1495. doi: 10.1093/brain/awh155. [DOI] [PubMed] [Google Scholar]
- 5.Ryu V, Gallaher Z, Czaja K. Plasticity of nodose ganglion neurons after capsaicin- and vagotomy-induced nerve damage in adult rats. Neuroscience. 2010;167(4):1227–1238. doi: 10.1016/j.neuroscience.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Gallaher ZR, Ryu V, Larios RM, et al. Neural proliferation and restoration of neurochemical phenotypes and compromised functions following capsaicin-induced neuronal damage in the nodose ganglion of the adult rat. Front Neurosci. 2011;5:12. doi: 10.3389/fnins.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari CC, Tarelli R. Parkinson's disease and systemic inflammation. Parkinsons Dis. 2011:436–813. doi: 10.4061/2011/436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21(5):624–633. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diez M, Abdelmagid N, Harnesk K, et al. Identification of gene regions regulating inflammatory microglial response in the rat CNS after nerve injury. J Neuroimmunol. 2009;212(1-2):82–92. doi: 10.1016/j.jneuroim.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Hatashita S, Sekiguchi M, Kobayashi H, et al. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine (Phila Pa 1976) 2008;33(12):1344–1351. doi: 10.1097/BRS.0b013e3181733188. [DOI] [PubMed] [Google Scholar]
- 11.Gallaher ZR, Ryu V, Herzog T, et al. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci Lett. 2012;513(1):31–36. doi: 10.1016/j.neulet.2012.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro LA, Perez ZD, Foresti ML, et al. Morphological and ultrastructural features of Iba1-immunolabeled microglial cells in the hippocampal dentate gyrus. Brain Res. 2009;1266:29–36. doi: 10.1016/j.brainres.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiessner C, Gehrmann J, Lindholm D, et al. Expression of transforming growth factor-beta 1 and interleukin-1 beta mRNA in rat brain following transient forebrain ischemia. Acta Neuropathol. 1993;86(5):439–446. doi: 10.1007/BF00228578. [DOI] [PubMed] [Google Scholar]
- 14.Fujita T, Yoshimine T, Maruno M, et al. Cellular dynamics of macrophages and microglial cells in reaction to stab wounds in rat cerebral cortex. Acta Neurochir (Wien) 1998;140(3):275–279. doi: 10.1007/s007010050095. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- 16.Niquet J, Ben-Ari Y, Represa A. Glial reaction after seizure induced hippocampal lesion: immunohistochemical characterization of proliferating glial cells. J Neurocytol. 1994;23(10):641–656. doi: 10.1007/BF01191558. [DOI] [PubMed] [Google Scholar]
- 17.Huttmann K, Sadgrove M, Wallraff A, et al. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18(10):2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 18.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20(3):269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 19.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 20.Banati RB, Gehrmann J, Czech C, et al. Early and rapid de novo synthesis of Alzheimer beta A4-amyloid precursor protein (APP) in activated microglia. Glia. 1993;9(3):199–210. doi: 10.1002/glia.440090305. [DOI] [PubMed] [Google Scholar]
- 21.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 22.Hiura A. Neuroanatomical effects of capsaicin on the primary afferent neurons. Arch Histol Cytol. 2000;63(3):199–215. doi: 10.1679/aohc.63.199. [DOI] [PubMed] [Google Scholar]
- 23.Ritter S, Dinh TT. Capsaicin-induced neuronal degeneration: silver impregnation of cell bodies, axons, and terminals in the central nervous system of the adult rat. J Comp Neurol. 1988;271(1):79–90. doi: 10.1002/cne.902710109. [DOI] [PubMed] [Google Scholar]
- 24.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol. 1987;253(2 Pt 2):R361–370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- 25.Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134(1-2):13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- 26.Brown JP, Couillard-Despres S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 27.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 28.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82(4):163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Jain N, Florence SL, Kaas JH. Reorganization of somatosensory cortex after nerve and spinal cord injury. News Physiol Sci. 1998;13:143–149. doi: 10.1152/physiologyonline.1998.13.3.143. [DOI] [PubMed] [Google Scholar]
- 30.Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci. 1991;14:137–167. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- 31.Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev. 2002;39(2-3):181–215. doi: 10.1016/s0165-0173(02)00192-3. [DOI] [PubMed] [Google Scholar]
- 32.Kaas JH, Collins CE. Anatomic and functional reorganization of somatosensory cortex in mature primates after peripheral nerve and spinal cord injury. Adv Neurol. 2003;93:87–95. [PubMed] [Google Scholar]
