Abstract
Quantitative real-time reverse transcription-polymerase chain reaction (qPCR) is widely used to investigate transcriptional changes following experimental manipulations to the nervous system. Despite the widespread utilization of qPCR, the interpretation of results is marred by the lack of a suitable reference gene due to the dynamic nature of endogenous transcription. To address this inherent deficiency, we investigated the use of an exogenous spike-in mRNA, luciferase, as an internal reference gene for the 2-∆∆Ct normalization method. To induce dynamic transcription, we systemically administered capsaicin, a neurotoxin selective for C-type sensory neurons expressing the TRPV-1 receptor, to adult male Sprague-Dawley rats. We later isolated nodose ganglia for qPCR analysis with the reference being either exogenous luciferase mRNA or the commonly used endogenous reference β-III tubulin. The exogenous luciferase mRNA reference clearly demonstrated the dynamic expression of the endogenous reference. Furthermore, variability of the endogenous reference would lead to misinterpretation of other genes of interest. In conclusion, traditional reference genes are often unstable under physiologically normal situations, and certainly unstable following the damage to the nervous system. The use of exogenous spike-in reference provides a consistent and easily implemented alternative for the analysis of qPCR data.
Keywords: exogenous reference gene, sensory ganglia, reverse transcription-polymerase chain reaction, normalization, injury, neural regeneration
Abbreviations
qPCR, quantitative real-time reverse transcription-polymerase chain reaction; GADPH, glyceraldehydes-3-phospate dehydrogenase; Tubb3, β-III Tubulin; NG, nodose ganglia
INTRODUCTION
Quantitative real-time reverse transcription-polymerase chain reaction (qPCR) is one of the most sensitive and powerful methods for investigating specific changes in DNA transcription[1], and still remains the gold standard for verification of microarray and next-generation chromatin-protection assay results[2,3]. However, despite the prolific use of qPCR, a universal method of analysis has proven elusive. Analysis methods fall into one of two groups: absolute and relative quantification. Absolute quantification is implemented using an independent[4,5] or absolute[6] standard calibration curve with experimentally determined amplification efficiencies of known initial transcript concentrations to determine absolute copy number. Further, analysis of genes of interests rests upon the assumption that the amplification efficiencies of the calibration and target RNAs are equal[7]. Even when amplification efficiencies are nearly equal and constant, absolute quantification is not always possible due to the compounding of systemic error during the chain reaction[8,9,10]. Relative quantification is implemented using a control group as a reference in an attempt to determine the relative change in transcript under experimental conditions. Each of these methods has its deficiencies.
Relative quantification compensates for this deficiency of absolute quantification by measuring only the relative change in gene expression without an effort to determine the exact copy number. This is accomplished by normalizing the target RNA to an internal reference or several references undergoing simultaneous and similar amplification, often an endogenous reference gene which is assumed to undergo constitutive transcription[1]. To determine the best internal reference, an abundance of methods have been developed. Examples of these include Normfinder, which measures inter- and intra-group variation[11]; geNorm, which measures gene-gene expression ratios[12]; and BestKeeper which selects the gene of least variability based upon the geometric mean[13]. After a suitable reference is determined, a relative analysis method such as the 2-∆∆Ct method[14] is implemented to compare relative change between target and reference transcripts.
Although careful consideration must be taken to ensure reference RNA and target RNA have similar amplification efficiencies, the 2-∆∆Ct method is one of the most-prolific quantification methods. This is due to the relative ease of using an endogenous reference and the fact that simultaneous amplification of the reference and the target transcript ensures that both are equally subjected to PCR's inherent inefficiencies[15,16]. However, the major pitfall of this method lies in the fact that the assumed stable expression of an endogenous reference gene has increasingly proven false[12,17].
While many traditional reference genes—such as glyceraldehydes-3-phospate dehydrogenase (GADPH), β-actin (Actb), 18S rRNA, and Histone H2A, among others—served as controls in conventional RT-PCR and Northern blots, they were selected as qualitative positive controls, not as quantitative standards. With their initial adoption in qPCR analyses, classic reference genes were not re-evaluated for quantitative variability[18]. In fact, a significant quantity of literature has demonstrated a need to re-evaluate the standard practice of assuming constitutive expression of reference genes in neural[19,20,21] as well as a variety of other tissues[22]. Of particular interest to neuronal injury models, common reference genes used as internal controls (such as GAPDH, hypoxanthine guanine phosphoribosyl transferase [HPRT], cyclophilin A [CycA], ribosomal protein L13A [RPL13A], Actb and β-III tubulin [Tubb3] cannot be assumed to be stable[23,24,25]. Further, it has been demonstrated that treatment-altered reference-gene regulation within an experiment results in incorrect findings[26,27].