- 33.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1(8):477–82. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 34.Schachter SC. Therapeutic effects of vagus nerve stimulation in epilepsy and implications for sudden unexpected death in epilepsy. Clin Auton Res. 2006;16(1):29–32. doi: 10.1007/s10286-006-0275-1. [DOI] [PubMed] [Google Scholar]
- 35.Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42(2-3):203–210. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 36.Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93–99. doi: 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- 37.Harden CL. The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology. 2002;59(6 Suppl 4):S48–55. doi: 10.1212/wnl.59.6_suppl_4.s48. [DOI] [PubMed] [Google Scholar]
- 38.Duan X, Kang E, Liu CY, et al. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18(1):108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18(3):311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 40.Czaja K, Burns GA, Ritter RC. Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats. Neuroscience. 2008;154(2):621–630. doi: 10.1016/j.neuroscience.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revesz D, Tjernstrom M, Ben-Menachem E, et al. Effects of vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214(2):259–265. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 42.des Portes V, Francis F, Pinard JM, et al. doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH) Hum Mol Genet. 1998;7(7):1063–1070. doi: 10.1093/hmg/7.7.1063. [DOI] [PubMed] [Google Scholar]
- 43.Gleeson JG, Allen KM, Fox JW, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92(1):63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 44.Couillard-Despres S, Winkler J, Uyanik G, et al. Molecular mechanisms of neuronal migration disorders, quo vadis? Curr Mol Med. 2001;1(6):677–688. doi: 10.2174/1566524013363195. [DOI] [PubMed] [Google Scholar]
- 45.Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14(4):629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- 46.Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 47.Czaja K, Czaja WE, Giacobini-Robecchi MG, et al. Injury-induced DNA replication and neural proliferation in the adult mammalian nervous system. In: Kusic-Tisma J, editor. DNA Replication and Related Cellular Processes. Rijeka (Croatia): InTech; 2011. [Google Scholar]
- 48.Morioka T, Kalehua AN, Streit WJ. The microglial reaction in the rat dorsal hippocampus following transient forebrain ischemia. J Cereb Blood Flow Metab. 1991;11(6):966–973. doi: 10.1038/jcbfm.1991.162. [DOI] [PubMed] [Google Scholar]
- 49.Moore S, Thanos S. The concept of microglia in relation to central nervous system disease and regeneration. Prog Neurobiol. 1996;48(4-5):441–460. doi: 10.1016/0301-0082(95)00051-8. [DOI] [PubMed] [Google Scholar]
- 50.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 51.Ito D, Imai Y, Ohsawa K, et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 52.Ito D, Tanaka K, Suzuki S, et al. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32(5):1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- 53.Mori I, Goshima F, Koshizuka T, et al. Iba1-expressing microglia respond to herpes simplex virus infection in the mouse trigeminal ganglion. Brain Res Mol Brain Res. 2003;120(1):52–56. doi: 10.1016/j.molbrainres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28(2):101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 56.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 57.Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jancso G, Kiraly E. Sensory neurotoxins: chemically induced selective destruction of primary sensory neurons. Brain Res. 1981;210(1-2):83–89. doi: 10.1016/0006-8993(81)90886-6. [DOI] [PubMed] [Google Scholar]
- 59.Yox DP, Stokesberry H, Ritter RC. Vagotomy attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1991;260(3 Pt 2):R503–508. doi: 10.1152/ajpregu.1991.260.3.R503. [DOI] [PubMed] [Google Scholar]
- 60.Burns GA, Ritter RC. Visceral afferent participation in delayed satiation following NMDA receptor blockade. Physiol Behav. 1998;65(2):361–366. doi: 10.1016/s0031-9384(98)00176-0. [DOI] [PubMed] [Google Scholar]
- 61.Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134(1-2):77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 62.Hayes NL, Nowakowski RS. Exploiting the dynamics of S-phase tracers in developing brain: interkinetic nuclear migration for cells entering versus leaving the S-phase. Dev Neurosci. 2000;22(1-2):44–55. doi: 10.1159/000017426. [DOI] [PubMed] [Google Scholar]
- 63.Eisch AJ, Barrot M, Schad CA, et al. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagace DC, Yee JK, Bolanos CA, et al. Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol Psychiatry. 2006;60(10):1121–1130. doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Noonan MA, Choi KH, Self DW, et al. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28(10):2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