As an alternative to dynamically transcribed endogenous genes, exogenous mRNA spike-in transcripts have proven to be reliable references for qPCR analysis. Such transcripts provide a stable reference while simultaneously undergoing reverse transcription and amplification with the target transcript[28,29,30,31,32,33,34,35,36]. If selected carefully, these references will inherit the same efficiencies as the target transcripts. Exogenous references have been particularly well adopted in embryonic development models where transcription cannot be assumed to be constitutive[28,29,37,38]. They have been further implemented in quantifying dynamic changes in a number of genes commonly used as references[30,31], across phylogeny[32,33,34,35,39,40,41], tissue type[36,42], and treatment[22,43]. As far as we know, the only time an exogenous reference has been applied to the study of adult nervous tissue is in the examination of transcriptional changes within a neuronal culture using conventional PCR[43].
It was in our examinations of damage to the nervous system that we began to investigate the use of exogenous reference genes. We previously found a significant decrease in the number of neurons within the rat nodose ganglia (NG) following systemic administration of the neurotoxin, capsaicin, which selectively destroys small, unmyelinated C-type sensory neurons expressing the TRPV-1 receptor. However, at 60 days post-recovery, neuronal numbers returned to control levels[44,45]. Due to the dynamic nature of neurons within the ganglia, we expected the stability of all standard neuronal reference genes to decrease. Consequently, we chose to implement an exogenous spike-in of luciferase mRNA as an internal reference to investigate the differential expression of gene profiles within the NG using qPCR.
To the best of our knowledge, this is the first application of an exogenous reference transcript used to normalize qPCR results in neural tissue. To determine the impact that this method would have on the interpretation of qPCR results, we compared the exogenous luciferase reference data to those gathered using a standard neuronal reference gene, Tubb3. Our results indicate that the use of an exogenous reference provides increased stability and experimentally meaningful analysis during dynamic endogenous transcription.
RESULTS
Validation of luciferase as a suitable reference for real-time RT-PCR
Verification of luciferase efficiency was measured by running a titration series of luciferase using the method outlined above, varying the spike-in concentration of luciferase, diluting by magnitudes of ten. Each concentration was performed with three replicates. The qPCR assay for luciferase yielded an optimal efficiency of E = 103% (E = 10-1/m -1, where m is the slope of fitted line, Figure 1). This allows the relative quantification of RNA by the 2-∆∆CT method avoiding the elaborate amplification of standards in parallel[46].
Figure 1.
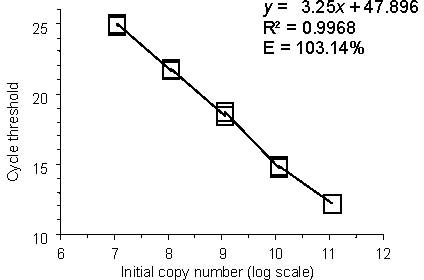
Validation of luciferase as a suitable reference for real-time RT-PCR.
Titration curve showing the decrease in cycle threshold for luciferase as the number of mRNA transcripts added at the RNA isolation step is increased. Amplifications were performed in triplicate for each initial concentration; the value for each of these amplifications is shown as a hollow box.
The efficiency of the amplification calculated using the equation E = 10-1/m -1, where m is the slope of the fitted line-demonstrates that with each cycle, roughly 100% of the mRNA transcript is copied.
Additionally, qPCR-step efficiency was determined by omitting the Luciferase spike-in prior to the RNA isolation step and substituting equal amounts of luciferase mRNA in each individual qPCR step in separate trials, according to the method outlined above and comparing the results. Cell lysis efficiency was 85.2±5.3%, RNA isolation 67.6±4.2%, DNA Removal 74.1±3.0%; reverse transcription efficiency was not determined. These findings, with the exception of DNA removal, were consistent with previous findings[34]. Final experimental spike-in concentration was calculated to equal a threshold cycle (Ct) of approximately 20 in the final qPCR measurement, which lay near the mean Ct for other genes investigated.
Differential expression of Tubb3 mRNA
We hypothesized that expression of traditional endogenous reference genes would be unstable following a neurotoxic dose of capsaicin, which selectively destroys small, unmyelinated C-type sensory neurons expressing the capsaicin receptor, TRPV-1. To test this, we examined the expression of Tubb3 using the luciferase spike-in as the reference. As expected, Tubb3 mRNA expression was unstable following capsaicin treatment (Figure 2). When compared to luciferase expression using the 2-ΔΔCt method, Tubb3 mRNA expression is increased at early time points after capsaicin treatment and highly variable, while at later time points expression returns to control levels (1.27 ± 0.92, 1.64 ± 1.12, 1.71 ± 0.44, 1.17 ± 0.19, 0.92 ± 0.31, 0.92 ± 0.41 for 1, 3, 15, 30, 60, and 180 days respectively). The difference was only significant at day 15 (P = 0.05) given the large variability of mRNA expression at 1 and 3 days, and a return to vehicle expression levels at 30 and 60 days.
Figure 2.
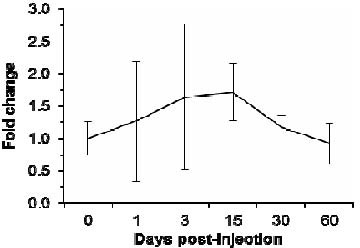
Differential expression of β-III tubulin (Tubb3) mRNA following capsaicin.
Fold change of Tubb3 mRNA in the nodose ganglia as shown by quantitative real-time reverse transcription-polymerase chain reaction with the exogenous luciferase reference. Capsaicin-treated rats (days 1, 3, 15, 30, and 60) are compared to time-matched vehicle-treated controls (graphically represented as day 0 to show normal amplification variability).
At early time points following the capsaicin injection, Tubb3 expression shows an increasing trend. However, the variability of the response to this injury results in widely varying expression between animals. At later post-injection time points, expression of this traditional reference gene returns to vehicle levels.
Comparison of luciferase and Tubb3 as reference genes
Given variable expression of the Tubb3 when using luciferase as a reference, we compared the results using either an endogenous reference, Tubb3, or an exogenous spike-in reference, luciferase, on relative expression of four other genes of interest: TRPV-1 (Trpv1), caspase-3 (Casp3), nestin (Nes), and glutamine synthetase (Glul). As previously stated, TRPV-1 is the capsaicin receptor. Caspase-3 expression increases with programmed cell death. Finally, nestin is expressed within neural progenitor cells, and glutamine synthetase is expressed in satellite glial cells. We expected to see a decrease in relative gene expression when comparing results obtained with the luciferase reference or the Tubb3 reference at early time points. In particular, we expected this change to be most significant at the 15 day time point when Tubb3 expression exhibits a 1.7 fold-change and the sample evaluation method is small.
When comparing results using either luciferase or Tubb3 as the reference, it is difficult to see a meaningful difference with regard to Trpv1 expression (Figure 3A). This may be because we are examining a decrease in expression that is approaching the lower physiological limit of expression. However, we do see some differences that may lead to varying interpretations. When Tubb3 is used as the reference, we see what appears to be a partial recovery in Trpv1 at 30 days post-capsaicin, where the difference between relative expression methods (luciferase and Tubb3) is significant at 15 days (P = 0.05), and the two demonstrate equal fold change at 30 and 60 days as the Tubb3 expression stabilizes. Alternatively, use of luciferase as a reference shows a decrease in Trpv1 that is not as drastic at early time points but is exceedingly stable throughout the remainder of the experiment.
Figure 3.
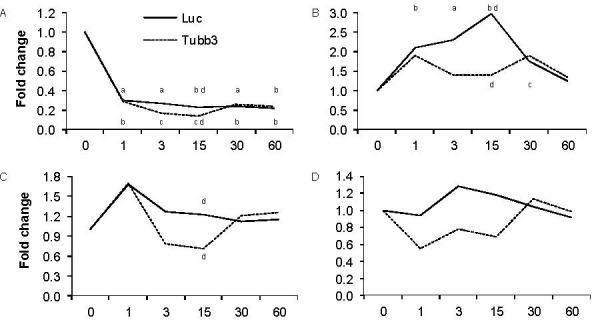
Consequences of choosing an endogenous reference.
Quantitative real-time reverse transcription-polymerase chain reaction fold changes for Trpv1 (A), Caspase-3 (B), Nestin (C), or Glutamine (D) when using the exogenous luciferase reference (Luc) or the endogenous Tubb3 reference. Tables specify the mean fold change ± SEM within capsaicin-treated nodose ganglia as compared to time-matched vehicle-treated controls for both reference genes. aP < 0.05, bP < 0.01, cP < 0.001 between capsaicin and vehicle controls; dP < 0.05 between time-matched Luc and Tubb3 reference genes.
As with Trpv1, the overall pattern of Casp3 expression is the same regardless of the reference gene, although in this case there is an increase in relative expression (Figure 3B). However, the increased Tubb3 expression at the middle time points effectively masks the real increase in Casp3 expression. This difference is best exemplified at 15 days when the Tubb3 reference displays a 1.4-fold increase in Casp3 expression compared with a 3.0-fold increase using luciferase as a reference (P = 0.023). At later time points, there is no significant difference between reference genes.
Unlike the other two genes observed here, the pattern of Nes expression depends on which reference gene is used (Figure 3C). When Tubb3 is used as a reference, Nes expression exhibits a transient increase 1 day after capsaicin administration before falling below baseline at 3 and 15 days. However, when luciferase is the reference, Nes expression exhibits a prolonged increase, slowly decreasing to baseline at 30 days of recovery. At day 15, comparison yields a significant difference between the 1.2 fold-increase with a luciferase reference and a 7 fold-change displayed with a Tubb3 reference (P = 0.033).
Glul similarly shows differential expression between reference genes (Figure 3D). Using luciferase as a reference, Glul is upregulated at 3 days and slowly decreases until it reaches control levels at day 60. Alternatively, with Tubb3 as a reference, Glul expression repeatedly increases and decreases until it reaches control levels at day 60. The latter is an effect of the instability of Tubb3 as a reference.
When comparing the results of each of the four genes of interest listed above, several interpretational errors occur: amplification of fold-change, decrease in fold-change toward baseline, or a combination of the two, which make results appear to oscillate. The effect of dynamic transcription can therefore lead to misinterpretation of genes of interest because endogenous transcripts do not provide appropriately stable controls. In all of the cases above, while the transcription environment remains variable, an exogenous reference proves more reliable. In fact, comparing variability of luciferase Ct values and variability of Tubb3 Ct values, the election to use an exogenous reference resulted in a 58.8±4.0% reduction in the variance of the reference gene, greatly increasing the overall reliability of the real-time RT-PCR analyses. On the other hand, at 30 and 60 days when the transcription environment has stabilized, there is demonstrably no difference between use of an endogenous or exogenous reference. This provides further credence to the ability of an exogenous reference to supplement the previously established 2-∆∆Ct relative quantification method.
DISCUSSION
The objective of this work was to demonstrate the efficacy of exogenous reference genes in qPCR analysis. This is salient to accurately quantify transcription in neuronal tissue following nerve injury when the constitutive expression of traditional reference genes is suspect. Accurate quantification of transcription is particularly limited by transcript losses during qPCR processing: cell lysis, RNA isolation, DNA removal, and reverse transcription[1,7,15]. Traditional methods attempt to compensate for these losses based upon the assumed relative stability of either total RNA, ribosomal RNA, tissue volume, or endogenous transcripts. However, this assumption has proven hasty[1]. The method outlined above-the spike-in of an exogenous mRNA transcript for relative RT-PCR normalization-mitigates these limitations by implementing a sample independent method and minimizing random error introduced by external calibration (Nordgard 2006). In this case, we were able to reduce reference Ct variability by using an exogenously added luciferase transcript rather than a traditional reference gene (i.e. β-III Tubulin, GAPDH,β actin), which have been shown to undergo dynamic transcription[19,20,21,22,23,24,25,26,27].
We previously observed a loss and recovery in the number of sensory neurons within the nodose ganglia following capsaicin-induced neuronal death[44], and we therefore hypothesized that Tubb3 and nestin expression would increase prior to neuronal recovery. Further, we previously found the loss of neurons to be mediated cleaved-caspase, and therefore we expected to see an increase in Casp3 mRNA expression following capsaicin. These results were consistent with previous findings with the exception that by 30 days cleaved-caspase immunoreactivity had returned to control levels whereas Casp3 was upregulated, though not significantly. Further, glutamine synthetase should also increase as satellite glial cells respond to systemic administration of capsaicin and proliferate[45]. On the other hand, TrpV1 mRNA expression and previous immunoreactivity results are difficult to compare. While TrpV1 mRNA expression is down-regulated, immunoreactivity shows a sharp decline in the total number of TrpV1-positive neurons, followed by increases until it reached control at 60 days[44]. We can offer one of several speculations: (1) immunoreactivity is not quantitative and while the number of TrpV1 neurons returns to control, the number of receptors per neuron is down-regulated and immunohistochemistry is unable to show this; (2) a translational change occurs that allows TrpV1 mRNA to be translated more efficiently with fewer transcripts required to produce an equivalent expression of TrpV1 receptors; or (3) the antibody used for TrpV1 immunoreactivity is not sufficiently specific to account for a functional change in TrpV1 receptors measured by the change in TrpV1 mRNA expression. Additional experiments should be performed to explain this phenomenon as well as the functional viability of TrpV1 receptors following the initial challenge of neurotoxic capsaicin.
A limitation of this method lay within the fact that it only provides information about the relative change in transcription. With greater effort, it is conceivable that an absolute method could be developed to provide information about initial target transcript quantities. First among these would be to account for differences in reverse transcription efficiencies. Before suggesting that housekeeping genes were constitutively expressed and suggesting their use as stable references[1], several attempts were made to develop exogenous reference methods for use in conventional PCR. In single transcript investigations, exogenous references nearly identical to the target transcript (with the exception of an inserted or deleted sequence[47,48,49] or a single base mutation[50,51]) proved beneficial in that they could utilize single primer sets. In the case of Becker-André et al. and Roy et al., these exogenous transcripts could be assumed to maintain efficiency nearly identical to the transcript of interest[50,51]. If added in known quantities, these methods could also provided information about the target's absolute input copy number. However, these methods required the addition of either a selective endonuclease digestion step or electrophoretic separation to differentiate between the two mRNAs and a sufficient primer concentration to mitigate reaction competition. Furthermore, the method would prove time consuming because it requires a nearly identical reference for each transcript of interest. The contemporary equivalent, a “dual spike-in method” was investigated by Zhang et al[35], which would answer many of these problems by attempting to quantify loses from reverse transcription. However, since the “dual spike-in method” comes with many of the benefits of earlier competitive PCRs, it also inherits similar undermining demands[49]; namely, the cost of investigating an additional cDNA transcript for each mRNA transcript of interest. Johnson et al[34]. developed a similar method hoping to achieve absolute quantification through the use of an exogenous reference and independent and absolute standard curves. However, the issue for qPCR analysis still remains one of balancing reliable results with ease of procedure.
In this study, a discrete volume of tissue-the isolated nodose ganglia-acted was uased as a control for total tissue volume to measure transcriptional changes relative to the entire ganglia. In other cases where the tissue of interest is not naturally defined, special care must be taken to ensure consistent volume and cell composition. In the case of cell-cultures, additional measures must be taken to normalize for cell number and volume[28,29,37,38]. None of the methods introduced thus far is able to accurately control for losses from incomplete cell lysis.
Absolute quantification should still be used in situations where it is necessary to determine absolute copy number of the transcript. However, in situations where knowledge about absolute copy number is not essential, relative quantification methods provide useful results. The use of an exogenous reference could be expanded similar to methods outlined above to allow for absolute quantification, however further characterization, such as its thermal stability, of the exogenous transcript would prove necessary. Ideally, quantification and comparison of reference and target transcript efficiencies should also be undertaken. These methods should be balanced to achieve the specific requirements of each experiment. The use of an endogenous transcript may still allow relative transcriptional quantification, although it may also limit the generalizability of findings.
Alternatively, an exogenous spike-in method provides several benefits. It is not actuated upon the assumed constitutive transcription of endogenous transcripts, an assumption which remains uncertain. Indeed, if the target and endogenous reference transcripts are not selected carefully, concordant regulation could lead to the erroneous conclusion of little to no change in transcription, or other mis-selection could lead to the conclusion that the target transcript changed when in reality it was the reference. The use of an unstable endogenous reference does not eliminate all useful information but it confines the relative comparability of results. In many instances it may only provide relationships confined in relevance to a given study or to single gene pair interactions.
The use of an exogenous spike-in reference is an efficient and easily implemented protocol to supplement known deficiencies in the well-established 2-∆∆Ct relative quantification method for qPCR analysis. Its greatest benefit lay within its ability to provide comparable results across studies if the same exogenous transcript is used. The use of an exogenous reference is validated by the fact that it reveals differential expression which is missed when using an unstable endogenous reference. Alternatively, when the endogenous reference is stable, as is the case in our example at later time points after the capsaicin injury, comparable results are yielded. Together, these benefits highlight the importance of including an exogenous reference for qPCR studies which seek to analyze mRNA expression following injury or other forms of dynamic expression.
MATERIALS AND METHODS
Materials
Male Sprague-Dawley rats (8-weeks old at the time of injections; Simonsen Laboratories, Gilroy, CA) were housed in groups of two, in a temperature-controlled vivarium with ad libitum access to food (Harlan Teklad F6 Rodent Diet W, Madison, WI) and water. Rats were maintained on a 12-hour light/dark schedule and habituated to laboratory conditions for 6 days prior to injections. All animal procedures were approved by the Washington State University Institutional Animal Care and Use Committee and conform to National Institutes of Health guidelines for the use of vertebrate animals of USA.
Methods
Capsaicin treatment
A total of 30 rats (n = 6 per time point) were injected intraperitoneally (i.p.) with capsaicin (lot. no. M2028, Sigma-Aldrich, St. Louis, MO). The total capsaicin dose (125 mg/kg) was administered as a series of three injections (25, 50, and 50 mg/mL dissolved in 10% ethanol and 10% Tween-80 in 0.9% saline) over 24 hours (0, 6, and 24 hours, respectively) at an injection volume of 1 mL/kg. Additionally, 30 rats (n = 6 per group) were injected with a vehicle solution (10% ethanol and 10% Tween-80 in 0.9% saline) using the same schedule and injection volumes. Rats were given an i.p. injection of 0.1 mL atropine (0.54 mg/mL) 5 minutes prior to all capsaicin or vehicle injections. Rats were under general inhalation anesthesia during capsaicin or vehicle treatment. Anesthesia was maintained with isoflurane in oxygen at an end-tidal concentration of 3.0%. Manual ventilation was provided following initial capsaicin injection until rats showed signs of breathing on their own, approximately 5-8 minutes. The effectiveness of the capsaicin treatment was determined by eye wipe response to a chemo-corneal stimulation test, mediated by capsaicin-sensitive innervation[44]. Briefly, a drop of 1% ammonium hydroxide was placed on the corneal surface of one eye. Control rats immediately wiped the eye following administration of ammonium hydroxide. All capsaicin-treated rats failed to wipe their eyes and were therefore included in the study.
Real-time RT-PCR
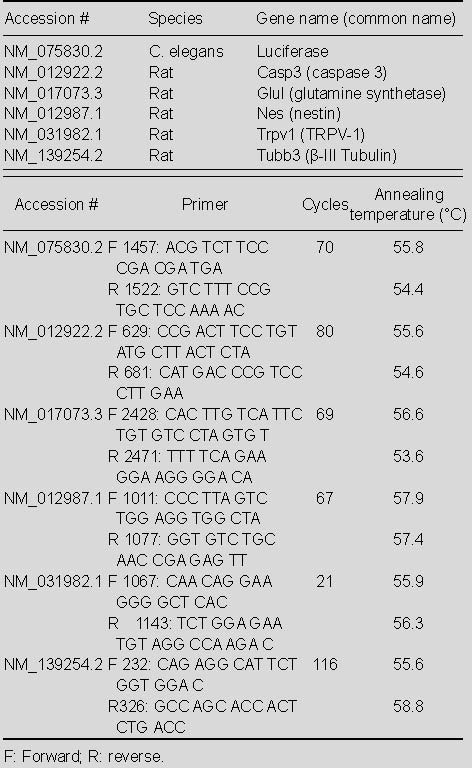
For each of the time points (1, 3, 15, 30, and 60 days), six capsaicin and six vehicle rats were anesthetized (ketamine 25, xylazine 2.5 mg/100 g) so that left and right NG could be collected before the animal was euthanized. NG was stored in RNALater (lot. no. AM7021, Applied Biosystems, Austin, TX) at -20°C until processing. RNA was isolated from pooled left and right NG with the Ambion RNAqueous kit (lot. no. AM1912, Applied Biosystems) according to the manufacturer's directions with a slight variation. Namely, preceding any tissue processing, 1 μg luciferase control mRNA (lot. No. L4561; Promega, Madison, WI, USA in a volume of 1 μL was added to pooled NG tissue in 200 μL cell lysis solution and 20 μL Proteinase K (Fermentas lot. No. EO0491, Glen Burnie, MD) for tissue homogenization and RNA isolation. The isolated RNA was then treated with an Ambion TURBO DNA-free kit (lot. No. AM1907, Applied Biosystems, Foster, CA, USA). First-strand cDNA synthesis was performed using the iScript cDNA synthesis kit (lot. No. 170-8890; Bio-Rad, Hercules, CA, USA). Intron-spanning probe-based PCR assays were designed using the Roche Universal Probe Library Assay Design Center (www.rocheapplied-science.com). Primers were obtained from Integrated DNA Technologies (Coralville, IA, USA), and probes were obtained from Roche Applied Science (Indianapolis, IN, USA). Primers and probes are listed in Table 1. Basic Logic Alignment Search Tool (BLAST) was used to verify that primers exhibited no homology with sequences other than those intended.
Table 1.
Comparison of the number of terminal deoxynuc primers and probes
qPCR reactions were performed in triplicate at 20 μL total volume in a Bio-Rad iCycler with iQ Supermix (lot. No. 170-8860). The amplification conditions were 1 cycle at 95°C for 4 minutes, followed by 50 cycles of oscillating between 94°C for 20 seconds and 60°C for 1 minute. Substitution of ddH2O for cDNA served as negative controls. A single fluorescence threshold was identified by the native iCycler software, which corresponded with exponential amplification in every sample for each target transcript. The threshold cycle (Ct) values were then used in accordance with the 2-∆∆Ct method to analyze qPCR data, with either luciferase or Tubb3 as the reference[14].
Statistical analysis
Data were analyzed by one-way repeated-measures analysis of variance (α = 0.05) using SigmaStat 3.5 software (Systat Software, Chicago, IL, USA). Where these tests yielded significant results, a Tukey Test was rerun with the same parameters for pairwise comparisons of means for different treatment groups.
Acknowledgments
The authors would like to thank Jeanne Jensen for assistance with editing.
Footnotes
Funding: This project was supported by the Washington State University Start-up Funds, George W. Bagby Research Fund.
Conflicts of interest: None declared.
Ethical approval: All animal procedures conformed to National Institutes of Health guidelines for the use of vertebrate animals of USA.
(Edited by oliveira JT/Lin NK/Zhao LJ/Song LP)
REFERENCES
- 1.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 2.Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 3.Haring M, Offermann S, Danker T, et al. Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods. 2007;3:11. doi: 10.1186/1746-4811-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W, Saint DA. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal Biochem. 2002;302(1):52–59. doi: 10.1006/abio.2001.5530. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Saint DA. Validation of a quantitative method for real time PCR kinetics. Biochem Biophys Res Commun. 2002;294(2):347–353. doi: 10.1016/S0006-291X(02)00478-3. [DOI] [PubMed] [Google Scholar]
- 6.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26(1):112. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- 8.Raeymaekers L. Quantitative PCR: theoretical considerations with practical implications. Anal Biochem. 1993;214(2):582–585. doi: 10.1006/abio.1993.1542. [DOI] [PubMed] [Google Scholar]
- 9.Raeymaekers L. A commentary on the practical applications of competitive PCR. Genome Res. 1995;5(1):91–94. doi: 10.1101/gr.5.1.91. [DOI] [PubMed] [Google Scholar]
- 10.Souazé F, Ntodou-Thomé A, Tran CY, et al. Quantitative RT-PCR: limits and accuracy. Biotechniques. 1996;21(2):280–285. doi: 10.2144/96212rr01. [DOI] [PubMed] [Google Scholar]
- 11.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 12.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfaffl MW, Tichopad A, Prgomet C, et al. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–515. doi: 10.1023/b:bile.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15(3):155–166. [PMC free article] [PubMed] [Google Scholar]
- 16.Bustin SA. Real-time, fluorescence-based quantitative PCR: a snapshot of current procedures and preferences. Expert Rev Mol Diagn. 2005;5(4):493–498. doi: 10.1586/14737159.5.4.493. [DOI] [PubMed] [Google Scholar]
- 17.Dheda K, Huggett JF, Bustin SA, et al. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37(1):112–114. doi: 10.2144/04371RR03. 116, 118-119. [DOI] [PubMed] [Google Scholar]
- 18.Huggett J, Dheda K, Bustin S, et al. Real-time RT-PCR normalisation: strategies and considerations. Genes Immun. 2005;6(4):279–684. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 19.Tohda M, Qi Z, Watanabe H. Influence of chronic treatment with imipramine on mRNA levels in rat brain: elevation of glyceraldehyde-3-phosphate dehydrogenase levels. Jpn J Pharmacol. 1999;81(4):393–396. doi: 10.1254/jjp.81.393. [DOI] [PubMed] [Google Scholar]
- 20.Drigues N, Poltyrev T, Bejar C, et al. cDNA gene expression profile of rat hippocampus after chronic treatment with antidepressant drugs. J Neural Transm. 2003;110(12):1413–1436. doi: 10.1007/s00702-003-0077-8. [DOI] [PubMed] [Google Scholar]
- 21.Pernot F, Dorandeu F, Beaup C, et al. Selection of reference genes for real-time quantitative reverse transcription-polymerase chain reaction in hippocampal structure in a murine model of temporal lobe epilepsy with focal seizures. J Neurosci Res. 2010;88(5):1000–1008. doi: 10.1002/jnr.22282. [DOI] [PubMed] [Google Scholar]
- 22.Sugden K, Pariante CM, McGuffin P, et al. Housekeeping gene expression is affected by antidepressant treatment in a mouse fibroblast cell line. J Psychopharmacol. 2010;24(8):1253–1259. doi: 10.1177/0269881108099690. [DOI] [PubMed] [Google Scholar]
- 23.Yao L, Chen X, Tian Y, et al. Selection of housekeeping genes for normalization of RT-PCR in hypoxic neural stem cells of rat in vitro. Mol Biol Rep. 2012;39(1):569–576. doi: 10.1007/s11033-011-0772-8. [DOI] [PubMed] [Google Scholar]
- 24.Bangaru ML, Park F, Hudmon A, et al. Quantification of gene expression after painful nerve injury: validation of optimal reference genes. J Mol Neurosci. 2012;46(3):497–504. doi: 10.1007/s12031-011-9628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn K, Huh JW, Park SJ, et al. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol Biol. 2008;9:78. doi: 10.1186/1471-2199-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bas A, Forsberg G, Hammarström S, et al. Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol. 2004;59(6):566–573. doi: 10.1111/j.0300-9475.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- 27.Tricarico C, Pinzani P, Bianchi S, et al. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309(2):293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 28.Lequarre AS, Traverso JM, Marchandise J, et al. Poly(A) RNA is reduced by half during bovine oocyte maturation but increases when meiotic arrest is maintained with CDK inhibitors. Biol Reprod. 2004;71(2):425–431. doi: 10.1095/biolreprod.103.026724. [DOI] [PubMed] [Google Scholar]
- 29.Vigneault C, McGraw S, Massicotte L, et al. Transcription factor expression patterns in bovine in vitro-derived embryos prior to maternal-zygotic transition. Biol Reprod. 2004;70(6):1701–1709. doi: 10.1095/biolreprod.103.022970. [DOI] [PubMed] [Google Scholar]
- 30.Bower NI, Moser RJ, Hill JR, et al. Universal reference method for real-time PCR gene expression analysis of preimplantation embryos. Biotechniques. 2007;42(2):199–206. doi: 10.2144/000112314. [DOI] [PubMed] [Google Scholar]
- 31.Bettegowda A, Patel OV, Ireland JJ, et al. Quantitative analysis of messenger RNA abundance for ribosomal protein L-15, cyclophilin-A, phosphoglycerokinase, beta-glucuronidase, glyceraldehyde 3-phosphate dehydrogenase, beta-actin, and histone H2A during bovine oocyte maturation and early embryogenesis in vitro. Mol Reprod Dev. 2006;73(3):267–278. doi: 10.1002/mrd.20333. [DOI] [PubMed] [Google Scholar]
- 32.Baker PJ, O’Shaughnessy PJ. Expression of prostaglandin D synthetase during development in the mouse testis. Reproduction. 2001;122(4):553–559. doi: 10.1530/rep.0.1220553. [DOI] [PubMed] [Google Scholar]
- 33.Smith RD, Brown B, Ikonomi P, et al. Exogenous reference RNA for normalization of real-time quantitative PCR. Biotechniques. 2003;34(1):88–91. doi: 10.2144/03341st05. [DOI] [PubMed] [Google Scholar]
- 34.Johnson DR, Lee PK, Holmes VF, et al. An internal reference technique for accurately quantifying specific mRNAs by real-time PCR with application to the tceA reductive dehalogenase gene. Appl Environ Microbiol. 2005;71(7):3866–3871. doi: 10.1128/AEM.71.7.3866-3871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Wei Z, Li YY, et al. Transcription level of messenger RNA per gene copy determined with dual-spike-in strategy. Anal Biochem. 2009;394(2):202–208. doi: 10.1016/j.ab.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 36.Revilla-Fernández S, Wallner B, Truschner K, et al. The use of endogenous and exogenous reference RNAs for qualitative and quantitative detection of PRRSV in porcine semen. J Virol Methods. 2005;126(1-2):21–30. doi: 10.1016/j.jviromet.2005.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrenzycki C, Herrmann D, Carnwath JW, et al. Alterations in the relative abundance of gene transcripts in preimplantation bovine embryos cultured in medium supplemented with either serum or PVA. Mol Reprod Dev. 1999;53(1):8–18. doi: 10.1002/(SICI)1098-2795(199905)53:1<8::AID-MRD2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Yaseen MA, Wrenzycki C, Herrmann D, et al. Changes in the relative abundance of mRNA transcripts for insulin-like growth factor (IGF-I and IGF-II) ligands and their receptors (IGF-IR/IGF-IIR) in preimplantation bovine embryos derived from different in vitro systems. Reproduction. 2001;122(4):601–610. [PubMed] [Google Scholar]
- 39.Myers MB, Mittelstaedt RA, Heflich RH. Using phiX174 DNA as an exogenous reference for measuring mitochondrial DNA copy number. Biotechniques. 2009;47(4):867–869. doi: 10.2144/000113222. [DOI] [PubMed] [Google Scholar]
- 40.Jelaso AM, Lehigh-Shirey E, Means J, et al. Gene expression patterns predict exposure to PCBs in developing Xenopus laevis tadpoles. Environ Mol Mutagen. 2003;42(1):1–10. doi: 10.1002/em.10173. [DOI] [PubMed] [Google Scholar]
- 41.McMaugh SJ, Lyon BR. Real-time quantitative RT-PCR assay of gene expression in plant roots during fungal pathogenesis. Biotechniques. 2003;34(5):982–986. doi: 10.2144/03345st04. [DOI] [PubMed] [Google Scholar]
- 42.Futamata H, Kaiya S, Sugawara M, et al. Phylogenetic and transcriptional analyses of a tetrachloroethene-dechlorinating “Dehalococcoides” enrichment culture TUT2264 and its reductive- dehalogenase genes. Microbes Environ. 2009;24(4):330–337. doi: 10.1264/jsme2.me09133. [DOI] [PubMed] [Google Scholar]
- 43.Chelly J, Montarras D, Pinset C, et al. Quantitative estimation of minor mRNAs by cDNA-polymerase chain reaction. Application to dystrophin mRNA in cultured myogenic and brain cells. Eur J Biochem. 1990;187(3):691–698. doi: 10.1111/j.1432-1033.1990.tb15355.x. [DOI] [PubMed] [Google Scholar]
- 44.Czaja K, Burns GA, Ritter RC. Capsaicin-induced neuronal death and proliferation of the primary sensory neurons located in the nodose ganglia of adult rats. Neuroscience. 2008;154(2):621–630. doi: 10.1016/j.neuroscience.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallaher ZR, Ryu V, Larios RM, et al. Neural proliferation and restoration of neurochemical phenotypes and compromised functions following capsaicin-induced neuronal damage in the nodose ganglion of the adult rat. Front Neurosci. 2011;5:12. doi: 10.3389/fnins.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Futscher BW, Blake LL, Gerlach JH, et al. Quantitative polymerase chain reaction analysis of mdr1 mRNA in multiple myeloma cell lines and clinical specimens. Anal Biochem. 1993;213(2):414–421. doi: 10.1006/abio.1993.1440. [DOI] [PubMed] [Google Scholar]
- 48.Barthelson RA. Quantitation of IL-4 expression in small numbers of cells from mice. J Immunol Methods. 1993;161(1):67–76. doi: 10.1016/0022-1759(93)90198-g. [DOI] [PubMed] [Google Scholar]
- 49.Wang AM, Doyle MV, Mark DF. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker-André M, Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY) Nucleic Acids Res. 1989;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy G, Roy R, Mitra S. Quantitative reverse transcriptase polymerase chain reaction for measuring the N-methylpurine-DNA glycosylase mRNA level in rodent cells. Anal Biochem. 1997;246(1):45–51. doi: 10.1006/abio.1996.9992. [DOI] [PubMed] [Google Scholar]


